Introduction
Marginal zone B-cell lymphoma (MZL), a type of
low-grade B-cell lymphoma, accounts for ~7% of all cases of mature
patients with non-Hodgkin lymphoma (NHL) in the United States and
15% of cases in Asian/Pacific Island populations. Extranodal MZL
(EMZL) accounts for 61% of patients with MZL, with the stomach
being the most frequently involved site (1). In a study by Chuang et al
(2), EMZL accounted for 9.34% of
cases of B-cell NHL in Taiwan between 2000 and 2015. Primary
hepatic lymphoma (PHL) is extremely rare, constituting only 0.016%
of cases of NHL, with the MZL subtype comprising only 2–4% of cases
of PHL (3). PHL presents diagnostic
challenges as outlined in the ESMO Clinical Practice Guidelines for
MZL (4); it commonly manifests as
non-specific symptoms, such as abdominal pain. Liver biopsies are
essential for diagnosis; however, the optimal therapeutic approach
remains uncertain. Given its rarity, there is no consensus
regarding its treatment. The present study describes a case of
primary hepatic MZL in an older adult female patient with Sjögren's
syndrome and common variable immunodeficiency (CVID). Sjögren's
syndrome is an autoimmune disease characterized by chronic
inflammation of the lacrimal and salivary glands, leading to dry
eyes and dry mouth (5). CVID is a
primary immunodeficiency disorder marked by low levels of serum
immunoglobulins and recurrent infections (6). Hepatic MZL, a rare subtype of PHL, is
frequently misdiagnosed as a hepatocellular carcinoma. The present
study describes a case of primary hepatic MZL in an older adult
female patient complicated with Sjögren's syndrome and CVID.
Case report
A 61-year-old woman with a history of Sjögren's
syndrome, managed with hydroxychloroquine for 38 years, was
diagnosed with CVID, and had been receiving intravenous
immunoglobulin therapy since February 2023. In December 2023, the
patient presented to the Emergency Department of Penghu Branch,
Tri-Service General Hospital (Taiwan), with shortness of breath
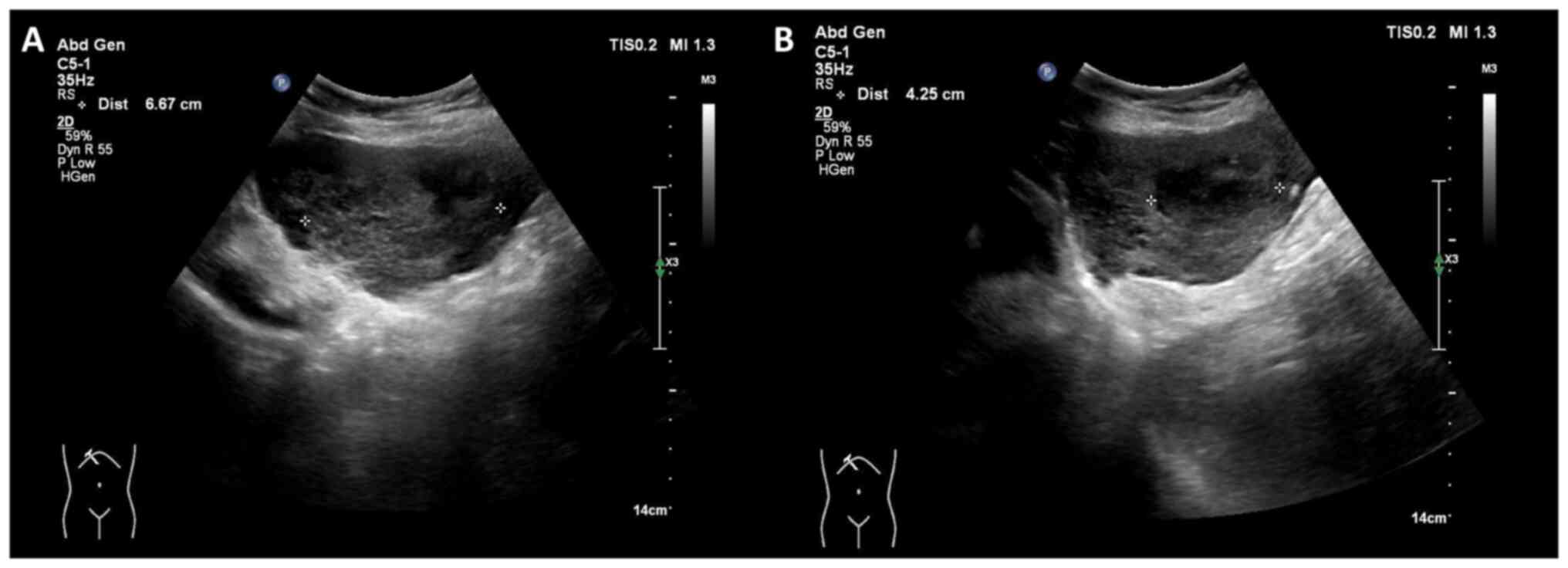
that had progressed over several days. Abdominal ultrasonography
incidentally revealed two hypoechoic masses in the left lobe of the
liver (Fig. 1). Subsequent dynamic
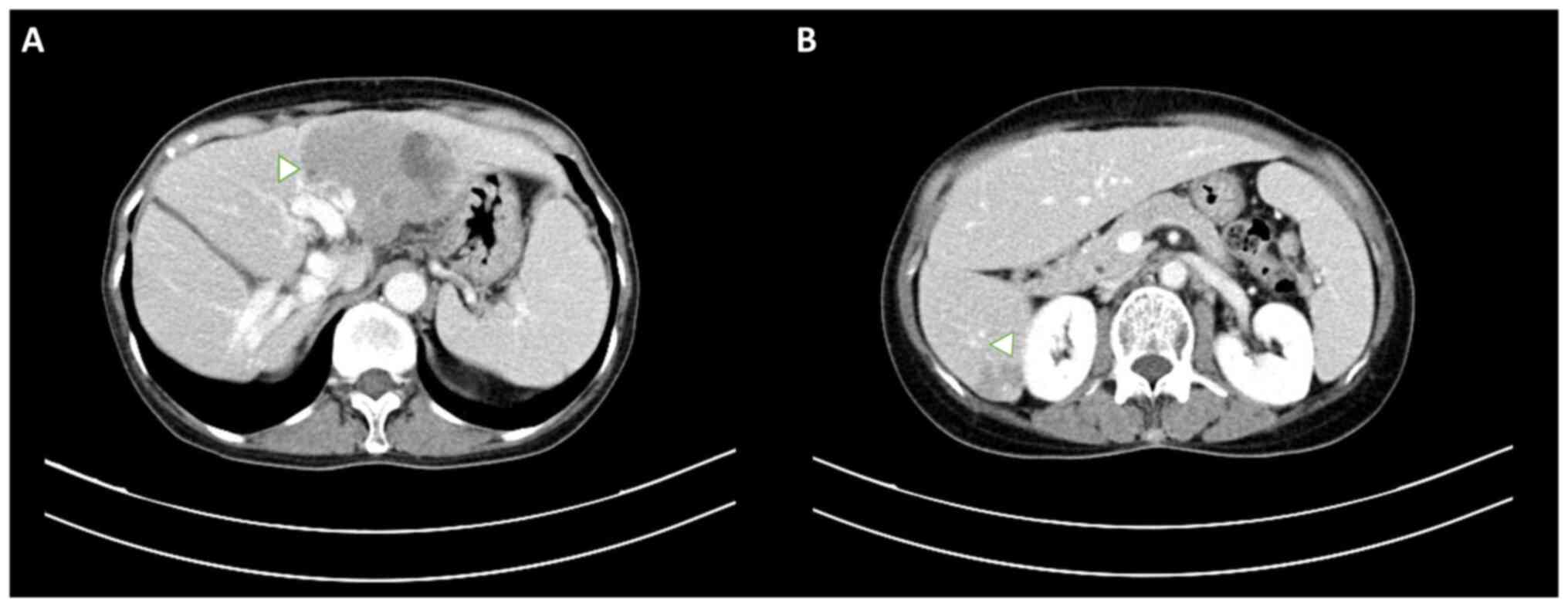
abdominal computed tomography (CT) revealed two hypo-enhanced
lesions located in liver segments 2/4a and 6 (Fig. 2), which were initially misdiagnosed
as hepatocellular carcinoma due to their non-specific radiological
features. The patient's tumor markers, including α-fetoprotein and
carbohydrate antigen 19-9, were within normal limits. Additionally,
liver function tests, including those for alanine aminotransferase
(76 U/l; normal range, 7–56 U/l), aspartate aminotransferase (56
U/l; normal range, 10–40 U/l) and alkaline phosphatase (277 U/l;
normal range, 44–147 U/l), showed mildly elevated levels, while the
bilirubin levels (0.5 mg/dl; normal range, 0.1–1.2 mg/dl) remained
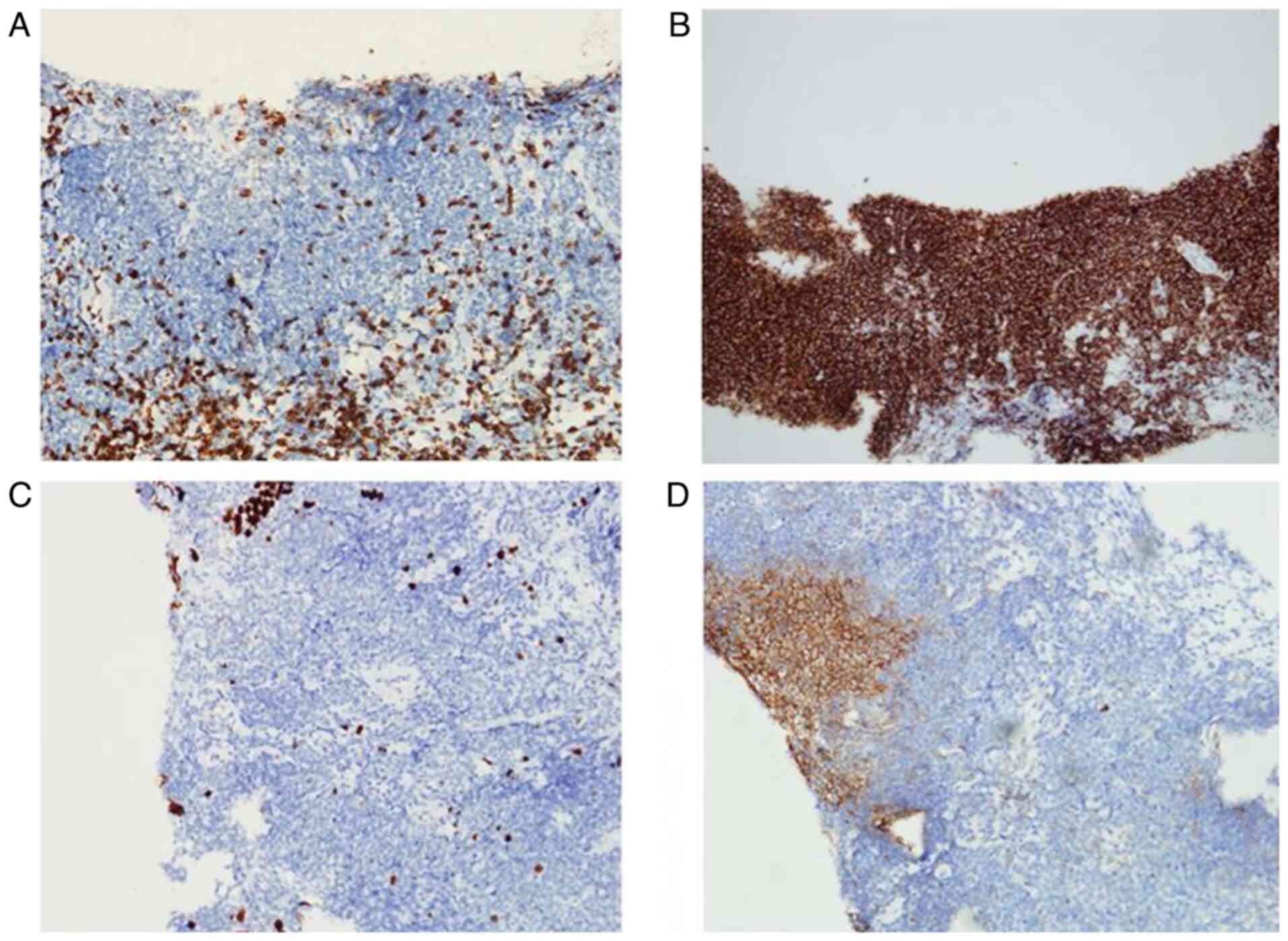
normal. A sonography-guided liver biopsy was performed, and hepatic
MZL was histologically confirmed based on strong CD20 positivity, a
low Ki-67 proliferation index (~5%) and the absence of CD3 in
neoplastic cells. Focal positivity for CD10 was also observed
(Fig. 3). Samples were fixed in 10%
neutral buffered formalin at room temperature for 24, sectioned to
4-µm and then stained with hematoxylin (room temperature for 5 min)
and eosin (room temperature for 2 min), before being observed by
light microscopy. For immunohistochemistry, the sections were
incubated with anti-CD20 (dilution 1:100; cat. no. ab9475; Abcam),
anti-CD3 (dilution 1:100; cat. no. ab16669; Abcam), anti-CD10
(dilution 1:100; cat. no. NCL-L-CD10-270; Leica Biosystems) and
anti-Ki-67 (dilution 1:200; cat. no. ab16667; Abcam) primary
antibodies at room temperature for 1 h and then with biotinylated
secondary antibody (horseradish peroxidase-conjugated; dilution
1:200; cat. no. ab6720; Abcam) at room temperature for 30 min.
Light microscopy was used for observation.
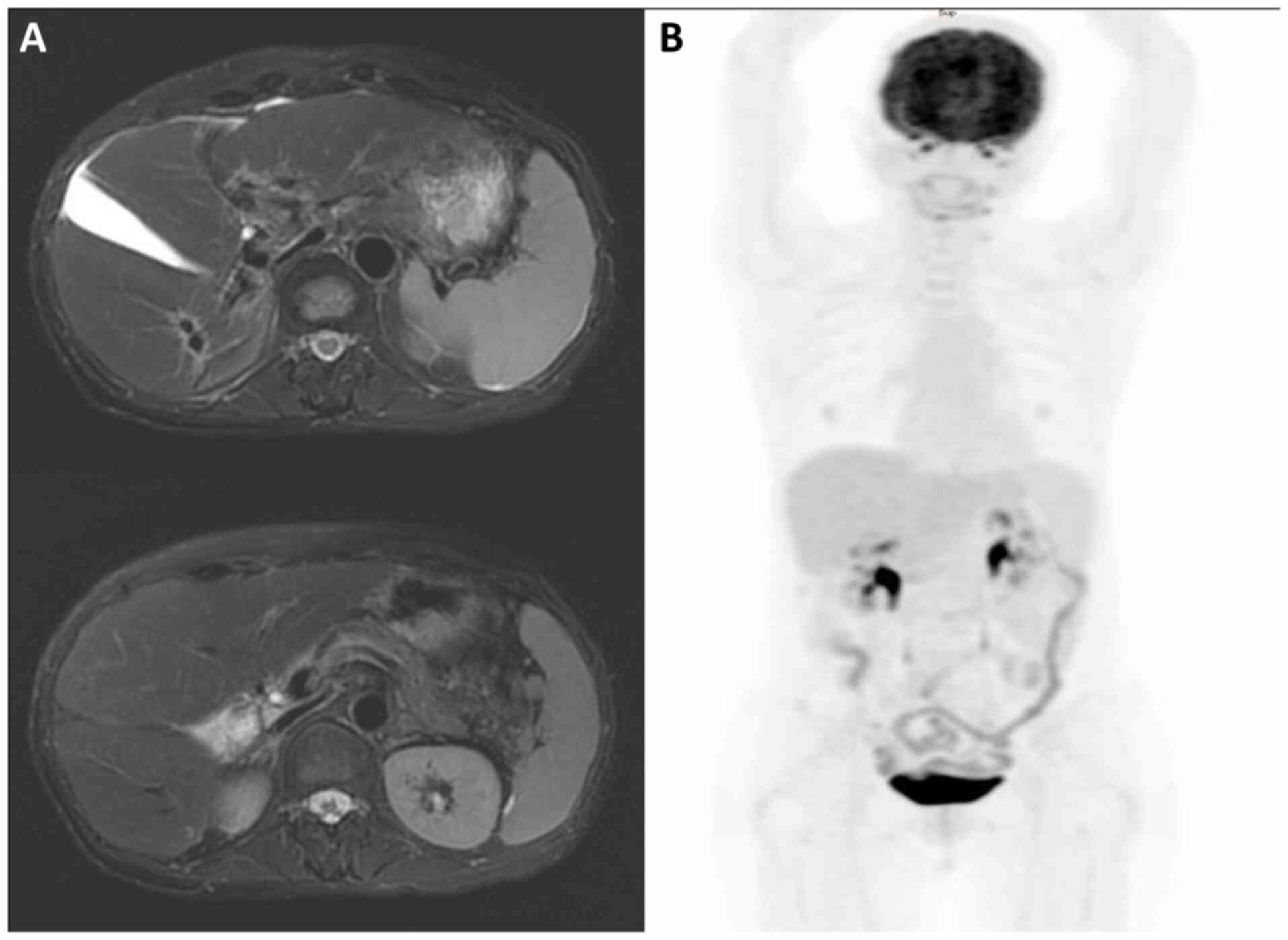
Bone marrow aspiration revealed no evidence of
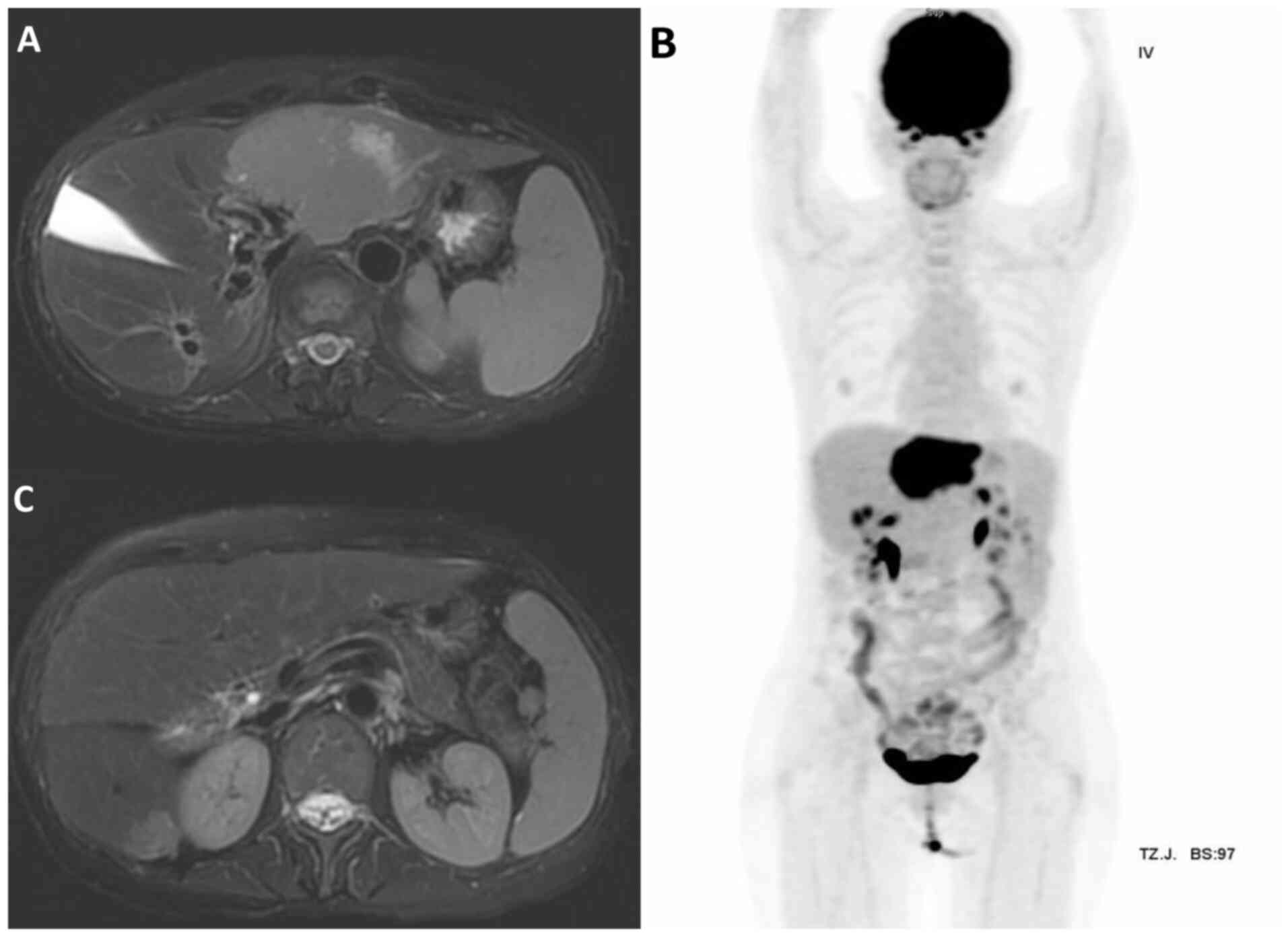
lymphoma. Abdominal magnetic resonance imaging (MRI) and whole-body
positron emission tomography (PET) scans showed two ill-defined
fluorodeoxyglucose (FDG)-avid mass lesions of 7.8 and 1.8 cm in
diameter in segments 2/4a and 6 of the liver, respectively
(Fig. 4), classified as stage IV
disease according to the Lugano Staging System (7).
Owing to immunodeficiency and the risk of
pancytopenia, chemotherapy was not suggested by the hematologist.
Considering the size and location of the hepatic lesions, as well
as the patient's overall health and comorbidities, a
multidisciplinary team decided to proceed with involved site
radiotherapy (ISRT) as the primary modality. Given the bilateral
lobe involvement, huge tumor burden and relatively small normal
liver volume, a stepped response-adapted approach was planned. A
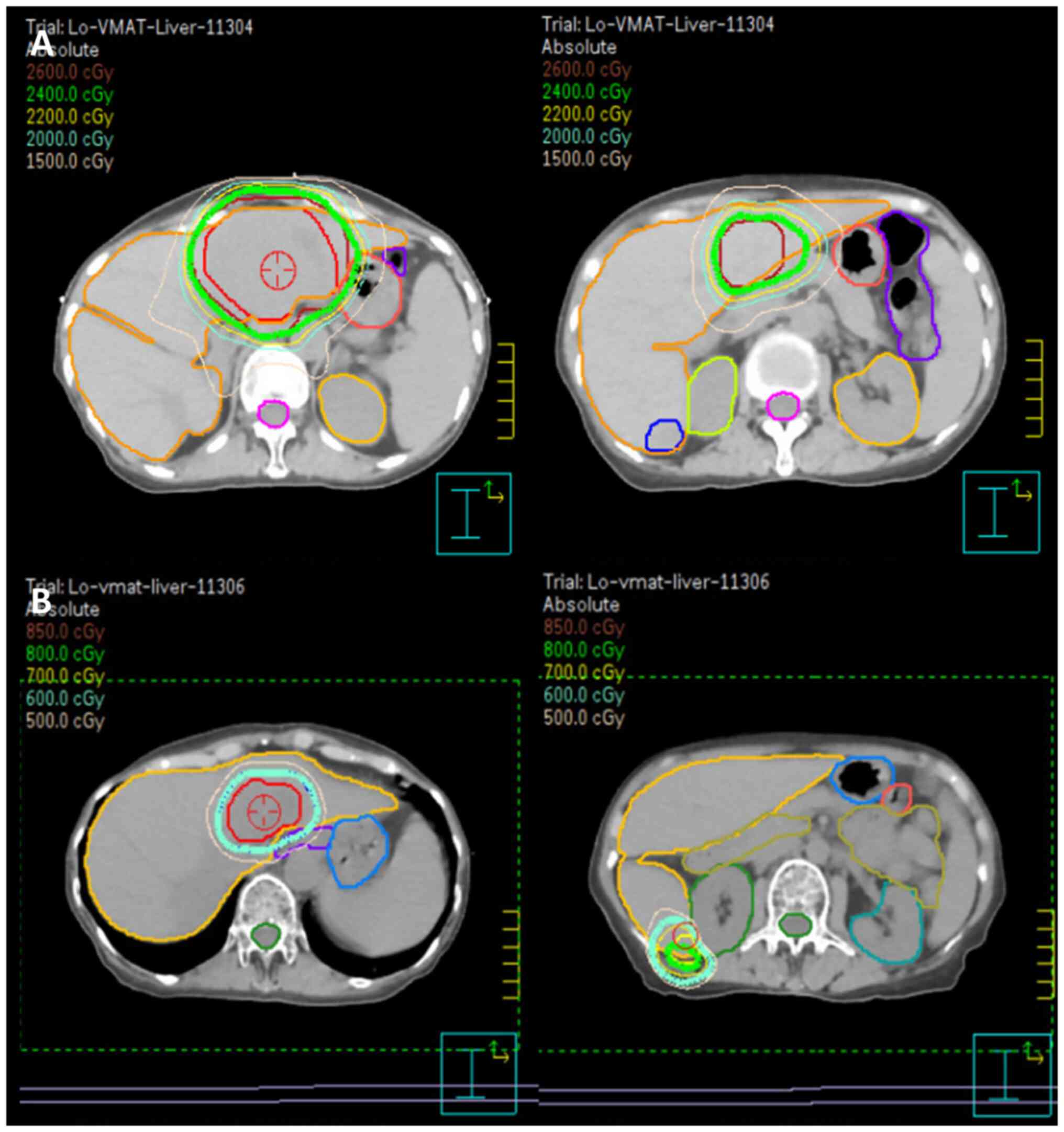
radiation therapy plan of 24 Gy in 16 fractions for the larger
tumor was first developed and administered in March 2024 (Fig. 5). Four-dimensional CT (4DCT) was
employed during the treatment planning phase to account for
respiratory motion. By capturing tumor movement across various
respiratory phases, 4DCT enabled the creation of a
motion-compensated treatment plan, ensuring precise targeting of
hepatic lesions while minimizing the radiation dose to surrounding
normal tissues. The larger tumor was irradiated first, with the
smaller tumors primarily receiving a scatter dose (mean dose, 447.4
cGy). During the radiotherapy course, significant tumor shrinkage
was observed, with the tumor diameter decreasing from 154.9 to
131.4 cm3 and the volume from 6.6 to 5.1 cm3.
This prompted the first adaptation of the radiation plan, which
involved re-defining the clinical target volume to reflect the
reduced tumor size. The updated plan was applied for the last four
fractions of radiotherapy, starting from the 13th
fraction. The patient tolerated radiotherapy well, with only grade
I gastrointestinal upset, graded according to the Common
Terminology Criteria for Adverse Events (CTCAE), version 4.0
(8). The patient showed no signs of
immunosuppression or the deterioration of pre-existing conditions.
Throughout the radiation therapy period, laboratory assessments,
including hematological, liver and kidney function tests,
demonstrated results within normal limits. Follow-up MRI at 1 month
after the first radiotherapy plan showed a marked reduction in both
directly irradiated and non-irradiated lesions. For the initial
ISRT plan (Fig. 5A), the tumor
volumes were 154.9 and 6.6 cm3 for the larger and
smaller tumors, respectively. The normal liver volume was 798
cm3. For the response-adapted plan (Fig. 5B), after significant tumor
shrinkage, the tumor volumes reduced 38.98 and 2.78 cm3,
respectively. The normal liver volume increased to 913.13
cm3. These changes were observed during follow-up
imaging, which prompted the response-adapted plan prescribing boost
doses of 6 and 8 Gy to the residual lesions in segments 2/4a and 6,
respectively (Fig. 5).
Consequently, a local radiation boost to the bilateral hepatic
lesions was planned, delivering an additional 6 Gy to the segment
2/4a tumor and 8 Gy to the segment 6 tumor in four fractions. At 4
months post-treatment, follow-up MRI (Fig. 6) demonstrated no evidence of
residual malignancy. At 7 months post-treatment, whole-body PET
revealed no metabolic evidence of malignancy (Deauville criteria
score 1) (9) (Fig. 6). No abnormal indicators were
observed in the physical, hematological or hepatological
examinations at the follow-up evaluations, which were performed
every 3 months. This schedule will continue for at least 2 years
and may transition to every 6 months based on the patient's
condition and physician's assessment. The patient remained healthy
and without signs of relapse following radiotherapy.
Discussion
The present case highlights the complexity of
managing hepatic MZL in patients with multiple comorbidities,
including autoimmune diseases and immunodeficiencies. Sjögren's
syndrome and CVID are known to be associated with an increased risk
of lymphoma (5,6). Low-grade MZL is the most common
lymphoid neoplasm in patients with Sjögren's syndrome (5). The majority of Sjögren's
syndrome-associated lymphomas are characterized by a localized
stage, an indolent clinical course and recurrence at other
extranodal sites. Sjögren's syndrome increases the risks of MZL and
parotid gland extranodal MZL by 30-fold and a factor of 1,000,
respectively (5). Specific
pathogens are associated with extranodal MZLs involving certain
anatomical sites: Helicobacter pylori (gastric),
Chlamydia psittaci (ocular adnexal), Borrelia
burgdorferi (cutaneous), Campylobacter jejuni (small
intestine) and Mycobacterium species (bronchus) (10). Chronic antigenic stimulation by
exoantigens or autoantigens is considered to play a key role in the
pathogenesis of Sjögren's syndrome-associated lymphoproliferation.
In a retrospective, single-center study (6), among 647 patients with CVID aged
>45 years, 45 patients (7%) developed lymphomas, predominantly
B-cell type (96%), compared to a 2.1% risk in the general
population. The present study highlights immunological and clinical
phenotypes, treatments and outcomes, showing high lymphoma
prevalence and diagnostic challenges in CVID due to immune
dysregulation. Therefore, patients with immune diseases should be
closely monitored for a high risk of lymphoma.
The present patient's clinical course underscores
the necessity for a comprehensive diagnostic evaluation of atypical
liver mass presentations. Initially, the patient was misdiagnosed
with hepatocellular carcinoma based on the CT and MRI results.
Betianu et al (11) reported
that PHL often lacks distinct radiological features. Post-contrast
imaging showed that >50% of the PHLs had no enhancement, while
~30% exhibited patchy enhancement and ~15% displayed ring
enhancement. On MRI, PHL appears as hypointense or isointense on
T1-weighted images, and hyperintense on T2-weighted images.
Consequently, in cases where liver masses are detected without a
corresponding increase in tumor markers, lymphoma should be
considered a differential diagnosis, with liver biopsy being a
viable diagnostic option. Bao et al (12) suggested that 18F-FDG PET is
particularly useful for identifying the early stages of MZL,
although pathological confirmation is essential for a definitive
diagnosis. This imaging modality aids in staging, assessing the
treatment response and monitoring relapse. The present patient
presented with a slightly hypermetabolic mass, consistent with an
indolent nature.
The treatment options for extranodal MZL include
chemotherapy, radiotherapy and surgery (13), with the choice depending on the
disease stage, patient performance status and underlying
conditions. Table I (13–19)
summarizes the published cases, highlighting the various management
approaches and outcomes for lesions in different liver segments.
Most patients presented with solitary lesions, typically <2 cm
in size and confined to a single liver segment. One case was of a
70-year-old man with two lesions in segment 2, measuring 3.3 and
3.6 cm, which were treated successfully with a left lateral segment
hepatectomy, with no recurrence at 8 months (13). Another patient, a 60-year-old woman,
had a 1-cm solitary lesion in segment 8 and underwent segmental
hepatectomy with adjuvant chemotherapy, resulting in no recurrence
over 4 years (14). Furthermore,
radiofrequency ablation (RFA) is a significant modality, offering a
quick and cost-effective treatment for unresectable liver tumors,
particularly for cases with a single tumor ≤5 cm or up to three
tumors, each ≤3 cm (20). However,
its long-term efficacy in lymphoma remains unclear, and the
surgical margins are inaccessible. A 63-year-old woman with a
solitary 1.7-cm lesion in segment 6 underwent RFA with no
recurrence after 1 year (15). In
the present case, the larger tumor burden and bilateral involvement
precluded liver surgery or RFA. By contrast, radiotherapy offers
localized treatment that minimizes systemic exposure, an essential
consideration for patients with CVID, where reducing the risk of
treatment-related immunosuppression is crucial. The positive
outcome, in the present case, suggests that radiotherapy is a
viable option for managing hepatic MZL in a similar manner as
extranodal MZL in other organs, particularly in patients with
contraindications for systemic chemotherapy or surgery.
Additionally, radiotherapy is more effective for controlling
multifocal lesions, which are often challenging to ablate
completely. In the case of extranodal disease, particularly
indolent lymphoma, the whole organ comprises the clinical target
volume, including the stomach, salivary glands and thyroid. Partial
organ irradiation may be appropriate for other organs, including
the orbit (involving the bony orbit and adjacent structures),
breast, lung, bone, ocular region (involving the eyeball) and
localized skin, as well as under certain circumstances when
radiotherapy is used for consolidation after chemotherapy (21). In the present case, due to the
localized nature of the hepatic tumors and the use of image-guided
radiation therapy combined with 4DCT for motion management, partial
irradiation was selected to minimize radiation exposure to normal
tissues while maintaining therapeutic effectiveness.
 | Table I.Published cases of primary hepatic
marginal zone B-cell lymphoma in the last 6 years. |
Table I.
Published cases of primary hepatic
marginal zone B-cell lymphoma in the last 6 years.
| First author,
year | Age, years | Sex | Comorbidities | Number of
lesions | Tumor size, cm | Positions | Nodal
involvement | Treatment | Outcome/follow-up
time | (Refs.) |
|---|
| Choi et al,
2020 | 70 | M | Intracerebral
hemorrhage; myocardial infarction | 2 | 3.3/3.6 | Segment 2 | No | Left lateral segment
hepatectomy | No recurrence/8
months | (13) |
| Okura et al,
2023 | 60 | F | Sigmoid colon cancer
with liver metastasis; HBV | 1 | 1 | Segment 8 | No | Hepatectomy of
segment 8, adjuvant chemotherapy | No recurrence/4
years | (14) |
| Xu et al,
2021 | 63 | F | None | 1 | 1.7 | Segment 6 | No | RFA | No recurrence/1
year | (15) |
| Yamashita et
al, 2021 | 66 | F | Uterine cervical
cancer; HBV | 2 | 3.5/3 | Segment 8, 5 | No | Patient refused (only
tenofovir for HBV control) | Not mentioned | (16) |
| Xie et al,
2019 | 73 | M | HBV | 1 | 1.8 | Segment 2 | No | Left lateral segment
hepatectomy (segments 2 and 3) | No recurrence/6
months | (17) |
| Koh et al,
2023 | Mid-40s | M | None | 1 | 1.8 | Segment 5 | No | Inferior segments
hepatectomy (segments 5 and 6) | No recurrence/2
years | (18) |
| Liu et al,
2024 | 77 | F | Repeated paroxysmal
palpitations | 1 | 1.4 | Segment 4a | No | Hepatectomy of
segment 4 | No recurrence/20
months | (19) |
| Present case | 61 | F | Sjogren's syndrome,
CVID | 2 | 7.8/1.8 | Segment 2/4a, 6 | No | Radiotherapy | No recurrence/7
months |
|
In the present case, response-adapted radiation
therapy was used to optimize the dose delivery. Typically, this
approach includes assessing the tumor response to an initial
radiation course or another treatment modality using imaging or
diagnostic tools. Based on this assessment, the radiation plan is
adjusted in terms of dose, target area or technique to maximize
treatment efficacy while minimizing damage to the surrounding
healthy tissues. A similar response-based approach was demonstrated
in a phase II trial by Pinnix et al (22), which investigated radiotherapy for
orbital indolent B-cell lymphoma, employing a response-adapted
ultra-low-dose protocol initiated with a 4-Gy dose, followed by an
additional 20 Gy for cases of persistent disease. The trial
demonstrated a 2-year local control rate of 89.4%, with 90% of
patients achieving a complete response and no grade 3 or higher
adverse effects. In the present case, radiation therapy dosages and
normal tissue constraints were guided by recommendations provided
by the International Lymphoma Radiation Oncology Group (23). Considering the bilateral lobe
involvement and limited normal liver volume (798 cm3;
compared with the average normal adult liver volume of 1,000-1,400
cm3 in females), the larger tumor was irradiated first,
with the smaller tumors primarily receiving a scatter dose (mean
dose, 447.4 cGy). Using scatter doses for smaller tumors can be
appropriate in certain scenarios, given the highly radiosensitive
nature of lymphoma (as low as 4 Gy). Although no significant
toxicities were observed during the 7-month follow-up, the
potential for late toxicity, such as radiation-induced liver
disease (RILD) or central biliary tract toxicity, persists, and
this is more common in cases with large liver volume irradiation or
central lesions. Toesca et al (24,25)
highlighted strategies for predicting and mitigating RILD and
central biliary tract toxicity, emphasizing the importance of
personalized treatment planning and careful dose management to
minimize risks. In the present study, subsequent MRI follow-up
indicated a partial response, leading to the administration of
additional radiation (6 Gy to the segment 2/4a tumor and 8 Gy to
the segment 6 tumor, delivered in four fractions). This approach
minimizes radiation exposure to the uninvolved normal liver while
ensuring adequate treatment for both tumors. In a previous study,
in patients with gastric mucosa-associated lymphoid tissue lymphoma
treated with definitive radiotherapy, the median time to achieve
complete remission was 3.9 months, with some cases requiring >12
months for confirmation through follow-up biopsies (26). This variability underscores the
importance of careful, long-term monitoring to evaluate treatment
outcomes and ensure sustained disease control.
In conclusion, the present case illustrates the
successful use of response-adapted ISRT to treat hepatic MZL in a
patient with Sjögren's syndrome and CVID. The role of radiotherapy
in the management of hepatic MZL has not been well established
owing to the rarity of this disease; thus, a multidisciplinary
approach and careful consideration of the patient's unique clinical
profile are crucial to achieving an individualized approach.
Further research and case studies are needed to establish
standardized treatment protocols for similar cases; however, this
report provides valuable insights into the management of a complex
lymphoma presentation in an immunocompromised patient.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data that support the findings of this case
report are not publicly available due to privacy and ethical
restrictions. However, they may be requested from the corresponding
author and obtained with permission from the Institutional Review
Board of Tri-Service General Hospital (Taipei, Taiwan).
Authors' contributions
SC contributed to the conception and design of the
study, analysis of clinical data, and drafting of the manuscript.
SC was responsible for interpreting the findings and preparing the
initial manuscript. CL supervised the study, provided critical
revisions for intellectual content and contributed to the
interpretation of data. CL also approved the final version of the
manuscript. YC and WH contributed to data collection and analysis.
SC and CL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Tri-Service General Hospital (approval no. A202415126).
The patient provided written informed consent to participate prior
to their inclusion in the study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of their clinical data an accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cerhan JR and Habermann TM: Epidemiology
of marginal zone. Lymphoma. Ann Lymphoma. 5:2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chuang SS, Chen SW, Chang ST and Kuo YT:
Lymphoma in Taiwan: Review of 1347 neoplasms from a single
institution according to the 2016 Revision of the World Health
Organization Classification. J Formos Med Assoc. 116:620–625. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Fattah MA: Non-hodgkin lymphoma of the
liver: A US population-based analysis. J Clin Transl Hepatol.
5:83–91. 2017.PubMed/NCBI
|
|
4
|
Zucca E, Arcaini L, Buske C, Johnson PW,
Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K,
Thieblemont C, et al: Marginal zone lymphomas: ESMO clinical
practice guidelines. Ann Oncol. 31:17–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Routsias JG, Goules JD, Charalampakis G,
Tzima S, Papageorgiou A and Voulgarelis M: Malignant lymphoma in
primary Sjögren's syndrome: An update on the pathogenesis and
treatment. Semin. Arthritis Rheum. 43:178–186. 2013.PubMed/NCBI
|
|
6
|
Smith T and Cunningham-Rundles C: Lymphoid
malignancy in common variable immune-deficiency in a single-center
cohort. Eur J Haematol. 107:503–516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th Edition.
Springer Cham; Switzerland: pp. 9542017
|
|
8
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE).
Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdfFebruary
12–2025
|
|
9
|
Meignan M, Gallamini A, Meignan M,
Gallamini A and Haioun C: Report on the first international
workshop on interim-PET-scan in lymphoma. Leuk Lymphoma.
50:1257–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zucca E, Bertoni F, Vannata B and Cavalli
F: Emerging role of infectious etiologies in the pathogenesis of
marginal zone B-cell lymphomas. Clin Cancer Res. 20:5207–5216.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Betianu CI, Dima A and Pavaloiu G: Primary
hepatic mucosa-associated lymphoid tissue lymphoma in a patient
with no chronic liver disease: Case report. J Radiol Case Rep.
12:715–719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao C, Wei J, Zhao X, Lin L, Chen D, Liu
K, Qian W, Anas JM and Zhao K: Prognostic value of
fluorine-18-fluorodeoxyglucose positron emission
tomography/computed tomography in primary hepatic mucosa-associated
lymphoid tissue lymphoma: A case report and review of the
literature. Medicine (Baltimore). 97:98772018. View Article : Google Scholar
|
|
13
|
Choi S, Kim JH, Kim K, Kim M, Choi HJ, Kim
YM, Suh JH, Seo MJ and Cha HJ: Primary hepatic extranodal marginal
zone lymphoma of mucosa-associated lymphoid tissue. J Pathol Transl
Med. 54:340–345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okura K, Seo S, Shimizu H, Nishino H, Yoh
T, Fukumitsu K, Ishii T, Hata K, Haga H and Hatano E: Primary
hepatic extranodal marginal zone B-cell mucosa-associated lymphoid
tissue lymphoma treated by laparoscopic partial hepatectomy: A case
report. Surg Case Rep. 9:292023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Z, Pang C, Sui J and Gao Z: A case of
primary hepatic extranodal marginal zone B-cell mucosa-associated
lymphoid tissue (MALT) lymphoma treated by radiofrequency ablation
(RFA), and a literature review. J Int Med Res. 49:300060521999539.
2021.
|
|
16
|
Yamashita Y, Joshita S, Kobayashi H,
Wakabayashi SI, Sugiura A, Yamazaki T and Umemura T: Primary
hepatic extranodal marginal zone lymphoma of mucosa-associated
lymphoid tissue in a patient with chronic hepatitis B virus
infection: Case report and summary of the literature. Medicina
(Kaunas). 57:2802021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie H, Lv J, Ji Y, Du X and Yang X:
Primary hepatic mucosa-associated lymphoid tissue lymphoma.
Medicine (Baltimore). 98:e150342019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh HD, Choi JW, Kim EK, Park S, Kim MJ
and Lee CK: Primary hepatic mucosa-associated lymphoid tissue
lymphoma mimicking hepatocellular carcinoma in a patient with
chronic hepatitis B: A case report. J Int Med Res.
51:30006052311543992023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Liu Y, Zhang J, Chai Y, Lu B and
Tang H: Primary hepatic mucosa-associated lymphoid tissue lymphoma
complicated with atrial fibrillation: A case report and literature
review. Medicine (Baltimore). 103:e369262024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benson AB III, D'Angelica MI, Abrams T,
Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Baker M, Binder D,
et al: NCCN clinical practice guidelines in oncology:
Hepatocellular carcinoma. National Comprehensive Cancer Network,
Version. 1:512024.
|
|
21
|
Zelenetz AD, Gordon LI, Abramson JS,
Advani RH, Andreadis B, Bartlett NL, Budde LE, Caimi PF, Chang JE,
Christian B, et al: NCCN clinical practice guidelines in oncology:
B-cell lymphomas. National Comprehensive Cancer Network, Version.
2:1442024.
|
|
22
|
Pinnix CC, Dabaja BS, Gunther JR, Fang PQ,
Wu SY, Nastoupil LJ, Strati P, Nair R, Ahmed S, Steiner R, et al:
Response-adapted ultralow-dose radiation therapy for orbital
indolent B-Cell lymphoma A phase 2 nonrandomized controlled trial.
JAMA Oncol. 10:1195–1203. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yahalom J, Illidge T, Specht L, Hoppe RT,
Li YX, Tsang R and Wirth A; International Lymphoma Radiation
Oncology Group, : Modern radiation therapy for extranodal
lymphomas: Field and dose guidelines from the international
lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys.
92:11–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toesca DAS, Ibragimov B, Koong AJ, Xing L,
Koong AC and Chang DT: Strategies for prediction and mitigation of
radiation-induced liver toxicity. J Radiat Res. 59:40–49. 2018.
View Article : Google Scholar
|
|
25
|
Toesca DA, Osmundson EC, Eyben RV, Shaffer
JL, Lu P, Koong AC and Chang DT: Central liver toxicity after SBRT:
An expanded analysis and predictive nomogram. Radiother Oncol.
122:130–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katano A and Yamashita H: Definitive
radiotherapy for stage I gastric mucosa-associated lymphoid tissue
lymphoma: A retrospective cohort of unique-dose administration of
30 Gy in 15 fractions and analysis of remission duration. World J
Oncol. 15:506–510. 2024. View Article : Google Scholar : PubMed/NCBI
|