Introduction
Wernicke's encephalopathy (WE) is an acute or
subacute neuropsychiatric syndrome that is caused by thiamine
(vitamin B1) deficiency. This vitamin plays indispensable roles in
various physiological processes, including functioning as a
coenzyme in the metabolism of carbohydrates, fats and proteins,
making it a crucial enzyme regulating the proper functioning of the
nervous system, muscles and cells (1). In humans, intestinal bacteria can
synthesize only small amounts of thiamine, and thus, dietary intake
is necessary (2). Generally, the
occurrence of WE is associated with alcohol abuse. Evidence from
numerous clinical case reports has indicated that WE is also common
in patients with cancer, particularly those with advanced cancer.
Reduced thiamine availability and storage capacity in patients with
cancer may arise from starvation, malabsorption, malnutrition,
chemotherapeutic agents use (e.g., 5-fluorouracil) and disease
progression (3–5). Moreover, in patients with cancer
undergoing gastrointestinal surgeries or experiencing complications
such as vomiting, diarrhea or intestinal obstruction, thiamine
absorption may be compromised, leading to thiamine deficiency
(4). There are also rare factors
contributing to thiamine deficiency, such as prolonged
hospitalization and administration of total parenteral nutrition
(TPN) without thiamine support supplementation.
The classic clinical triad of WE comprises
alterations in consciousness, eye movement disorders and ataxia. In
terms of diagnosis, WE is primarily based on the presence of one or
two core manifestations. Magnetic resonance imaging (MRI) is
currently considered the most reliable tool for diagnosing WE,
providing high specificity and accurate positive predictive value,
but low sensitivity (6). The
prevalence of WE is in the range of 0.4 to 2.8%, as determined by
several autopsy studies (7,8). However, in patients with cancer, WE
may be underdiagnosed due to factors such as the under-recognition
of early symptoms and the lack of specific diagnostic criteria for
critically ill patients. The present study describes the case of a
woman with advanced primary cervical cancer who developed WE,
highlighting the need for early recognition and monitoring of
thiamine levels in patients with cancer, particularly those with
malnutrition or undergoing prolonged hospitalization.
Case report
In June 2014, a 44-year-old woman underwent
laparoscopic radical hysterectomy with endoscopic pelvic
lymphadenectomy for the treatment of primary cervical
adenocarcinoma [pathological T1b2N0M0 stage 1B, according to the
7th edition of the Union for International Cancer Control-American
Joint Committee on Cancer staging system (9)] at the Cancer Hospital, Chinese Academy
of Medical Sciences (Beijing, China). In June 2016, multiple
metastases were detected in the pelvic and abdominal cavity, right
iliopsoas muscle and iliac wing during a follow-up positron
emission tomography-computed tomography (PET-CT) examination at the
Cancer Hospital, Chinese Academy of Medical Sciences. Subsequently,
multiple cycles of various chemotherapy regimens were administered
as follows: Paclitaxel liposomes (240 mg) combined with nedaplatin
(120 mg) was administered over 6 cycles via intravenous infusion;
oral altretamine was administered for 4 months (specific dosage
unavailable); liposome-encapsulated doxorubicin (60 mg) combined
with lobaplatin (50 mg) was administered over 3 cycles via
intravenous infusion; and albumin-bound paclitaxel (300 mg)
combined with oxaliplatin (200 mg) was administered over 2 cycles
by intravenous infusion. In June 2019, the patient developed a
fever of 38.5°C, and in July, TPN was provided at an external
hospital due to intestinal obstruction. At the Department of
Oncology, Guang'anmen Hospital, China Academy of Chinese Medical
Sciences (Beijing, China), symptoms of a fever, abdominal pain and
vomiting were observed, which were ascribed to the unresolved
intestinal obstruction. The patient was treated with an enema and
anti-infective drugs, including levornidazole and sodium chloride
injection (0.5 g, every 12 h) combined with piperacillin sodium and
tazobactam sodium injection (4.5 g, every 8 h) via intravenous
infusion, which restored the temperature to normal level and
alleviated the abdominal pain. Subsequently, the patient was
discharged with a tolerable small liquid diet.
By August, the patient had lost ~4.6 kg, which
represented a loss of 5–7.5% of total body weight occurring in
<2 months, and they now returned to the hospital due to the
spontaneous rupture of a mass in the right lower abdomen. Based on
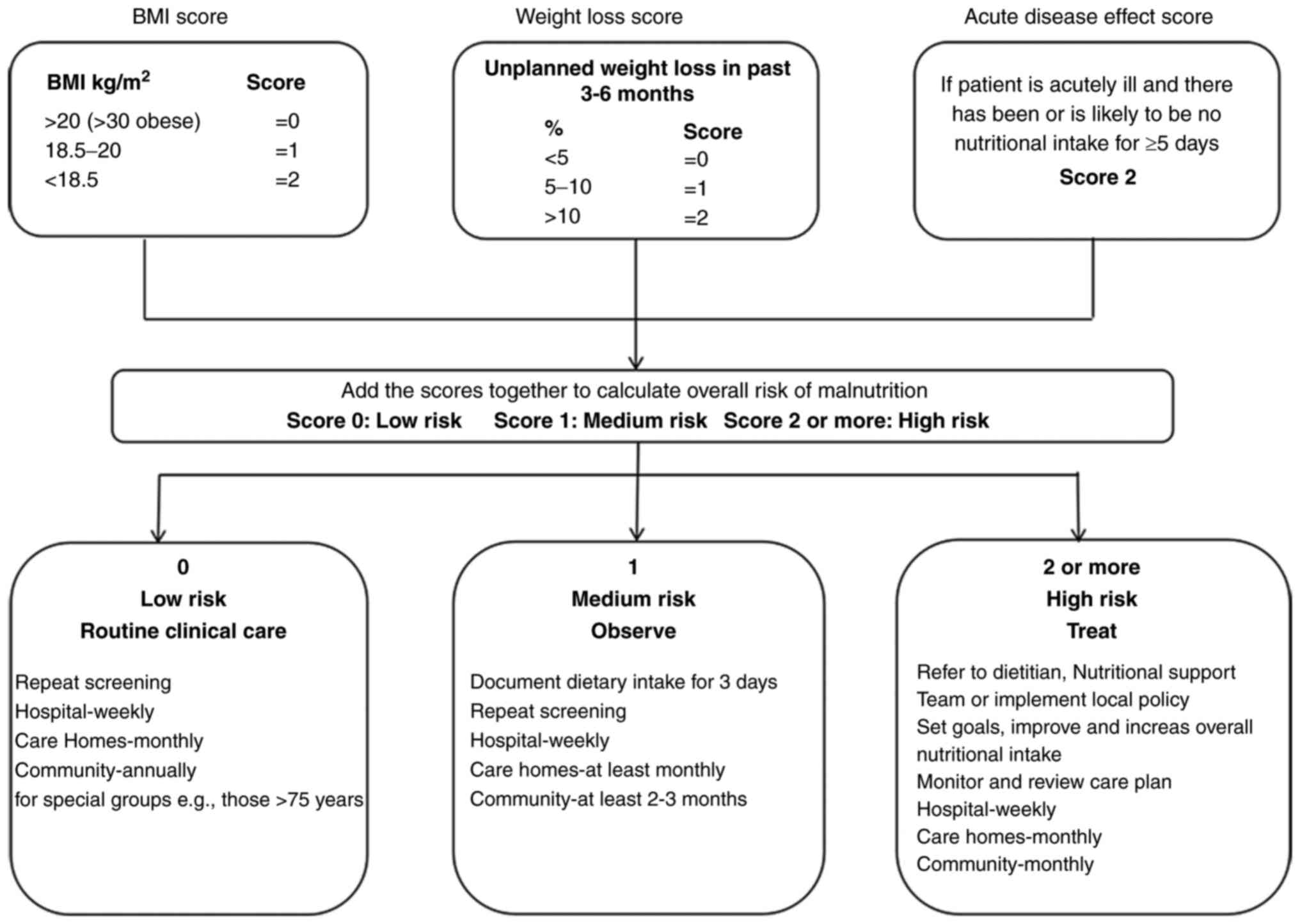
the Malnutrition Universal Screening Tool assessment (Fig. 1), the patient was considered to be
at high risk of malnutrition (score 3) due to substantial weight
loss and the current acute disease status, despite having a body
mass index of 23.03 (10). A
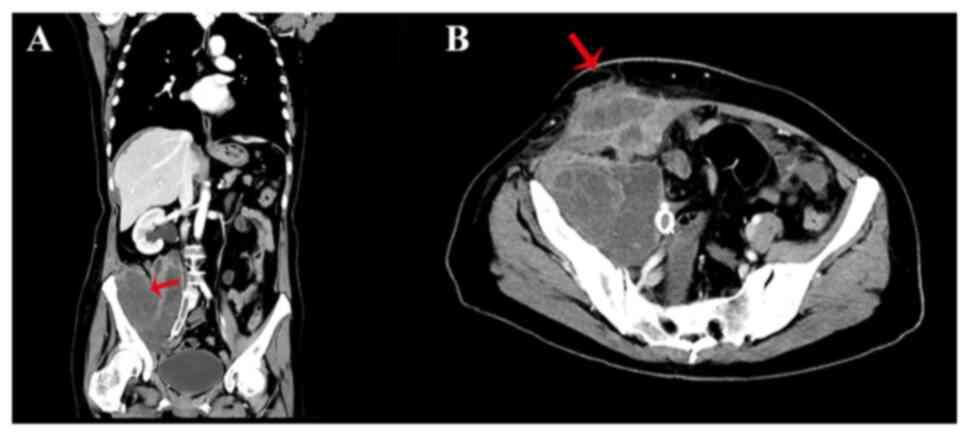
computed tomography scan confirmed that the primary cervical cancer
had spread to the right lower abdomen and broken through the skin
(Fig. 2). The wound, with
significant exudate, was cleaned twice daily, and the patient
continued to receive TPN due to poor oral intake. At 10 days after
admission, the patient exhibited sudden cognitive impairment,
especially in recent and immediate memory. An examination performed
by a neurologist showed that she was conscious but disoriented with
regard to time, place and person. The Mini-Mental State Exam (MMSE)
and Montreal Cognitive Assessment (MoCA) scores were 19/30 and
17/30, respectively (11,12). In addition, ophthalmoplegia with
bilateral sustained nystagmus was observed. Eye movement was normal
to the left side, but abnormal to the right side. The patient could
not walk due to cancer metastases within the right iliopsoas muscle
and iliac wing. Consequently, a gait assessment was not performed.
Mild upper-limb ataxia was observed and tendon reflexes exhibited a
slight response. All other neurological examinations, including
assessments of muscle tone and strength, were normal. Brain MRI and
magnetic resonance angiography (MRA) were performed immediately.
The MRA demonstrated normal blood vessels without restricted
diffusion, which excluded the possibility of ischemic or
hemorrhagic stroke. No significant abnormalities were detected on
T1-weighted imaging and diffusion-weighted imaging, which further
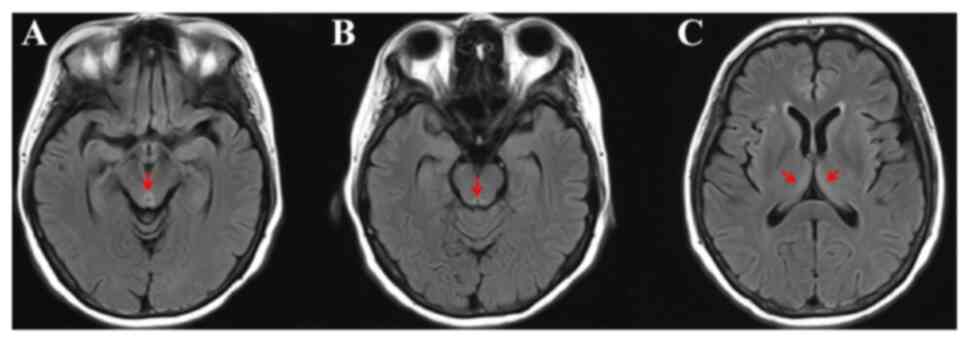
ruled out the possibility of brain metastases. However, brain MRI
demonstrated hyperintensity in the periaqueductal midbrain on T2
fluid-attenuated inversion recovery imaging (Fig. 3). The patient's laboratory results
were as follows: White blood cell count, 6.34×109/l
(normal range, 3.5–9.5×109/l); red blood cell count,
3.69×1012/l (normal range, 4.3–5.8×10¹2/l);
hemoglobin, 86 g/l (normal range, 120–160 g/l); platelets,
277×109/l (normal range, 125–350×109/l);
alanine aminotransferase, 14.4 U/l (normal range, 7–40 U/l);
aspartate aminotransferase, 15.9 U/l (normal range, 13–35 U/l);
serum albumin, 32.62 g/dl (normal range, 40–55 g/l); potassium,
4.88 mmol/l (normal range, 3.5–5.3 mmol/l); magnesium, 0.90 mmol/l
(normal range, 0.77–1.03 mmol/l); folate, 5.3 ng/ml (normal range,
3.38–5.38 ng/ml); and vitamin b12, 605 pg/ml (normal range, 211–911
pg/ml). Serum thiamine levels were not tested due to laboratory
constraints. Several other conditions, including brain metastases,
acute cerebrovascular disease and electrolyte imbalance, were
considered and excluded based on clinical symptoms, imaging
examination and laboratory results. Furthermore, the TPN regimen
did not include routine thiamine supplementation, which may have
promoted the development of WE. Thiamine (100 mg) was immediately
administered intramuscularly three times a day. After 1 week of
treatment, the patient's eye movement disorder and recent memory
improved, with the MMSE and MoCA scores changing to 23/30 and
20/30, respectively. The patient was subsequently discharged and
returned to her hometown. After 3 months, a telephone follow-up was
conducted. It was noted that the patient showed rapid progression
of the primary tumor, along with ongoing partial recent memory
impairment and spatial disorientation. Ultimately, the patient
passed away due to complications related to the tumor. The timeline
of the disease course is presented in Fig. 4.
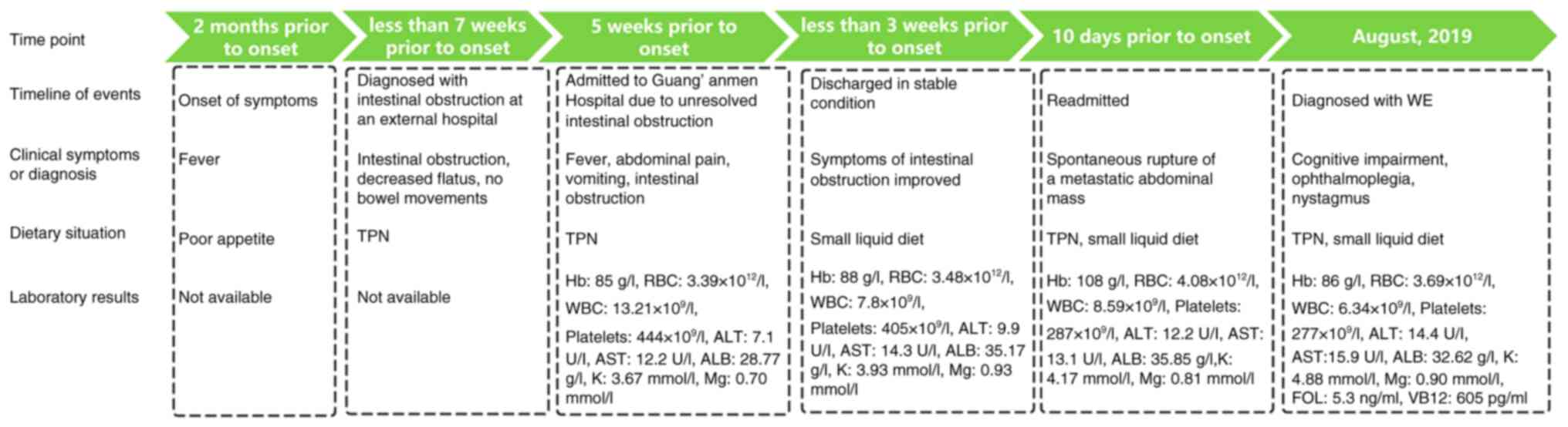 | Figure 4.Case summary timeline. TPN, total
parenteral nutrition; Hb, hemoglobin; RBC, red blood cell; WBC,
white blood cell; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; ALB, albumin; K, potassium; Mg, magnesium; WE,
Wernicke's encephalopathy. |
Discussion
Thiamine is a water-soluble vitamin absorbed in the
small intestine, with a daily requirement of 1–2 mg in healthy
adults (13). The body's thiamine
reserves can be nearly exhausted within ~18 days. The deficiency
primarily arises from insufficient dietary intake, reduced
gastrointestinal absorption or impaired utilization. Natural
sources of thiamine include whole grains, legumes, leafy greens,
nuts and seeds, fish and pork, which are essential for maintaining
adequate thiamine levels in the body. Thiamine has essential roles
in the regulation of cerebral metabolism, synapse formation and
neurotransmitter synthesis (6,14,15).
In neuronal and glial cells, thiamine pyrophosphate, the active
form of thiamine, acts as a cofactor that modulates the complete
oxidation of nutrients via the Krebs cycle to influence energy
production. Thiamine is also involved in the synthesis of various
neurotransmitters, such as glutamate and γ-aminobutyric acid, and
its deficiency leads to cellular damage, impairing cerebellar
activity (14,15). Thiamine deficiency induces
neuropathological damage, including neuronal loss,
micro-hemorrhages, endothelial swelling and gliosis in selective
brain regions, primarily affecting the mammillary bodies, thalamus,
cerebellum, cerebral aqueduct, and the third and fourth ventricles
(16). Several mechanisms, such as
cellular energy failure, lactic acidosis, oxidative and nitrosative
stress, and N-methyl-D-aspartate receptor-mediated excitotoxicity,
have been proposed to explain the association of thiamine
deficiency with neuronal cell damage and loss (17).
In patients with advanced cancer, the risk of
developing of WE is increased due to multiple factors, including
poor nutritional intake, vomiting, severe malnutrition and the use
of chemotherapy agents. A literature review of all WE cases in
patients with cancer from studies that were published in the last 3
years and retrieved from PubMed (https://pubmed.ncbi.nlm.nih.gov/) is presented in
Table I (18–28).
Key terms used in the search included ‘Wernicke's Encephalopathy’,
‘cancer’, ‘chemotherapy’, ‘neurological complications’ and ‘case
report’. Inclusion criteria were studies that reported on patients
with cancer diagnosed with WE and were available through PubMed.
Exclusion criteria included studies focusing on non-cancer-related
causes of WE or those with insufficient data on patient treatment
or outcomes. Table I summarizes a
series of cases of WE in patients with cancer, emphasizing several
key findings. Most patients had advanced cancer, particularly
gastrointestinal, lung and hematological malignancies, and were
undergoing chemotherapy or had significant nutritional
deficiencies. Common symptoms of WE included confusion, ataxia and
ophthalmoplegia, although atypical symptoms, such as isolated
cognitive changes or motor disturbances, were also observed.
Diagnosis was primarily clinical, with MRI providing typical
imaging findings in some cases. Treatment involved thiamine
supplementation, though the timing and dosage of administration
varied. Patient outcomes ranged from full recovery to long-term
neurological deficits, with faster diagnosis and prompt thiamine
treatment generally associated with better outcomes (18–28).
Clinically, WE is often underdiagnosed or diagnosed late due to the
unclear clinical guidelines and criteria, especially in patients
with advanced cancer. This challenge is particularly complicated by
the significant overlap between advanced cancer symptoms and those
of WE. The classic triad of symptoms has been reported in 16.5% of
patients (29). This suggests that
regular monitoring of thiamine levels in patients with cancer,
particularly those with persistent nausea, vomiting or weight loss,
is imperative to improve early detection and prevention of WE. In a
number of cases, thiamine supplementation is administered after the
development of neurological damage. However, early intervention may
reduce the degree of neurological damage. This highlights the
significance of incorporating regular nutritional assessments,
including thiamine levels, in the routine care protocols,
especially for patients at high risk for severe malnutrition.
 | Table I.Review of cases of Wernicke's
encephalopathy in patients with cancer from studies published in
PubMed over the last 3 years. |
Table I.
Review of cases of Wernicke's
encephalopathy in patients with cancer from studies published in
PubMed over the last 3 years.
| First author,
year | Malignancy | Age, years | Sex | Nutritional
status | Medication
history | Treatment | Prognosis | (Refs.) |
|---|
| Zhang et al,
2022 | Esophageal
cancer | 64 | Male | Underweight
(BMI=19.88 kg/m2) | Radiotherapy | Intravenous thiamine
500 mg/day for 3 days, then 200 mg/week, then oral thiamine 100
mg/day | Neurological symptoms
improved, mild memory impairment | (18) |
| Nikjoo et al,
2022 | Gastric cancer | 38 | Male | Malignancy | Gastric stent
placement | Intravenous thiamine
250 mg/8 h for 2 days, then 250 mg/day | Neurological
improvement | (19) |
| Lin et al,
2023 | Gastric cancer | 67 | Female | Malignancy, weight
loss | Post-gastrectomy,
TPN | Intravenous
thiamine 100 mg/day, 200 mg/8 h, 500 mg/8 h | Died from secondary
respiratory failure | (20) |
| Gross et al,
2023 | Duodenal
adenocarcinoma | 50 | Female | Obese, Poor
nutritional intake | Laparoscopic
gastric band placement 11 years ago, TPN | Intravenous
thiamine 500 mg/8 h for 2 days, then 250 mg/day for 5 days, then
100 mg orally daily | Dizziness, diplopia
and nausea largely resolved | (21) |
| Koca et al,
2022 | Cholangiocellular
carcinoma | 65 | Male | Not mentioned | Gastrojejunostomy,
radiotherapy, chemotherapy (gemcitabine), TPN | Intravenous
thiamine 200 mg/8 h, then 100 mg/day | Orientation and
cognitive function recovered, nystagmus persisted | (22) |
| Ibnawadh et
al, 2023 | Pancreatic cancer
with colonic fistula | 39 | Male | Obese, weight loss
(BMI=38 kg/m2) | Surgical
intervention for fistula and abscess, gastrojejunostomy, TPN | Intravenous
thiamine 500 mg/day for 5 days, then 250 mg/day for 7 days, then
100 mg/day | Transitioned to
palliative care and died | (23) |
| Slim et al,
2022 | Sigmoid colon
tumor | 66 | Male | Underweight (BMI=17
kg/m2) | Post-surgical,
TPN | Intravenous
thiamine 1,000 mg/day for 3 days | Died on the 24th
day after surgery | (24) |
| Brown and
Hutt-Williams, 2022 | Metastatic breast
cancer | 54 | Female | Malnutrition,
prolonged vomiting | Chemotherapy
(docetaxel, trastuzumab, pertuzumab) targeted therapy,
(trastuzumab, emtansine), radiotherapy | Intravenous
Pabrinex for 48 h, then oral thiamine | Speech returned,
memory and mobility improved | (25) |
| Azapagasi et
al, 2024 | Acute myeloid
leukemia | 7 | Female | Not mentioned | Chemotherapy,
TPN | 500 mg thiamine via
nasogastric enteral tube | Symptom
remission | (26) |
| Feng et al,
2022 | Diffuse large
B-cell lymphoma | 67 | Male | Malnourished | History of chronic
alcohol abuse, 1 year of sobriety | Intramuscular
thiamine 200 mg/12 h | Metamorphopsia,
ataxia and cognitive improvement, with persistent ataxia after
discharge | (27) |
| Malik et al,
2024 | Malignant
melanoma | 79 | Male | Poor nutritional
intake, weight loss | Immunotherapy
(nivolumab) | Intravenous
thiamine 200 mg/8 h for 3 days | Mental status
improved | (28) |
In the current case, the patient presented with a
nearly 2-month illness, including fever, vomiting and infection.
Although TPN was administered, with regimens comprising the
supplementation of 12 vitamins, thiamine was not included. The
patient experienced a sudden onset of anterograde amnesia,
characterized by an inability to recall the reason for the
hospitalization, repeatedly asking the same questions and failing
to recognize the attending physician. The anterograde amnesia was
associated with the occurrence of lesions in the mammillary bodies
and thalamus. The mammillary bodies, which regulate memory, are
directly connected to the hippocampus, thalamus and midbrain. The
mammillary bodies receive memory information from the hippocampus,
and thalamic connections transmit the information from the
mammillary bodies to the thalamus (30). In the present case, analysis of the
MoCA and MMSE scores demonstrated cognitive deficits in the spatial
and temporal orientations, immediate memory and calculation
ability. These signs, and symptoms such as eye movement abnormality
and confusion, along with the MRI scan findings, were consistent
with WE.
Although several double-blind randomized clinical
trials have explored the effective treatment strategies, there is
no consensus regarding the treatment of WE. Pharmacokinetic studies
have suggested that administration of thiamine twice or three times
daily may induce a satisfactory clinical response compared with a
single daily dose, due to the short plasma half-life of free
thiamine (31). So far, few
guidelines for WE treatment have been proposed, and most of them
have not been updated. The Royal College of Physicians of the
United Kingdom recommends intravenous administration of two pairs
of high-potency B-complex vitamins (each pair containing 250 mg of
thiamine) twice daily for 3 days in patients with confirmed or
suspected WE (32). However,
according to the European Federation of Neurological Societies,
patients with suspected or confirmed WE should receive intravenous
administration of 200 mg thiamine three times daily, until the
clinical signs and symptoms stabilize (7). A recent systematic review reported
that the most frequently used regimen in case studies is a dose of
500 mg thiamine administered intravenously three times daily
(33). It has been reported that
early and adequate thiamine supplementation can alleviate symptoms
in 90% of patients, with mental status showing the most rapid
improvement (33). However, another
randomized controlled trial showed that different dosing regimens
(100, 300 and 500 mg) did not significantly improve cognitive or
neurological outcomes in both asymptomatic at-risk patients and
symptomatic WE patients (34). For
effective treatment of WE, several factors should be considered. In
most cases, thiamine administered via the intravenous route has a
high safety profile. However, anaphylactic responses may occur
during or shortly after the intravenous injection, especially with
multiple administrations (35).
Therefore, intravenous injections should be administered over 30
min (36). Cases of WE induced by
magnesium depletion have been reported, as magnesium functions as a
co-factor promoting the phosphorylation of thiamine (37). Consequently, magnesium depletion may
exacerbate thiamine deficiency, implying that clinicians should
correct magnesium deficiencies and pay attention to other
nutritional deficiencies (38). In
the present case, the patient's TPN comprised 12 vitamins, and the
serum magnesium and other vitamin levels were within the normal
range. Therefore, supplementation with additional magnesium or
other vitamins was deemed unnecessary. Data indicates that only 25%
of patients with WE achieve a full recovery, while 50% show gradual
improvement and the remaining 25% fail to show any improvement
(39). It should be noted that the
prognosis of WE in patients with advanced cancer is complex and
influenced by several factors, such as the stage of malignancy, the
degree of tumor progression, the timeliness of diagnosis and
adequate thiamine supplementation (23–26).
In the present study, it was observed that the patient's glassy
eyes improved gradually after treatment for 1 week. At this point,
the patient had partial recent memory recall, but the immediate
memory was still impaired. The patient returned to her hometown
after 1 week of thiamine treatment. After 3 months, a follow-up
phone call revealed that the patient had experienced rapid tumor
progression, accompanied with partial impairment of recent memory
and disorientation in terms of place.
Thiamine deficiency is often not detected by
oncologists, which results in delays or missed treatments. For
high-risk cancer patients, regular monitoring of thiamine levels,
early nutritional support and timely recognition of deficiency
symptoms are essential to improve outcomes. To improve patient
outcomes, future research should focus on creating comprehensive
prevention and treatment guidelines for high-risk or WE patients.
These guidelines should be easily accessible and readily
implemented by healthcare providers to minimize the risk of
underdiagnosis and undertreatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Guang'anmen Hospital, China Academy of Chinese Medical Sciences
(Beijing, China).
Patient consent for publication
Written consent for publication was obtained from
the patient's guardian.
Authors' contributions
HZ wrote the manuscript and participated in the
management of the patient. YMZ designed the case report, and
participated in the diagnosis and management of the patient. SLW
participated in the diagnosis of the patient and revised the
manuscript for important intellectual content. All authors have
read and approved the final manuscript. YMZ and SLW confirm the
authenticity of all the raw data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MMSE
|
mini-mental state exam
|
|
MoCA
|
montreal cognitive assessment
|
|
MRA
|
magnetic resonance angiography
|
|
MRI
|
magnetic resonance imaging
|
|
TPN
|
total parenteral nutrition
|
|
WE
|
Wernicke's encephalopathy
|
References
|
1
|
Mrowicka M, Mrowicki J, Dragan G and
Majsterek I: The importance of thiamine (vitamin B1) in humans.
Biosci Rep. 43:BSR202303742023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soto-Martin EC, Warnke I, Farquharson FM,
Christodoulou M, Horgan G, Derrien M, Faurie JM, Flint HJ, Duncan
SH and Louis P: Vitamin biosynthesis by human gut
butyrate-producing bacteria and cross-feeding in synthetic
microbial communities. mBio. 11:e00886–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cobilinschi C, Andrei CA, Grinţescu IM and
Mirea L: Metabolic failure due to thiamine deficiency during
critical illness. Curr Opin Clin Nutr Metab Care. 27:155–162. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hutcheon DA: Malnutrition-induced
Wernicke's encephalopathy following a water-only fasting diet. Nutr
Clin Pract. 30:92–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasu T, Iimura Y, Momo K and Kuroda S:
Potential thiamine deficiency in elderly patients with
gastrointestinal cancer undergoing chemotherapy. Int J Clin
Pharmacol Ther. 58:174–176. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sechi G and Serra A: Wernicke's
encephalopathy: New clinical settings and recent advances in
diagnosis and management. Lancet Neurol. 6:442–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galvin R, Bråthen G, Ivashynka A, Hillbom
M, Tanasescu R and Leone MA; EFNS: EFNS guidelines for diagnosis,
therapy and prevention of Wernicke encephalopathy. Eur J Neurol.
17:1408–1418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ota Y, Capizzano AA, Moritani T, Naganawa
S, Kurokawa R and Srinivasan A: Comprehensive review of Wernicke
encephalopathy: Pathophysiology, clinical symptoms and imaging
findings. Jpn J Radiol. 38:809–820. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stratton RJ, Hackston A, Longmore D, Dixon
R, Price S, Stroud M, King C and Elia M: Malnutrition in hospital
outpatients and inpatients: Prevalence, concurrent validity and
ease of use of the ‘malnutrition universal screening tool’ (‘MUST’)
for adults. Br J Nutr. 92:799–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aiello EN, Pasotti F, Appollonio I and
Bolognini N: Equating mini-mental state examination (MMSE) and
montreal cognitive assessment (MoCA) scores: Conversion norms from
a healthy Italian population sample. Aging Clin Exp Res.
34:1721–1724. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roheger M, Xu H, Hoang MT, Eriksdotter M
and Garcia-Ptacek S: Conversion between the mini-mental state
examination and the montreal cognitive assessment for patients with
different forms of dementia. J Am Med Dir Assoc. 23:1986–1989.e1.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomson AD, Guerrini I and Marshall EJ:
Wernicke's encephalopathy: Role of thiamine. Pract Gastroenterol.
33:21–30. 2009.
|
|
14
|
Huskisson E, Maggini S and Ruf M: The
influence of micronutrients on cognitive function and performance.
J Int Med Res. 35:1–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baltrusch S: The Role of Neurotropic B
Vitamins in Nerve Regeneration. Biomed Res Int. 2021.9968228. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kril JJ: Neuropathology of thiamine
deficiency disorders. Metab Brain Dis. 11:9–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butterworth RF: Thiamin deficiency and
brain disorders. Nutr Res Rev. 16:277–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Wang L, Jiang J and Chen WY:
Non-alcoholic Wernicke encephalopathy in an esophageal cancer
patient receiving radiotherapy: A case report. World J Clin Cases.
10:5810–5815. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikjoo A, Rashid H, Chung R and Sadat MA:
A rare case of Wernicke encephalopathy in stage IV gastric cancer.
Neurocase. 28:123–125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Q, Li G, Wang Z and Zhang Y: Case
Report: Wernicke's encephalopathy after gastric surgery presenting
as lactic acidosis and refractory thrombocytopenia. Front Surg.
10:10163472023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gross A, Yu AT, Lara-Reyna J, Park K and
Harvey EJ: Duodenal adenocarcinoma in the setting of bariatric
surgery: A perfect storm for Wernicke's encephalopathy. Cureus.
15:e337652023.PubMed/NCBI
|
|
22
|
Koca O, Demir B, Derin S and Turna ZH: A
case report of Wernicke Korsakoff syndrome in a patient with
cholangiocellular carcinoma: An underestimated cause of
encephalopathy in cancer patients. Medicine (Baltimore).
101:e319042022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ibnawadh AK, Alashgar HI, Peedikayil M and
Amin T: Pancreatic cancer presenting with acute pancreatitis
complicated by Wernicke's encephalopathy and a colonic fistula: A
case report. Ann Med Surg (Lond). 85:574–578. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slim S, Ayed K, Triki W, Baccar A, Baraket
O, Rahal K, Ganzoui I and Bouchoucha S: Gayet-Wernicke's
encephalopathy complicating prolonged parenteral nutrition in
patient treated for colonic cancer-a case report. BMC Nutr.
8:832022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brown S and Hutt-Williams S: Wernicke's
encephalopathy in advanced cancer. BMJ Support Palliat Care.
12:460–462. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azapagasi E, Kunt Baykal N, Kirkiz Kayali
S, Salimli Mirzayeva L, Topuz Türkcan B, Uysal Yazici M, Serdaroğlu
E and Kaya Z: Severe lactic acidosis, Wernicke's encephalopathy,
and wet beriberi due to thiamine deficiency in a child with
leukemia. Klin Padiatr. 236:193–196. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng Y, Long X and Li X: Wernicke
encephalopathy with extensive cortical lesions combined with
diffuse large B-cell lymphoma. Neuro Endocrinol Lett. 43:361–365.
2022.PubMed/NCBI
|
|
28
|
Malik S, White W and Dasanu CA: Unusual
encephalopathy with Wernicke-Korsakoff syndrome-like features due
to adjuvant nivolumab for malignant melanoma. J Oncol Pharm Pract.
30:408–411. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harper CG, Giles M and Finlay-Jones R:
Clinical signs in the Wernicke-Korsakoff complex: A retrospective
analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg
Psychiatry. 49:341–345. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nardone R, Höller Y, Storti M, Christova
M, Tezzon F, Golaszewski S, Trinka E and Brigo F: Thiamine
deficiency induced neurochemical, neuroanatomical, and
neuropsychological alterations:a reappraisal.
ScientificWorldJournal. 2013:3091432013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Donnino MW, Vega J, Miller J and Walsh M:
Myths and misconceptions of Wernicke's Encephalopathy: What every
emergency physician should know. Ann Emerg Med. 50:715–721. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomson AD, Cook CC, Touquet R and Henry
JA; Royal College of Physicians, : London: The Royal College of
Physicians report on alcohol: Guidelines for managing Wernicke's
encephalopathy in the accident and emergency department. Alcohol
Alcohol. 37:513–521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cantu-Weinstein A, Branning R, Alamir M,
Weleff J, Do M, Nero N and Anand A: Diagnosis and treatment of
Wernicke's encephalopathy: A systematic literature review. Gen Hosp
Psychiatry. 87:48–59. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dingwall KM, Delima JF, Binks P, Batey R
and Bowden SC: What is the optimum thiamine dose to treat or
prevent Wernicke's encephalopathy or Wernicke-Korsakoff syndrome?
Results of a randomized controlled trial. Alcohol Clin Exp Res.
46:1133–1147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stephen JM, Grant R and Yeh CS:
Anaphylaxis from administration of intravenous thiamine. Am J Emerg
Med. 10:61–63. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tjugum SL, Hedrick TL, Jean SJ, Heeney SA,
Rohde KA and Campbell-Bright SL: Evaluation of the safety of
intravenous thiamine administration in a large academic medical
center. J Pharm Pract. 34:397–402. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ott M and Werneke U: Wernicke's
encephalopathy - from basic science to clinical practice. Part 1:
Understanding the role of thiamine. Ther Adv Psychopharmacol.
10:20451253209781062020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dyckner T, Ek B, Nyhlin H and Wester PO:
Aggravation of thiamine deficiency by magnesium depletion. A case
report. Acta Med Scand. 218:129–131. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Victor M, Adams RD and Collins GH: The
Wernicke-Korsakoff syndrome. A clinical and pathological study of
245 patients, 82 with post-mortem examinations. Contemp Neurol Ser.
7:1–206. 1971.PubMed/NCBI
|