Introduction
Globally, colorectal carcinoma (CRC) is the third
most common type of cancer and also the third highest cause of
tumor-associated mortalities, representing a serious health hazard
to the population (1). It is
estimated that CRC incidence and mortality will increase globally
by 2035, which may be related to risk factors including cigarette
smoking, physical inactivity, red meat consumption and obesity
(2). Although surgical resection is
still the most effective treatment for patients diagnosed with CRC,
chemotherapy serves an important postoperative role (3). Currently, oxaliplatin-based adjuvant
chemotherapy is recommended for stage III and high-risk stage II
patients with CRC who are undergoing radical resection, which can
markedly prolong overall survival (OS) (4). Currently, T stage 4, poor histological
differentiation, vascular infiltration, perineural invasion,
preoperative intestinal obstruction, tumor perforation, incisal
positive, insufficient distance for cut edge and examining <12
lymph nodes are considered to be high-risk factors for tumor
recurrence; however, a high tumor-stroma ratio (TSR) is not
(5). The TSR has been regarded as
an essential factor associated with for tumor metastasis based on
the theory of ‘seed (carcinoma cell) and soil (tumor stroma)’
(6). Research suggests that during
cancer progression, normal stromal compartments transform due to
increased cancer-associated fibroblasts, which were generated from
normal fibroblasts and epithelial cells in response to
platelet-derived growth factor, fibroblast growth factor and
transforming growth factor-β. Crosstalk between signaling molecules
contributes to the production of a number of cytokines and growth
factors, creating an environment conducive to tumor growth and
invasion (7,8). Therefore, the TSR may be an improved
prognostic predictor for CRC, which can guide the selection of the
chemotherapy regimen.
Several studies have suggested a potential
prognostic role of the TSR in CRC; however, controversy remains
regarding its use (9,10). Although a meta-analysis focusing on
the prognostic value of the TSR in patients with CRC was previously
performed, it had a small sample size (11). Furthermore, the application of
visual assessment (‘eyeballing’), systematic point counting and
tissue section [whole-slide images (WSI)] scanning has improved the
accuracy of TSR calculations (12).
Therefore, computer-aided quantification of tumor-stroma is more
credible than manual estimation. However, there is no meta-analysis
on the prognostic value of computer-aided quantification of
tumor-stroma in CRC, to the best of our knowledge. Therefore, the
current study aimed to perform a meta-analysis of all eligible
published studies to evaluate the prognostic value of the TSR in
CRC, especially for the TSR calculated by computer.
Materials and methods
The present meta-analysis is registered with
PROSPERO (www.crd.york.ac.uk/prospero; registration no.
CRD42022364340).
Search strategy
The meta-analysis was performed in accordance with
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (13). A
comprehensive search strategy was developed to screen eligible
peer-reviewed articles, associated with several specific key words:
‘Colorectal cancer’, ‘tumor stromal ratio’ and ‘prognosis’.
Furthermore, the free text associated with the key words was
retrieved from the PubMed MeSH-database, which was used to form
search strings with key words (colorectal neoplasms, colorectal
tumors, colorectal cancer, colorectal OR carcinomas, tumor stroma,
stroma score, tumour-stroma ratio, carcinoma-stromal ratio,
tumor-stroma proportion, tumour stroma percentage, proportion of
tumour cells, stromal part of adenocarcinomas and high amount of
stroma) to allow for a thorough search of the databases [Web of
Science (https://www.webofscience.com), PubMed
(https://pubmed.ncbi.nlm.nih.gov),
Cochrane (https://www.cochranelibrary.com) and Embase
(https://www.embase.com)] for all relevant
studies. The last search was updated on December 13, 2023. Research
lists of articles passing the initial screening process were also
used as auxiliary sources to improve the search strategy. The
reference lists of the collected studies were manually searched to
further increase the robustness of the search results.
Study selection
All relevant articles were screened by two
independent authors (AS and PCY) according to the included criteria
after blind screening of titles and abstracts. All studies that met
the inclusion criteria were included. If a disagreement occurred, a
third co-author (JYX) was consulted to carefully scrutinize the
papers and decide whether a study was to be included in the final
research selection.
Inclusion criteria
The inclusion criteria were as follows: i) Patients
diagnosed with CRC who underwent radical resection; ii) assessment
of the association of the TSR and survival data; iii) evaluation of
survival-associated outcomes, such as OS and disease-free survival
(DFS); and iv) studies published in English.
Exclusion criteria
The exclusion criteria were as follows: i)
Non-research references, such as case reports, letters or
systematic reviews; ii) studies with duplicate data; iii) key
information extraction was not available; and iv) research using
non-human models.
Data extraction
The data extracted from the tables and figures of
the selected articles were tabulated in Microsoft Excel 16.18
software (Microsoft Corporation). In the present meta-analysis, the
extracted data elements were as follows: First author/s, publishing
year, study design, sample size, cohort characteristics of the
study (such as age and sex), tumor position, estimated method of
determining the TSR, treatment, histopathological stage, cut-off
value, follow-up time and hazard ratio (HR) estimates with 95%
confidence interval (CI) for OS, DFS, relapse-free survival (RFS)
and disease-specific survival (DSS).
Quality assessment
Quality assessment of the final selection articles
was performed using the Newcastle-Ottawa Scale (NOS) by two
independent authors (GH and LPL). The NOS contains three parts:
Selection (0–4 points); comparability (0–2 points); and outcome
assessment (0–3 points). A study was considered to be high-quality
if the scores were >6.
Statistical analysis
Stata software version 15.1 for Mac (StataCorp LP)
was used to perform the present meta-analysis to generate forest
plots by evaluating the HRs and associated 95% CIs of OS, DFS, DSS
and RFS from the included articles directly or estimated using the
methods by Parmar et al (14). To assess the results of
meta-analysis it is important to determine the effect size and its
impact (15). Therefore, Cochran's
Q-test and Higgins' I2 statistic were used to evaluate
the heterogeneity of the pooled results. Significant heterogeneity
was considered if P<0.1 for the Q-test or I2>50%
(16). According to the
recommendations provided by the Cochrane Handbook for Systematic
Reviews of Interventions (https://training.cochrane.org/handbook), the choice
between a fixed-effects and a random-effects meta-analysis should
never be made on the basis of a statistical test for heterogeneity.
Therefore, a random-effects model was adopted in the present
meta-analysis regardless of the I2 values found.
To assess the potential source of heterogeneity
among studies, subgroup analysis and meta-regression were applied
utilizing variables such as ethnicity, cancer position, carcinoma
stage, treatment, TSR calculation method and regression analysis
type.
Sensitivity analysis was performed to evaluate the
credibility of outcomes in the present meta-analysis. To assess the
publication bias of selection literature, visual inspection of the
Begg's funnel plot and Egger's test were performed, and P<0.05
was considered to indicate statistical significance.
Results
Search results and study
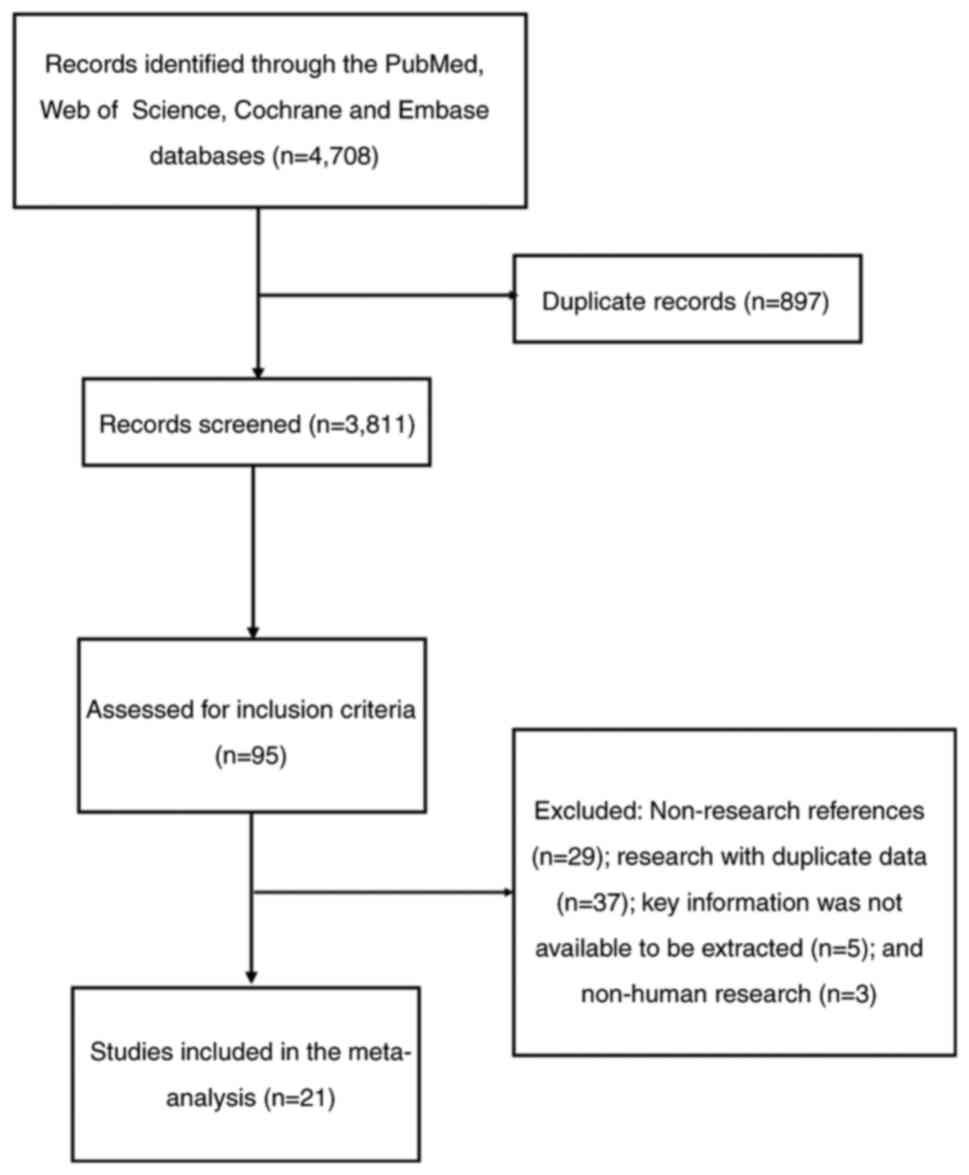
characteristics
A total of 4,708 articles were identified from the
database searches, with 3,811 studies obtained after removing
duplicate records. Subsequently, a further 3,716 studies were
eliminated by reviewing the titles and abstract according to the
PICOS principle (Population, Intervention, Comparison, Outcomes and
Study framework; http://training.cochrane.org/handbook). A total of 74
studies were then excluded after the articles were read
[non-research references (n=29); research with duplicate data
(n=37); key information extraction unavailable (n=5); and non-human
research (n=3)]. Finally, a total of 21 studies published between
August 13, 2007 and January 15, 2023 were included in the present
meta-analysis (9,10,12,17–34).
Notably, the study by Zhao et al (33) contained two independent
sub-datasets, which were considered two independent studies in the
present article. Therefore, there were 22 studies included in the
present meta-analysis (Fig. 1).
Characteristics of the 22 eligible studies are summarized in
Table I. Among the 22 included
articles, there was a total of 7,934 patients, with the number of
patients in individual studies ranging from 88 to 1,212. A total of
nine studies were performed in the Netherlands, seven in China, two
in the United Kingdom, and one in Germany, Turkey and Egypt,
respectively. Moreover, eight studies included patients with colon
carcinoma, 12 studies with patients with CRC and one study with
patients with rectal cancer. The prognostic value of the TSR was
assessed in the eligible studies: 16 studies evaluated the
association between the TSR and OS; eight studies assessed the
association of the TSR and DFS; three studies evaluated the
association between the TSR and cancer-specific survival (CSS); and
four studies assessed the prognostic impact of the TSR on RFS. The
cut-off values ranged from 40.0–65.5%. A total of 19 studies were
retrospective and two were prospective trials. The NOS scores of
all studies ranged from 6–9 (Table
I), which indicates that they are of a high quality (NOS scores
≥6).
 | Table I.Major features of the studies
included in the present meta-analysis. |
Table I.
Major features of the studies
included in the present meta-analysis.
| First author/s,
year | Country | Study design | Sample size | Histology | Sex, % male | TSR estimate
method | Treatment | Stage | Cut-off value,
% | Follow up,
months | Survival
analysis | NOS score | (Refs.) |
|---|
| Dang et al,
2020 | Netherlands | Retrospective | 223 | CRC | 56.1 | Artificial | Surgery | I | 50 | 43
(18–84)c | OS | 9 | (9) |
| Zhao et al,
2021 | China | Retrospective | 179 | CRC | 60.9 | Computer | Surgery + a djuvant
chemotherapy | II | 50 | 59d | OS/DFS | 8 | (10) |
| Geessink et
al, 2019 | Netherlands | Retrospective | 129 | RC | 67 | Computer | Surgery + adjuvant
chemotherapy + radiotherapy | I–III | 65.47 | 67.2
(27.6–99.6)a | CSS/DFS | 9 | (12) |
| Aboelnasr et
al, 2023 | Egypt | Retrospective | 103 | CRC | 30.9 | Artificial | NA | I–IV | 50 | NA | OS/RFS | 8 | (17) |
| Fan et al,
2022 | China | Retrospective | 207 | CRC | 60.9 | Artificial | Surgery + adjuvant
chemotherapy | II | 50 | NA | RFS | 7 | (18) |
| Fu et al,
2020 | China | Retrospective | 353 | CRC | 42 | Artificial | Surgery | I–III | 50 | 24
(16–37)c | OS | 7 | (19) |
| Huijbers et
al, 2013 | Netherlands | Perspective | 710 | CC | 50 | Artificial | Surgery + adjuvant
chemotherapy | II–III | 50 | 55.4
(0–84.9)a | OS/DFS | 9 | (20) |
| Huijbers et
al, 2018 | Netherlands | Retrospective | 965 | CRC | 56.8 | Artificial | Surgery + adjuvant
chemotherapy | II–III | 50 | NA | DFS | 8 | (21) |
| Hynes et al,
2017 | United.
Kingdom | Retrospective | 445 | CC | 53.3 | Artificial | Surgery + adjuvant
chemotherapy | II–III | 50 | 66
(1.2–120)a | OS/CSS | 9 | (22) |
| Li et al,
2021 | China | Retrospective | 996 | CRC | 58 | Artificial | NA | I–IV | 50 | NA | OS | 8 | (23) |
| Li et al,
2023 | China | Retrospective | 198 | CC | 52.5 | Computer | Surgery + adjuvant
chemotherapy | III | 50 | NA | OS/DFS | 7 | (24) |
| Mesker et
al, 2007 | Netherlands | Retrospective | 122 | CC | 59 | Artificial | Surgery | I–III | 50 | 148d | OS/DFS | 7 | (25) |
| Miller et
al, 2021 | Germany | Retrospective | 253 | CC | 57 | Computer | Surgery + adjuvant
chemotherapy | II–IV | 40 | 43.2d | RFS | 9 | (26) |
| Park et al,
2016 | United.
Kingdom | Retrospective | 246 | CRC | 52 | Artificial | Surgery + adjuvant
chemotherapy | I–III | 50 | 150
(87–206)a | CSS | 9 | (27) |
| Sandberg et
al, 2019 | Netherlands | Retrospective | 201 | CRC | 50.7 | Artificial | Surgery + adjuvant
chemotherapy | I–IV | 50 | 68
(0–229)a | OS/DFS | 9 | (28) |
| Smit et al,
2021 | Netherlands | Retrospective | 246 | CC | 54 | Artificial | Surgery + adjuvant
chemotherapy | II–III | 50 | 47
(4–158)a | OS/DFS | 9 | (29) |
| Vogelaar et
al, 2016 | Netherlands | Retrospective | 97 | CC | NA | Artificial | Surgery + adjuvant
chemotherapy | I–III | NA | 78
(0–144)a | OS | 8 | (30) |
| Zengin 2019 | Turkey | Retrospective | 88 | CC | 60 | Artificial | Surgery | I | 50.4 | 84
(10–205)a | OS/RFS | 8 | (31) |
| Zhang et al,
2021 | China | Retrospective | 147 | CRC | 61 | Artificial | Surgery + adjuvant
chemotherapy | I–IV | 50 | 48
(1–91)a | OS | 6 | (32) |
| Zhao et al,
2020 | China | Retrospective | 499 | CRC | 60.3 | Computer | Surgery | I–IV | 48.80 | 89
(79–96)b | OS | 9 | (33) |
|
| China | Retrospective | 315 | CRC | 59.7 | Computer | Surgery | I–IV | 48.80 | 51
(50–53)b | OS | 9 |
|
| Zunder et
al, 2018 | Netherlands | Perspective | 1212 | CRC | 55.5 | Artificial | Surgery + adjuvant
chemotherapy | II–III | 50 | NA | OS/DFS | 9 | (34) |
Prognostic impact of the TSR on OS in
patients with CRC
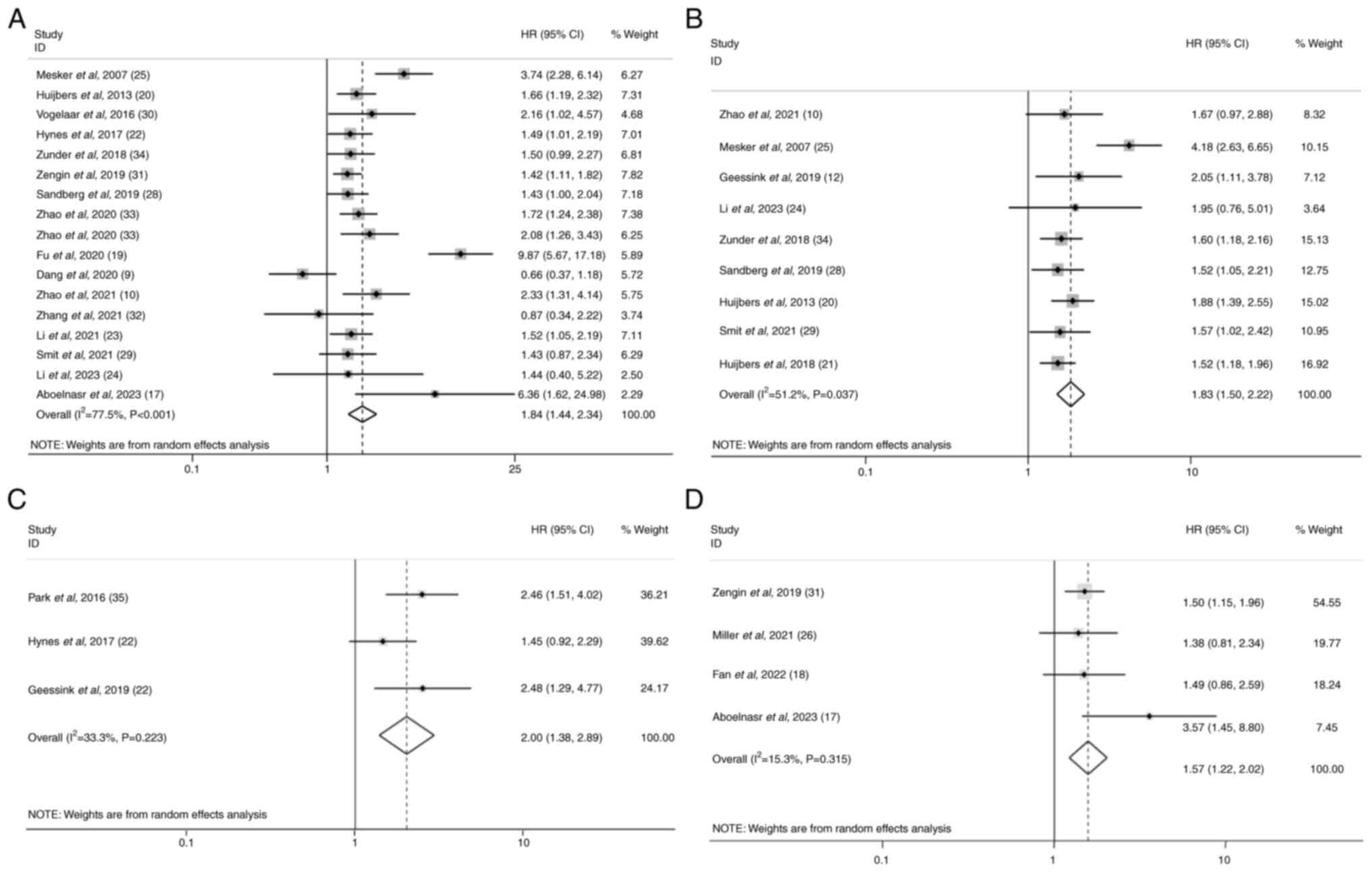
A total of 16 studies provided data from 6,134
patients for the OS analysis (9,10,17,19,20,22–25,28–34). A
random-effects model was performed with a significant heterogeneity
detected in these data (I2=77.5%; P<0.001; Table II). It was demonstrated that an
elevated TSR predicted a decreased OS with a combined HR of 1.84
(95% CI, 1.44–2.34; P<0.001; Fig.
2A). Subgroup analysis by ethnicity indicated that the TSR was
a negative predictor of overall survival both in Asian (HR=2.17;
95% CI, 1.44–3.28; P<0.001) and Caucasian populations (HR=1.58;
95% CI, 1.20–2.10; P=0.001). When the TSR estimation method was
considered, both computer-aided calculations (HR=1.88; 95% CI,
1.48–2.40; P<0.001) and artificial estimations (HR=1.82; 95% CI,
1.34–2.48; P<0.001) of the TSR were negative prognostic factors.
When performing subgroup analyses stratified by analysis method
(multivariate analysis), an increased TSR was revealed to be a
negative predictor for OS (HR=1.81; 95% CI, 1.41–2.32; P<0.001).
However, there was no statistical significance for the univariate
analysis (HR=1.80; 95% CI, 0.65–4.99; P=0.26). Considering
different cancer types, the TSR was a negative prognostic marker
for colon cancer (HR=1.75; 95% CI, 1.36–1.26; P<0.001) and CRC
(HR=1.90; 95% CI, 1.27–2.83; P=0.002). Furthermore, an increased
TSR predicted a worse outcome both in patients undergoing surgery
only (HR=2.21; 95% CI, 1.24–3.96; P=0.008) and surgery +
chemotherapy (HR=1.56; 95% CI, 1.33–1.83; P<0.001). However,
although an elevated TSR had a negative prognostic value for
patients with stage II–III CRC (HR=1.60; 95% CI, 1.33–1.93;
P<0.001), this was not demonstrated for those with stage I
(H=1.01; 95% CI, 0.48–2.14; P=0.97).
 | Table II.Subgroup analysis of pooled hazard
ratios and 95% confidence intervals for the association between the
tumor-stroma ratio and overall survival, disease-free survival,
cancer-specific survival and recurrence free survival in patients
with colorectal cancer. |
Table II.
Subgroup analysis of pooled hazard
ratios and 95% confidence intervals for the association between the
tumor-stroma ratio and overall survival, disease-free survival,
cancer-specific survival and recurrence free survival in patients
with colorectal cancer.
| A, OS |
|---|
|
|---|
|
|
|
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Studies, n | Patients, n | Effects model | HR (95% CI) | P-value | I2,
% | P-value | (Refs.) |
|---|
| Total OS | 17 | 6,134 | Random | 1.84
(1.44–2.34) | <0.001 | 77.5 | <0.001 | (9,10,17,19,20,22–25,28–34) |
| Ethnicity |
|
|
|
|
|
|
|
|
|
Asian | 9 | 2,878 | Random | 2.17
(1.44–3.28) | <0.001 | 83.1 | <0.001 | (10,17,19,23,24,31–33) |
|
Caucasian | 8 | 3,256 | Random | 1.58
(1.20–2.10) | 0.001 | 67.7 | 0.003 | (9,20,22,25,28–30,34) |
| TSR estimation
method |
|
|
|
|
|
|
|
|
|
Computer-aided | 4 | 1,191 | Random | 1.88
(1.48–2.40) | <0.001 | 0.0 | 0.770 | (10,24,33) |
|
Artificial estimation | 13 | 4,943 | Random | 1.82
(1.34–2.48) | <0.001 | 82.6 | <0.001 | (9,17,19,20,22,23,25,28–32,34) |
| Analysis
method |
|
|
|
|
|
|
|
|
|
Univariate | 3 | 4,67 | Random | 1.80
(0.65–4.99) | 0.260 | 75.4 | 0.007 | (24,25,32) |
|
Multivariate | 14 | 5,667 | Random | 1.81
(1.41–2.32) | <0.001 | 78.0 | <0.001 | (9,10,17,19,20,22,23,28–31,33,34) |
| Treatment |
|
|
|
|
|
|
|
|
| Surgery
+ chemotherapy | 9 | 3,435 | Random | 1.56
(1.33–1.83) | <0.001 | 0.0 | 0.792 | (10,20,22,24,28–30,32,34) |
| Surgery
only | 6 | 1,600 | Random | 2.21
(1.24–3.96) | 0.008 | 91.6 | <0.001 | (9,19,25,31,33) |
| Cancer type |
|
|
|
|
|
|
|
|
|
Colon | 7 | 1,906 | Random | 1.75
(1.36–1.26) | <0.001 | 54.0 | 0.043 | (20,22,24,25,29–31) |
|
Colorectal | 10 | 4,228 | Random | 1.90
(1.27–2.83) | 0.002 | 84.4 | <0.001 | (9,10,17,19,23,28,32–34) |
| Stage |
|
|
|
|
|
|
|
|
| I | 2 | 311 | Random | 1.01
(0.48–2.14) | 0.970 | 82.4 | 0.017 | (9,31) |
|
II–III | 6 | 2,990 | Random | 1.60
(1.33–1.93) | <0.001 | 0.0 | 0.830 | (10,20,22,24,29,34) |
|
Other | 9 | 2,833 | Random | 2.37
(1.54–3.64) | <0.001 | 84.0 | <0.001 | (17,19,23,25,28,30,32,33) |
|
| B, DFS |
|
|
|
|
|
|
|
|
Heterogeneity |
|
|
|
|
|
|
|
|
|
|
|
Variable | Studies,
n | Patients,
n | Effects
model | HR (95%
CI) | P-value | I2,
% | P-value | (Refs.) |
|
| Total DFS | 9 | 3,962 | Random | 1.83
(1.50–2.22) | <0.001 | 51.2 | 0.037 | (10,12,20,21,24,25,28,29,34) |
| Ethnicity |
|
|
|
|
|
|
|
|
|
Asian | 2 | 377 | Random | 1.73
(1.08–2.78) | 0.022 | 0.0 | 0.779 | (10,24) |
|
Caucasian | 7 | 3,585 | Random | 1.85
(1.47–2.32) | <0.001 | 63.2 | 0.012 | (12,20,21,25,28,29,34) |
| TSR estimation
method |
|
|
|
|
|
|
|
|
|
Computer-aided | 3 | 506 | Random | 1.85
(1.27–2.68) | 0.001 | 0.0 | 0.879 | (10,12,24) |
|
Artificial estimation | 6 | 3,456 | Random | 1.84
(1.43–2.36) | <0.001 | 68.9 | 0.007 | (20,21,25,28,29,34) |
| Analysis
method |
|
|
|
|
|
|
|
|
|
Univariate | 2 | 320 | Random | 3.20
(1.576.53) | 0.001 | 50.5 | 0.155 | (24,25) |
|
Multivariate | 7 | 3,642 | Random | 1.64
(1.43–1.87) | <0.001 | 0.0 | 0.933 | (10,12,20,21,28,29,34) |
| Treatment |
|
|
|
|
|
|
|
|
| Surgery
+ chemotherapy | 8 | 3,840 | Random | 1.64
(1.44–1.88) | <0.001 | 0.0 | 0.961 | (10,12,20,21,24,28,29,34) |
| Surgery
only | 1 | 122 | - | 4.18
(2.63–6.65) | <0.001 | - | - | (25) |
| Cancer type |
|
|
|
|
|
|
|
|
|
Colon | 4 | 1,276 | Random | 2.22
(1.42–3.48) | <0.001 | 72.0 | 0.013 | (20,24,25,29) |
|
Colorectal | 4 | 2,557 | Random | 1.56
(1.32–183) | <0.001 | 0.0 | 0.986 | (10,21,28,34) |
|
Rectal | 1 | 129 | - | 2.05
(1.11–3.78) | 0.022 | - | - | (12) |
| Stage |
|
|
|
|
|
|
|
|
|
II–III | 6 | 3,510 | Random | 1.64
(1.42–1.90) | <0.001 | 0.0 | 0.934 | (10,20,21,24,29,34) |
|
Other | 3 | 452 | Random | 2.34
(1.23–4.48) | 0.010 | 82.1 | 0.004 | (12,25,28) |
|
| C, CSS |
|
|
|
|
|
|
|
|
Heterogeneity |
|
|
|
|
|
|
|
|
|
|
|
Variable | Studies,
n | Patients,
n | Effects
model | HR (95%
CI) | P-value | I2,
% | P-value | (Refs.) |
|
| Total CSS | 3 | 820 | Random | 2.00
(1.38–2.89) | <0.001 | 33.3 | 0.220 | (12,22,27) |
|
| D, RFS |
|
|
|
|
|
|
|
|
Heterogeneity |
|
|
|
|
|
|
|
|
|
|
|
Variable | Studies,
n | Patients,
n | Effects
model | HR (95%
CI) | P-value | I2,
% | P-value | (Refs.) |
|
| Total RFS | 4 | 651 | Random | 1.57
(1.22–2.02) | <0.001 | 15.3 | 0.315 | (17,18,26,31) |
| Ethnicity |
|
|
|
|
|
|
|
|
|
Asian | 3 | 398 | Random | 1.70
(1.16–2.48) | <0.001 | 39.7 | 0.190 | (17,18,31) |
|
Caucasian | 1 | 253 | - | 1.38
(0.81–2.34) | 0.232 | - | - | (26) |
| Cancer type |
|
|
|
|
|
|
|
|
|
Colon | 2 | 341 | Random | 1.47
(1.16–1.87) | 0.001 | 0.0 | 0.783 | (26,31) |
|
Colorectal | 2 | 310 | Random | 2.14
(0.92–4.97) | 0.077 | 61.8 | 0.106 | (17,18) |
Prognostic role of the TSR for DFS in
CRC
A total of nine studies provided data from 3,962
patients for the DFS analysis (10,12,20,21,24,25,28,29,34).
The combined data demonstrated that an increased TSR was associated
with worse DFS for patients with CRC (HR=1.83; 95% CI, 1.50–2.22;
P<0.001; Fig. 2B). Moreover,
heterogeneity existed among the studies (I2=51.2%;
P=0.037). A high TSR was associated with poor DFS irrespective of
ethnicity (HR=1.73; 95% CI, 1.08–2.78; P=0.022 vs. HR=1.85, 95% CI,
1.47–2.32; P<0.001), analysis method (HR=3.20, 95% CI,
1.57–6.53; P=0.001 vs. HR=1.64, 95% CI, 1.43–1.87; P<0.001) and
cancer type (HR=2.22, 95% CI, 1.42–3.48; P<0.001 vs. HR=1.56,
95% CI, 1.32–183; P<0.001 vs. HR=2.05, 95% CI, 1.11–3.78;
P=0.022). Moreover, a high TSR also predicted poor DFS in patients
receiving postoperative adjuvant chemotherapy (HR=1.64; 95% CI,
1.44–1.88; P<0.001) and in patients with stage II and III CRC
(HR=1.64; 95% CI, 1.42–1.90; P<0.001). Notably, an increased TSR
calculated by computer was also associated with worse DFS in
patients with CRC (HR=1.85; 95% CI, 1.27–2.68; P<0.001)
(Table II).
Prognostic role of TSR for CSS and RFS
in patients with CRC
A total of three studies reported the association of
CSS and the TSR, with 820 patients included (12,22,27).
The combined data indicated that an elevated TSR was associated
with worse CSS in patients with CRC (HR=2.00; 95% CI, 1.38–2.89;
P<0.001; Fig. 2C). No
significant heterogeneity was detected (I2=33.3%;
P=0.22). Moreover, the data from 651 patients extracted from four
studies were used to perform the meta-analysis focusing on the
prognostic role of the TSR for RFS in patients with CRC (17,18,26,31). A
random-effects model was adopted, although no significant
heterogeneity among the studies was detected (I2=15.3%;
P=0.315; Table II). Pooled HR from
the eligible studies was demonstrated to be 1.57 (95% CI,
1.22–2.02; P<0.001), indicating that a high TSR predicted a poor
RFS (Fig. 2D). Subgroup analysis
was performed based on ethnicity and cancer type, which revealed
that a high TSR was associated with worse RFS for Asian patients
(HR=1.59, 95% CI, 1.26–2.01 P<0.001) or patients with colon
cancer (HR=1.47, 95% CI, 1.16–1.87 P=0.001) (Table II).
Meta-regression analysis
The results of the meta-regression analysis
demonstrated that publication year (P=0.29), follow-up time
(P=0.58), analysis method (P=0.63), treatment (P=0.72), cancer type
(P=0.87), tumor stage (P=0.17) and ethnicity (P=0.29) did not
contribute to the source of heterogeneity (Table SI).
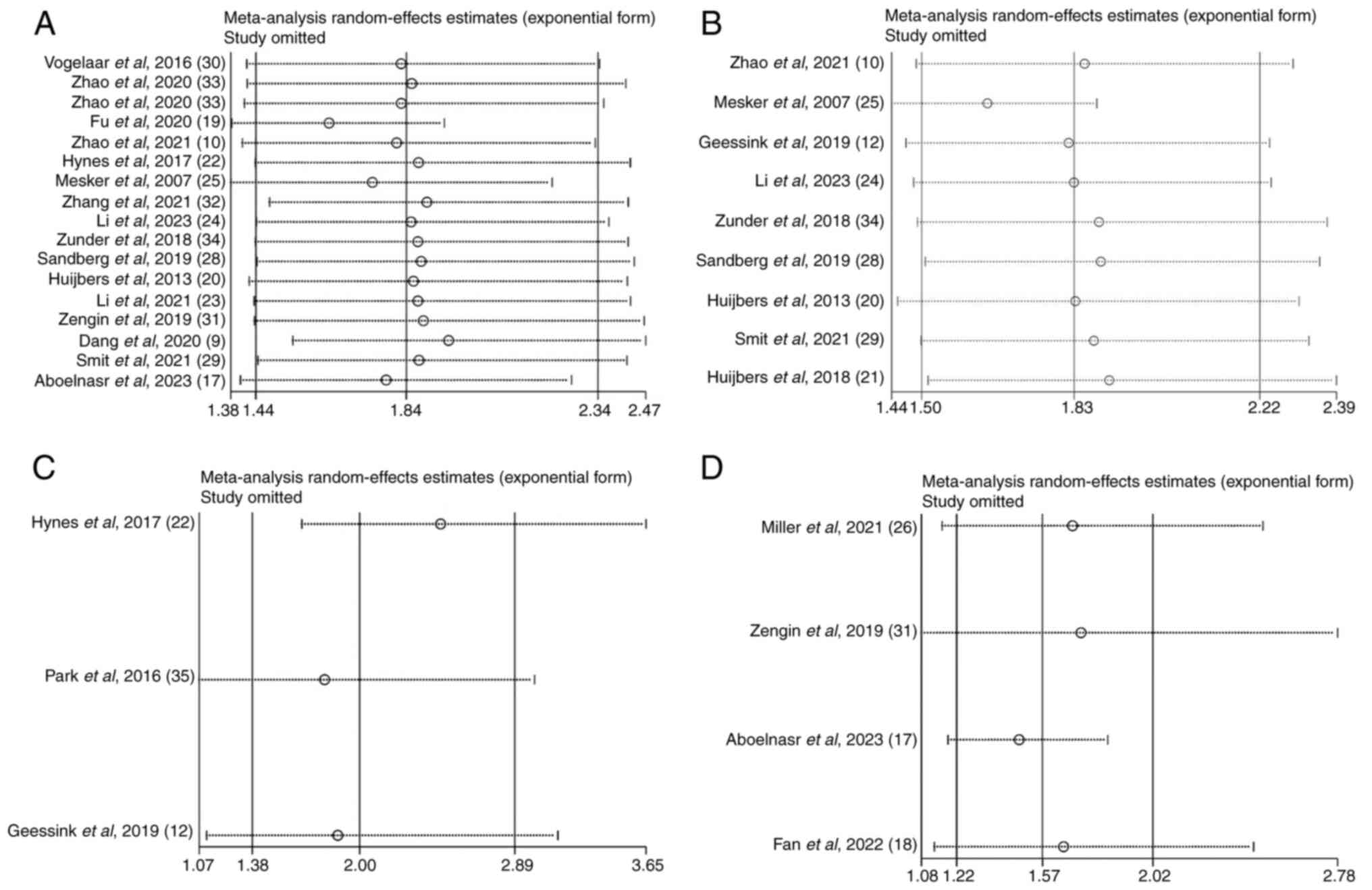
Sensitivity analysis
To assess the reliability of the pooled HR of OS,
DFS, CSS and RFS, a sensitivity analysis was performed (Fig. 3). There was no significant change in
overall HR when each eligible study from the present meta-analysis
was removed sequentially. Thus, the reliability of the results of
the present study was confirmed.
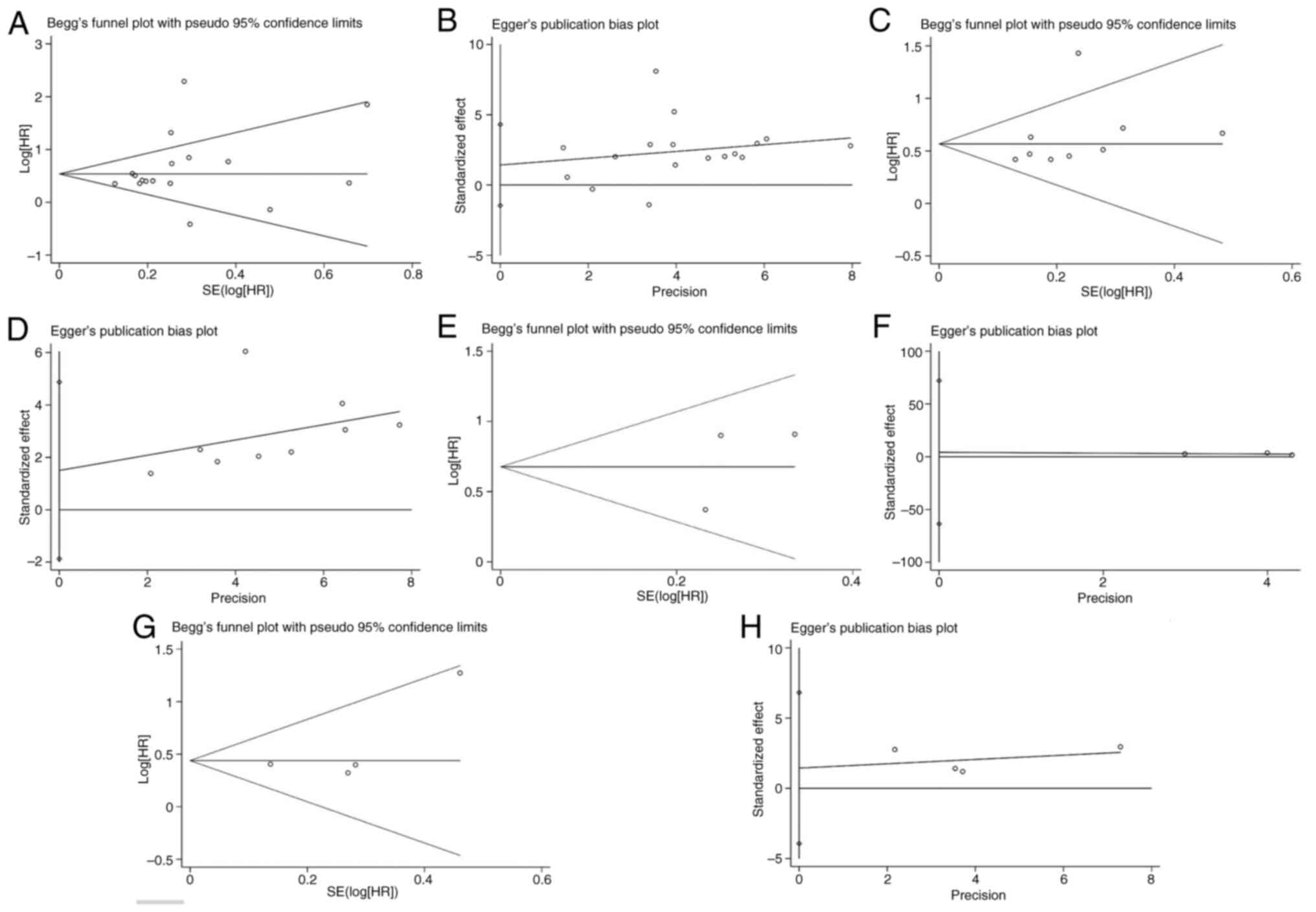
Publication bias
Begg's funnel and Egger's test were performed to
detect potential publication bias, with no significant bias
demonstrated in studies on the TSR with respect to OS (Begg's
P=0.266; Egger's P=0.310; Fig. 4A and
B), DFS (Begg's P=0.076; Egger's P=0.328; Fig. 4C and D), CSS (Begg's P=1.000;
Egger's P=0.574; Fig. 4E and F) or
RFS (Begg's P=0.308; Egger's P=0.368; Fig. 4G and H).
Association between the TSR and
clinicopathological features
Only 7/21 studies evaluated the relationship between
the TSR and clinicopathological features (Table III) (9,17–19,21,23,34). A
high TSR was reported to be markedly associated with increased T
stage in the studies by Fu et al (19) and Huijbers et al (21). Furthermore, these two studies
reported that patients with CRC with a high TSR had a higher
probability of lymphatic metastasis than those with a low TSR
(19,21); however, in the study by Aboelnasr
et al (17), the conclusion
was reversed. Unfortunately, it was not possible to generate a
pooled odds ratios (OR) value through meta-analysis for these
results as they were produced using the χ2 test.
 | Table III.Associations between
clinicopathological characteristics of colorectal cancer and the
tumor-stroma ratio. |
Table III.
Associations between
clinicopathological characteristics of colorectal cancer and the
tumor-stroma ratio.
|
|
| Tumor
differentiation |
| T stage |
| N stage |
| TNM stage |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | TSR | Well | Moderate | Poor | P-value | T1/T2 | T3/T4 | P-value | N0 | N+ | P-value | I/II | III/IV | P-value | (Refs.) |
|---|
| Aboelnasr et
al, 2023 | High | NA | NA | NA | NA | 19 | 48 | 0.187 | 44 | 23 | 0.001 | NA | NA | NA | (17) |
|
| Low | NA | NA | NA |
| 6 | 30 |
| 12 | 24 |
| NA | NA |
|
|
| Fan et al,
2022 | High | 17 | 69 | 28 | 0.221 | NA | NA | NA | NA | NA | NA | NA | NA | NA | (18) |
|
| Low | 13 | 47 | 33 |
| NA | NA |
| NA | NA |
| NA | NA |
|
|
| Fu et al,
2020 | High | 9 | 104 | 20 | 0.138 | 21 | 112 | 0.017 | 36 | 97 | <0.001 | NA | NA | NA | (19) |
|
| Low | 32 | 155 | 33 |
| 60 | 160 |
| 169 | 51 |
| NA | NA |
|
|
| Huijbers et
al, 2018 | High | NA | NA | NA | NA | 4 | 311 | 0.001 | 118 | 205 | 0.001 | 118 | 205 | 0.870 | (21) |
|
| Low | NA | NA | NA |
| 54 | 564 |
| 238 | 404 |
| 238 | 404 |
|
|
| Li et al,
2021 | High | 37 | 320a | 0.122 | 64 | 293 | 0.326 | 202 | 155 | 0.035 | 202 | 155 | 0.024 | (23) |
|
| Low | 48 | 591a |
| 131 | 508 |
| 405 | 234 |
| 408 | 231 |
|
|
| Smit et al,
2021 | High | NA | NA | NA | NA | 54 | 55 | 0.566 | 54 | 55 | 0.566 | 54 | 55 | 0.298 | (29) |
|
| Low | NA | NA | NA |
| 17 | 120 |
| 76 | 60 |
| 77 | 60 |
|
|
| Zunder et
al, 2018 | High | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 61 | 278 | 0.540 | (34) |
|
| Low | NA | NA | NA |
| NA | NA |
| NA | NA |
| 136 | 688 |
|
|
Discussion
To the best of our knowledge, the present research
is the first meta-analysis to assess the prognostic value of the
TSR on OS, DFS, CSS and RFS in CRC. As a novel prognostic marker,
the TSR can be calculated directly according to a systematic
evaluation process using pathological sections by pathologists
(35). Furthermore, with the
progression of convolutional neural networks (CNNs), the image
analysis field has been revolutionized. CNNs have been used to
classify medical images and detect TSR in histopathological images
(36). Fully automated TSR
assessments have also been applied on WSIs generated through
scanning the selected hematoxylin and eosin-stained tissue sections
using digital Whole Slide Scanning software (33). Therefore, the prognostic value of
the tumor-stroma percentage calculated using CNNs in CRC was more
objective.
The present meta-analysis collected data from 21
studies with 7,934 patients to assess the prognostic role of the
TSR in CRC. Significant prognostic efficacy in different subgroups
suggested that the TSR was a robust prognostic marker for long-term
survival outcomes, including OS, DFS, CSS and RFS. Furthermore,
computer-calculated TSR using WSIs was also an effective prognostic
marker for OS and DFS. However, although an elevated TSR was
associated with a negative prognosis in patients with stage II–III
CRC (HR=1.60; 95% CI, 1.33–1.93; P<0.001), it was not associated
with a negative prognosis in those with stage I CRC (HR=1.01; 95%
CI, 0.48–2.14; P=0.97).
The tumor microenvironment has attracted attention
in the field of carcinoma immunology. Neoplastic cells are not only
found in the tumor itself, but also in the surrounding stroma,
including immune cells, signaling molecules, the extracellular
matrix (ECM) and fibroblasts (37).
Several inflammatory cells and mediators have been reported to have
complex interactions with tumor cells (38). Stromal cells drive tumor progression
and invasion through modulation of the ECM, secretion of soluble
factors and stimulation of cell migration (39). Stromal cells, considered a scaffold
for tumor cells, provide survival signals such as C-X-C motif
chemokine ligand 12 and insulin growth factor, and lay down
extracellular elements including glycoprotein, integrins, collagen
and proteoglycans (7). ECM
deposition increases tumor-stromal density and tension, which may
generate a protective environment for cancer cells, preventing the
efficacy of anticancer agents such as biologics and chemotherapy
(7,39) Furthermore, several studies
evaluating the association between tumor-stroma percentage and
clinicopathological features in CRC have been published over
previous years, which have reported that a high TSR predicts worse
pathological outcomes, such as tumor budding, vessel invasion,
lymphatic invasion and microsatellite instability (18,20,23).
Therefore, a high tumor-stromal percentage may predict a poor
survival outcome. In addition, the process of assessing the TSR is
inexpensive, easily performed and reproducible (35), rendering the TSR a promising marker
to predict the survival outcome of CRC in clinical practice.
Several studies has reported the relationship
between the TSR and clinicopathological features previously
(9,17–19,21,23,34).
Unfortunately, the OR for the association between a high TSR and
tumor invasion, lymphatic metastasis and poor differentiation were
not available among the studies. Therefore, a meta-analysis on the
association between the TSR and tumor classification or stage
classification could not be performed. Moreover, among the included
studies, there were none that reported the relationship between the
TSR and genetic mutations. Therefore, it is necessary perform
further research exploring the association between the TSR and
tissue classification, stage classification, treatment selection or
genetic mutations.
The prognostic role of the TSR in solid tumors has
been reported in several meta-analyses in: i) A meta-analysis of
nine studies focusing on the clinical significance of the TSR in
head and neck cancers indicates that a high TSR is associated with
worse DFS or CSS in patients with head and neck cancers (40); ii) a meta-analysis of 12 studies
assessing the prognostic value of the TSR in women with breast
cancer reports that a high TSR predicts poor survival in women with
breast cancer (41); iii) a
meta-analysis of data from 2,031 patients with non-small cell lung
cancer indicates that stroma richness may be a predictor of poor
survival in patients with lung squamous cell carcinoma, but a
predictor of improved survival in patients with lung adenocarcinoma
(42); and iv) a meta-analysis of
the prognostic value of the TSR in rectal cancer, with data from
5,408 patients, demonstrated that a high TSR is notably associated
with worse survival outcomes (43).
The present meta-analysis evaluated the prognostic
value of the TSR in CRC; however, necessary subgroup analysis was
not performed; and a meta-analysis of 13 studies focusing on the
impact of the TSR on the prognosis of CRC indicated that a high TSR
was associated with worse DFS or OS in patients with CRC (44). Although subgroup analysis was
performed for tumor stage in the meta-analysis, it was not
performed for the TSR estimation method, analysis method, ethnicity
or cancer type. Furthermore, the prognostic value of the TSR for
CSS and RFS was not available in the meta-analysis by Gao et
al (44), therefore, the
conclusions are incomplete. The present meta-analysis demonstrated
the prognostic efficacy of the TSR for OS, DFS, RFS and CSS in CRC,
which is in-line with previous findings with other cancer types. In
addition, the present study also revealed that the TSR calculated
by computer using WSIs was also an effective prognostic marker for
OS and DFS. Furthermore, the present meta-analysis demonstrated
that an elevated TSR holds a negative prognostic value for patients
with stage II–III CRC, but not for those with stage I CRC. Lastly,
the references included in the present meta-analysis was quite
abundant, including 22 studies with 7,934 patients.
Based on the results of the present meta-analysis
and other published studies, the TSR may assist in the
determination of cancer prognosis and help develop treatment
regimens for patients with CRC. For example, patients with CRC with
a high TSR may benefit more from postoperative chemotherapy of
bevacizumab-capecitabine + oxaliplatin (XELOX) than XELOX alone.
However, administering postoperative chemotherapy of
bevacizumab-XELOX may lead to a worse OS in patients with CRC with
a low TSR (34). Moreover, although
patients with CRC with a high TSR may benefit from
bevacizumab-dependent adjuvant chemotherapy, whether the patient
benefits from other chemotherapy regimens is still unclear
(45). Furthermore, although the
present meta-analysis demonstrated that an increased TSR predicted
a worse outcome both in patients undergoing surgery alone and
surgery + chemotherapy, whether patients with CRC with a high TSR
benefit from chemotherapy was still unclear. Additionally, a
meta-analysis focusing on the association between the TSR and
treatment selection could be not performed due to a lack of
adequate data. Therefore, further investigations focusing on the
treatment of patients with CRC with a high TSR are needed to
explore more efficient postoperative chemotherapy regimens.
Although the present research is the first
meta-analysis to assess the prognostic value of the TSR on OS, DFS,
CSS and RFS in CRC, to the best of our knowledge, there are also
several limitations. First, most of the eligible studies adopted a
retrospective design, which led to heterogeneity among studies as
the selection criteria could not be strictly controlled. Second,
several HRs were extracted from univariate analyses performed
without consideration of confounding factors, which may have caused
an overestimation of effect sizes. Third, the definition of the TSR
cut-off values in the selected studies was inconsistent, which
could cause bias in the results.
In conclusion, the findings of the present
meta-analysis indicated that a higher TSR was associated with poor
OS, DFS, CSS and RFS in patients with CRC, especially for those
with stages II–IIIs. In addition, a TSR calculated by computer
using WSIs was also an effective prognostic marker for OS and DFS
in patients with CRC. Therefore, the TSR may serve an important
role in developing treatment regimens for CRC; however, further
prospective studies are needed to validate the results of the
present study due to its limitations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Guangxi Zhuang Autonomous
Region Health Committee self-funded project (grant no.
Z2016176).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AS, PCY, LPL, GH and JYX collected and extracted the
data and performed the quality assessment. AS, PCY, LPL and GH
analyzed the data. AS and JYX conceived and designed the present
study and wrote the paper. All authors read and approved the final
manuscript. AS and JYX confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L
and Zhou Z: VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt
and Hippo signalling in colorectal cancer. Nat Commun. 8:140582017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Araghi M, Soerjomataram I, Jenkins M,
Brierley J, Morris E, Bray F and Arnold M: Global trends in
colorectal cancer mortality: Projections to the year 2035. Int J
Cancer. 144:2992–3000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang K, Gao K, Hu G, Wen Y, Lin C and Li
X: CTGF enhances resistance to 5-FU-mediating cell apoptosis
through FAK/MEK/ERK signal pathway in colorectal cancer. Onco
Targets Ther. 9:7285–7295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crawford N, Salvucci M, Hellwig CT,
Lincoln FA, Mooney RE, O'Connor CL, Prehn JH, Longley DB and Rehm
M: Simulating and predicting cellular and in vivo responses of
colon cancer to combined treatment with chemotherapy and IAP
antagonist Birinapant/TL32711. Cell Death Differ. 25:1952–1466.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon Cancer, Version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dang H, van Pelt GW, Haasnoot KJC, Backes
Y, Elias SG, Seerden TCJ, Schwartz MP, Spanier BWM, de Vos Tot
Nederveen Cappel WH, van Bergeijk JD, et al: Tumour-stroma ratio
has poor prognostic value in non-pedunculated T1 colorectal cancer:
A multi-centre case-cohort study. United European Gastroenterol J.
9:20506406209753242020.PubMed/NCBI
|
|
10
|
Zhao Z, Zhang X, Li Z, Gao Y, Guan X,
Jiang Z, Liu Z, Yang M, Chen H, Ma X, et al: Automated assessment
of DNA ploidy, chromatin organization, and stroma fraction to
predict prognosis and adjuvant therapy response in patients with
stage II colorectal carcinoma. Am J Cancer Res. 11:6119–6132.
2021.PubMed/NCBI
|
|
11
|
Zhang R, Song W, Wang K and Zou S:
Tumor-stroma ratio (TSR) as a potential novel predictor of
prognosis in digestive system cancers: A meta-analysis. Clin Chim
Acta. 472:64–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geessink OGF, Baidoshvili A, Klaase JM,
Ehteshami Bejnordi B, Litjens GJS, van Pelt GW, Mesker WE,
Nagtegaal ID, Ciompi F and van der Laak JAWM: Computer aided
quantification of intratumoral stroma yields an independent
prognosticator in rectal cancer. Cell Oncol (Dordr). 42:331–341.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA; PRISMA-P Group,
: Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4:12015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jayaraj R, Kumarasamy C, Sabarimurugan S
and Baxi S: Diagnostic and prognostic value of microRNAs for
cancers-strategies and approaches to improve the clinical utility.
J Cancer. 10:1252–1253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aboelnasr LS, El-Rebey HS, Mohamed A and
Abdou AG: The prognostic impact of tumor border configuration,
tumor budding and tumor stroma ratio in colorectal carcinoma. Turk
Patoloji Derg. 39:83–93. 2023.PubMed/NCBI
|
|
18
|
Fan S, Cui X and Zheng L: Prognostic value
of desmoplastic stromal reaction, tumor budding and tumor-stroma
ratio in stage II colorectal cancer. J Gastrointest Oncol.
13:2903–2921. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu M, Chen D, Luo F, Li M, Wang Y, Chen J,
Li A and Liu S: Association of the tumour stroma percentage in the
preoperative biopsies with lymph node metastasis in colorectal
cancer. Br J Cancer. 122:388–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huijbers A, Tollenaar RA, v Pelt GW,
Zeestraten EC, Dutton S, McConkey CC, Domingo E, Smit VT, Midgley
R, Warren BF, et al: The proportion of tumor-stroma as a strong
prognosticator for stage II and III colon cancer patients:
Validation in the VICTOR trial. Ann Oncol. 24:179–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huijbers A, van Pelt GW, Kerr RS,
Johnstone EC, Tollenaar RAEM, Kerr DJ and Mesker WE: The value of
additional bevacizumab in patients with high-risk stroma-high colon
cancer. A study within the QUASAR2 trial, an open-label randomized
phase 3 trial. J Surg Oncol. 117:1043–1048. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hynes SO, Coleman HG, Kelly PJ, Irwin S,
O'Neill RF, Gray RT, McGready C, Dunne PD, McQuaid S, James JA, et
al: Back to the future: Routine morphological assessment of the
tumour microenvironment is prognostic in stage II/III colon cancer
in a large population-based study. Histopathology. 71:12–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li T, Yu Z, Yang Y, Fu Z, Chen Z, Li Q,
Zhang K, Luo Z, Qiu Z and Huang C: Rapid multi-dynamic algorithm
for gray image analysis of the stroma percentage on colorectal
cancer. J Cancer. 12:4561–4573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Liao L, Kong L, Jiang W, Tang J, Han
K, Hou Z, Zhang C, Zhou C, Zhang L, et al: DNA ploidy and stroma
predicted the risk of recurrence in low-risk stage III colorectal
cancer. Clin Transl Oncol. 25:218–225. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mesker WE, Junggeburt JM, Szuhai K, de
Heer P, Morreau H, Tanke HJ and Tollenaar RA: The carcinoma-stromal
ratio of colon carcinoma is an independent factor for survival
compared to lymph node status and tumor stage. Cell Oncol.
29:387–398. 2007.PubMed/NCBI
|
|
26
|
Miller S, Bauer S, Schrempf M, Schenkirsch
G, Probst A, Märkl B and Martin B: Semiautomatic analysis of tumor
proportion in colon cancer: Lessons from a validation study. Pathol
Res Pract. 227:1536342021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JH, McMillan DC, Edwards J, Horgan PG
and Roxburgh CS: Comparison of the prognostic value of measures of
the tumor inflammatory cell infiltrate and tumor-associated stroma
in patients with primary operable colorectal cancer.
Oncoimmunology. 5:e10988012016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandberg TP, Sweere I, van Pelt GW, Putter
H, Vermeulen L, Kuppen PJ, Tollenaar RAEM and Mesker WE: Prognostic
value of low CDX2 expression in colorectal cancers with a high
stromal content-a short report. Cell Oncol (Dordr). 42:397–403.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smit MA, van Pelt GW, Terpstra V, Putter
H, Tollenaar RAEM, Mesker WE and van Krieken JHJM: Tumour-stroma
ratio outperforms tumour budding as biomarker in colon cancer: A
cohort study. Int J Colorectal Dis. 36:2729–2737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogelaar FJ, van Pelt GW, van Leeuwen AM,
Willems JM, Tollenaar RA, Liefers GJ and Mesker WE: Are
disseminated tumor cells in bone marrow and tumor-stroma ratio
clinically applicable for patients undergoing surgical resection of
primary colorectal cancer? The Leiden MRD study. Cell Oncol
(Dordr). 39:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zengin M: Tumour budding and tumour stroma
ratio are reliable predictors for death and recurrence in elderly
stage I colon cancer patients. Pathol Res Pract. 215:1526352019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Liu Y, Qiu X and Yan B:
Concurrent comparison of the prognostic values of tumor budding,
tumor stroma ratio, tumor infiltrating pattern and
Lymphocyte-to-Monocyte ratio in colorectal cancer patients. Technol
Cancer Res Treat. 20:153303382110458262021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao K, Li Z, Yao S, Wang Y, Wu X, Xu Z,
Wu L, Huang Y, Liang C and Liu Z: Artificial intelligence
quantified tumour-stroma ratio is an independent predictor for
overall survival in resectable colorectal cancer. EBioMedicine.
61:1030542020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zunder SM, van Pelt GW, Gelderblom HJ,
Mancao C, Putter H, Tollenaar RA and Mesker WE: Predictive
potential of tumour-stroma ratio on benefit from adjuvant
bevacizumab in high-risk stage II and stage III colon cancer. Br J
Cancer. 119:164–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Pelt GW, Kjær-Frifeldt S, van Krieken
JHJM, Al Dieri R, Morreau H, Tollenaar RAEM, Sørensen FB and Mesker
WE: Scoring the tumor-stroma ratio in colon cancer: Procedure and
recommendations. Virchows Arch. 473:405–412. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ehteshami Bejnordi B, Veta M, Johannes van
Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak
JAWM; the CAMELYON16 Consortium, ; Hermsen M, Manson QF, et al:
diagnostic assessment of deep learning algorithms for detection of
lymph node metastases in women with breast cancer. JAMA.
318:2199–2210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arneth B: Tumor Microenvironment. Medicina
(Kaunas). 56:152019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Almangush A, Alabi RO, Troiano G, Coletta
RD, Salo T, Pirinen M, Mäkitie AA and Leivo I: Clinical
significance of tumor-stroma ratio in head and neck cancer: A
systematic review and meta-analysis. BMC Cancer. 21:4802021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang P, Chen Y and Liu B: Prognostic
efficacy of tumor-stroma ratio in women with breast cancer: A
Meta-analysis of cohort studies. Front Oncol. 11:7314092021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Ma H, Zhang L and Li F:
Predictive role of Tumor-stroma ratio for survival of patients with
Non-small cell lung cancer: A Meta-analysis. Pathol Oncol Res.
27:16100212021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu Y, Jin Z, Qian Y, Shen Y and Wang Z:
Prognostic value of tumor-stroma ratio in rectal cancer: A
systematic review and Meta-analysis. Front Oncol. 11:6855702021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao J, Shen Z, Deng Z and Mei L: Impact of
Tumor-stroma ratio on the prognosis of colorectal cancer: A
systematic review. Front Oncol. 11:7380802021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sullivan L, Pacheco RR, Kmeid M, Chen A
and Lee H: Tumor stroma ratio and its significance in locally
advanced colorectal cancer. Curr Oncol. 29:3232–3241. 2022.
View Article : Google Scholar : PubMed/NCBI
|