Introduction
Lung cancer is one of the most prevalent malignant
diseases in the world, accounting for >10% of new cancer cases,
and has the highest mortality rate among all cancer cases worldwide
(1,2). Osimertinib, a third-generation
epidermal growth factor receptor tyrosine kinase inhibitor
(EGFR-TKI), is widely used to treat EGFR-mutated non-small cell
lung cancer (NSCLC) (3). Although
osimertinib has high selectivity for EGFR-activating mutations and
the EGFR T790M mutation in patients with advanced NSCLC, the
development of drug resistance remains a major challenge (4). Drug resistance often indicates disease
progression. Delaying resistance can slow tumor growth and reduce
the risk of relapse and metastasis, allowing more patients to
benefit from treatment and improve their quality of life (5). Therefore, novel therapeutic strategies
for overcoming osimertinib resistance are urgently needed.
At present, various therapeutic approaches in
combination with the administration of osimertinib, including the
use of MET inhibitors, chemotherapy and immunotherapy, have been
shown to be effective at prolonging the time to the development of
resistance (6–8). Research has demonstrated that the
overamplification of MET is a significant contributor to
osimertinib resistance. The combination of MET inhibitors and
osimertinib has been shown to be more effective for managing tumor
progression (9). Chemotherapy
remains a primary therapeutic approach for NSCLC. In patients with
NSCLC that is resistant to EGFR inhibitors, timely chemotherapy
interventions can serve as an effective treatment strategy.
Evidence suggests that combining chemotherapy drugs, such as
pemetrexed or cisplatin, with EGFR inhibitors can improve treatment
effectiveness and prolong patient survival (10). In patients with resistant NSCLC,
immune checkpoint inhibitors, such as programmed cell death
1/programmed death-ligand 1 inhibitors, have been shown to have
significant efficacy (11). This
combined strategy can enhance the immune response to delay the
onset of drug resistance (12).
Increasing evidence shows that the use of natural
compounds can overcome osimertinib resistance in NSCLC (13,14).
Resveratrol, a natural polyphenolic compound found in various
plants, has gained considerable attention for its potential role in
cancer prevention and treatment (15). Resveratrol is widely found in
plants, including in blueberries, and the skins of grapes and
peanuts (16), and has multiple
beneficial characteristics, including antioxidant, anticancer,
anti-inflammatory and immune-modulating properties (17). Moreover, a previous study has
indicated that resveratrol has the potential to act as a regulator
of multidrug resistance by inhibiting efflux transporters such as
P-glycoprotein, multidrug resistance-associated proteins and breast
cancer resistance proteins (18).
Resveratrol can also enhance the antitumor ability of paclitaxel
against NSCLC by regulating PTEN-induced kinase 1/Parkin-mediated
mitophagy (19).
(Z)3,4,5,4′-trans-tetramethoxystilbene, a novel derivative of
resveratrol, selectively increases intracellular calcium levels to
effectively suppress gefitinib-resistant NSCLC (20). Nevertheless, the molecular mechanism
by which resveratrol delays osimertinib resistance remains to be
elucidated. Therefore, it is necessary to elucidate the precise
molecular targets of resveratrol to increase its specificity when
targeting osimertinib resistance while minimizing off-target
effects.
Network pharmacology, an emerging research field,
integrates bioinformatics and pharmacology to examine the interplay
between drugs and diseases (21).
In previous years, it has been employed primarily for analyzing the
potential mechanisms of action of natural products (22). Therefore, in the present study,
network pharmacology combined with molecular docking was utilized
to reveal the potential mechanisms by which resveratrol delays
osimertinib resistance, and in vitro experiments were
carried out to validate the results.
Materials and methods
Materials and reagents
Resveratrol (purity ≥98.0%) was purchased from
NatureStandard (Shanghai Standard Technology Co., Ltd.; cat. no.
ST00670120), prepared as a 100-mM stock solution with DMSO
(Beyotime Institute of Biotechnology; cat. no. ST038) and diluted
to different concentrations according to the experimental
requirements. Osimertinib, purchased from Selleck Chemicals (cat.
no. S7297), was reconstituted in DMSO. The solution was prepared to
a concentration of 200 mM and stored at −20°C to preserve its
stability.
Collection of the targets of
resveratrol and of osimertinib resistance in lung cancer
The potential targets of resveratrol were identified
via searches of the Traditional Chinese Medicine Systems
Pharmacology (TCMSP) database (https://www.tcmspw.com/tcmsp.php) (23). The disease-related genes were
obtained from the GeneCards (http://www.genecards.org) database (24), with the search term ‘lung cancer
osimertinib resistance’. The gene names were standardized via the
UniProtKB database (https://www.uniprot.org/) (25). Subsequently, the targets associated
with both resveratrol and osimertinib-resistant lung cancer were
obtained.
Construction of the protein-protein
interaction (PPI) network
The PPI network of the overlapping targets was
established via the STRING database (https://string–db.org/) (26). With ‘Homo sapiens’ as the
chosen species and a confidence score of ≥0.4, the PPI network was
visualized via Cytoscape (version 3.7.2) (27). The PPI network was subsequently
analyzed and the hub targets with degrees above the median were
filtered.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses
To further determine the mechanism by which
resveratrol delays osimertinib resistance in lung cancer, the
biological functions and potential pathways involved were
investigated. The Metascape database (www.metascape.org/) (28) was employed to perform GO and KEGG
analyses on the hub targets identified from the PPI network. The
results were visualized in the form of a bubble chart via the
ggplot2 package (version 3.5.1) (29). The network of resveratrol, the
common targets and the pathways were subsequently mapped via
Cytoscape.
Molecular docking
The 3D model of resveratrol was retrieved from the
PubChem database (30) and Chem3D
software (version 20.1;
library.bath.ac.uk/chemistry-software/chem3d) was utilized to
minimize the energy of the structure. The protein structures of
TP53 (4AGQ) (31), STAT3 (6NJS)
(32), IGF1R (1P4O) (33), MCL1 (6OQC) (34) and BCL2L11 (1PQ1) (35) were subsequently extracted from the
Protein Data Bank repository (www.rcsb.org)
(36) and converted into the pdbqt
format. Discovery Studio 2016 (Dassault Systèmes S.E.) was then
employed for the processes of dehydration and hydrogenation, the
modification of amino acid residues and energy minimization. The
CDOCKER protocol within the software was applied to estimate the
binding affinity between resveratrol and the target proteins.
Finally, PyMOL (version 2.2.0; pymol.org/2/) was selected to
visualize the molecular interactions.
Cell culture
PC9 cells, initially procured from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences, were
cultivated in RPMI-1640 medium (HyClone; Cytiva; cat. no.
SH30809.01) enriched with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.; cat. no. 10091148) and 1%
penicillin-streptomycin (HyClone; Cytiva; cat. no. SV30010) at 37°C
in a humidified incubator with 5% CO2 (Thermo Fisher
Scientific, Inc.). Subsequently, osimertinib-resistant PC9 cells
(PC9OR) were developed by gradually increasing the osimertinib
concentration from 5 to 3,000 nM over a period of 5 months, as
previously described (37). PC9OR
cells were treated with osimertinib at a concentration of 3,000 nM
to sustain the drug resistance.
Cell viability
The Cell Counting Kit-8 (CCK-8) assay (cat. no.
C6005; NCM Biotech) was utilized to evaluate the viability of PC9OR
cells after treatment with resveratrol. PC9OR cells were seeded in
96-well plates and exposed to different concentrations of
resveratrol (0, 10, 20, 40, 80 and 160 µM) for 48 h. A CCK-8
solution (10 µl/well) was subsequently added, and the cells were
incubated for 2 h, after which the absorbance at 450 nm was
measured with a spectrophotometer.
Colony formation assay
A total of 1,000 PC9OR cells were cultured in 6-well
plates and then treated with 60 µM resveratrol for 2 weeks. Once
visible colonies had formed (>50 cells per colony) (38), the plates were fixed with 4%
paraformaldehyde (cat. no. BL539A; Biosharp Life Sciences) at room
temperature for 30 min and stained with a 0.1% crystal violet
solution (cat. no. C0121; Beyotime Institute of Biotechnology) for
15 min at room temperature. After staining, the plates were washed
with PBS, dried and imaged, and then cells were counted using
ImageJ (v1.8.0; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
PC9OR cells were plated in 6-well plates at a
density of 5×103 cells per well. After the cells had
attached, 60 µM resveratrol was added for treatment for 48 h. Total
RNA was extracted from PC9OR cells with the EZ-press RNA
Purification Kit (EZBioscience; cat. no. B0004DP). cDNA was reverse
transcribed using the PrimeScript™ RT reagent Kit
(Takara Bio, Inc.; cat. no. RR037A). The reverse transcription
conditions were as follows: 37°C for 15 min, then 85°C for 5 sec
and finally cooling to 4°C. For qPCR, PerfectStart®
Green qPCR SuperMix (TransGen Biotech Co., Ltd.; cat. no. AQ601-02)
was utilized. The qPCR thermal cycling parameters were set as
follows: Preliminary denaturation at 95°C for 30 sec, followed by
40 cycles of 95°C for 15 sec and 60°C for 60 sec. The
quantification of mRNA levels was performed using the
2−ΔΔCq method (39), and
the results were further normalized against the expression levels
of GAPDH. The specific sequences of the primers used were as
follows: BCL2L11 forward, 5′-CTTGTGACCCCAGCCAT-3′ and reverse,
5′-CTTCCTTCCACTGCACTAA-3′; Bcl-2 forward, 5′-GATTGTGGCCTTCTTTGAG-3′
and reverse, 5′-GTTCCACAAAGGCATCC-3′; Bax forward,
5′-CCTGTGCACCAAGGTGCCGGAACT-3′ and reverse,
5′-CCACCCTGGTCTTGGATCCAGCCC-3′; and GAPDH forward,
5′-GGAAGCTTGTCATCAATGGAAATC-3′ and reverse,
5′-TGATGACCCTTTTGGCTCCC-3′. All primers were synthesized by Sangon
Biotech Co., Ltd.
Western blotting (WB) analysis
PC9OR cells were treated with 60 µM resveratrol for
48 h and then lysed in ice-cold RIPA lysis buffer (Beyotime
Institute of Biotechnology; cat. no. P0013B). The protein
concentrations within the lysates were measured with a BCA Protein
Assay Kit (Epizyme; Ipsen Pharma; cat. no. ZJ101). Proteins (20
µg/lane) were resolved through 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then
electrophoretically transferred onto polyvinylidene fluoride
membranes. The membranes were blocked with a 5% milk solution at
room temperature for 1 h, then incubated with primary antibodies
(1:5,000) at 4°C overnight. Afterwards, the membranes were
incubated with a 1:5,000 dilution of HRP-conjugated goat
anti-rabbit IgG secondary antibody (Proteintech Group, Inc.; cat.
no. SA00001-2) for 1 h at 37°C and visualized using an enhanced
chemiluminescence solution (New Cell Molecular Biotech; cat. no.
P10200). The analysis of the bands was conducted with ImageJ
(v1.8.0; National Institutes of Health). The BCL2L11 antibody (cat.
no. ET1608-14) was obtained from HUABIO, and the β-actin antibody
(cat. no. 20536-1-AP) was purchased from Proteintech Group,
Inc.
Statistical analysis
All data are displayed as the mean ± SD derived from
a minimum of three separate experimental trials. GraphPad Prism 8.0
software (Dotmatics) was utilized for both plotting graphs and
statistical analysis. All the results were calculated using the
unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Collection of drug-disease overlapping
targets
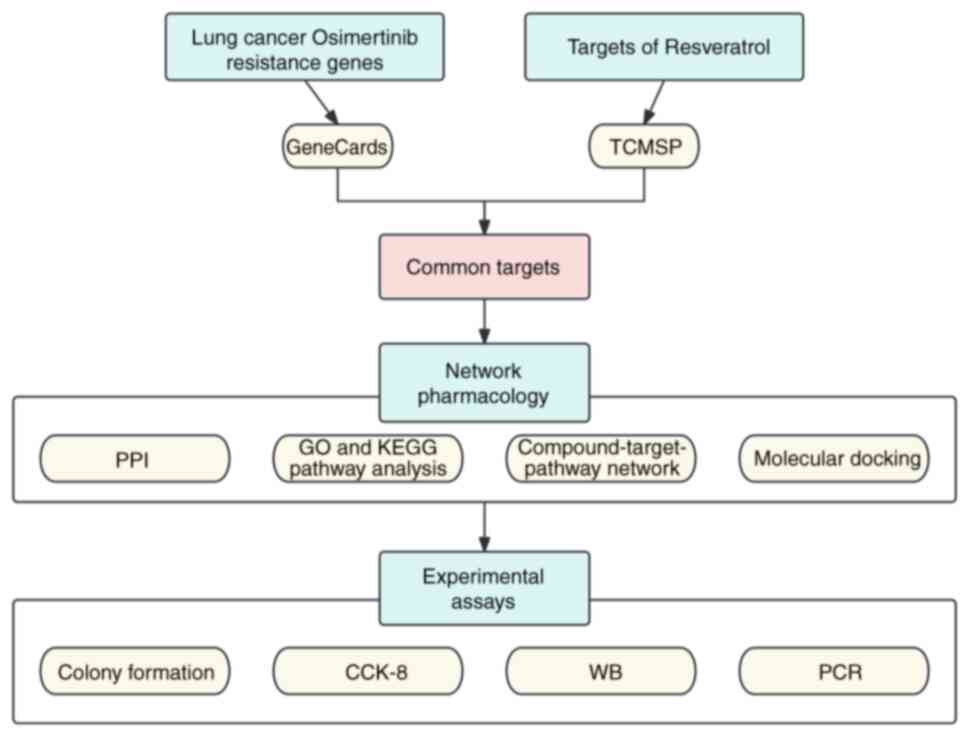
A flowchart illustrating the design of the present
study is depicted in Fig. 1. A
total of 151 target genes were initially obtained from the TCMSP
database and 150 genes were retained from the UniProt database with
standardized gene symbols. Specifically, the standardized gene
symbols for the target genes PKA catalytic subunit C-α and protein
kinase C α type were consistent, both being PRKCA. Simultaneously,
131 disease-related genes were identified from the GeneCards
database. A total of 13 overlapping targets (TP53, BRCA2, IGF1R,
STAT3, ABCG2, HGF, MCL1, CFLAR, BCL2L11, TNFRSF10A, TGFB2, SREBF1
and CDK7) were obtained when the resveratrol targets were compared
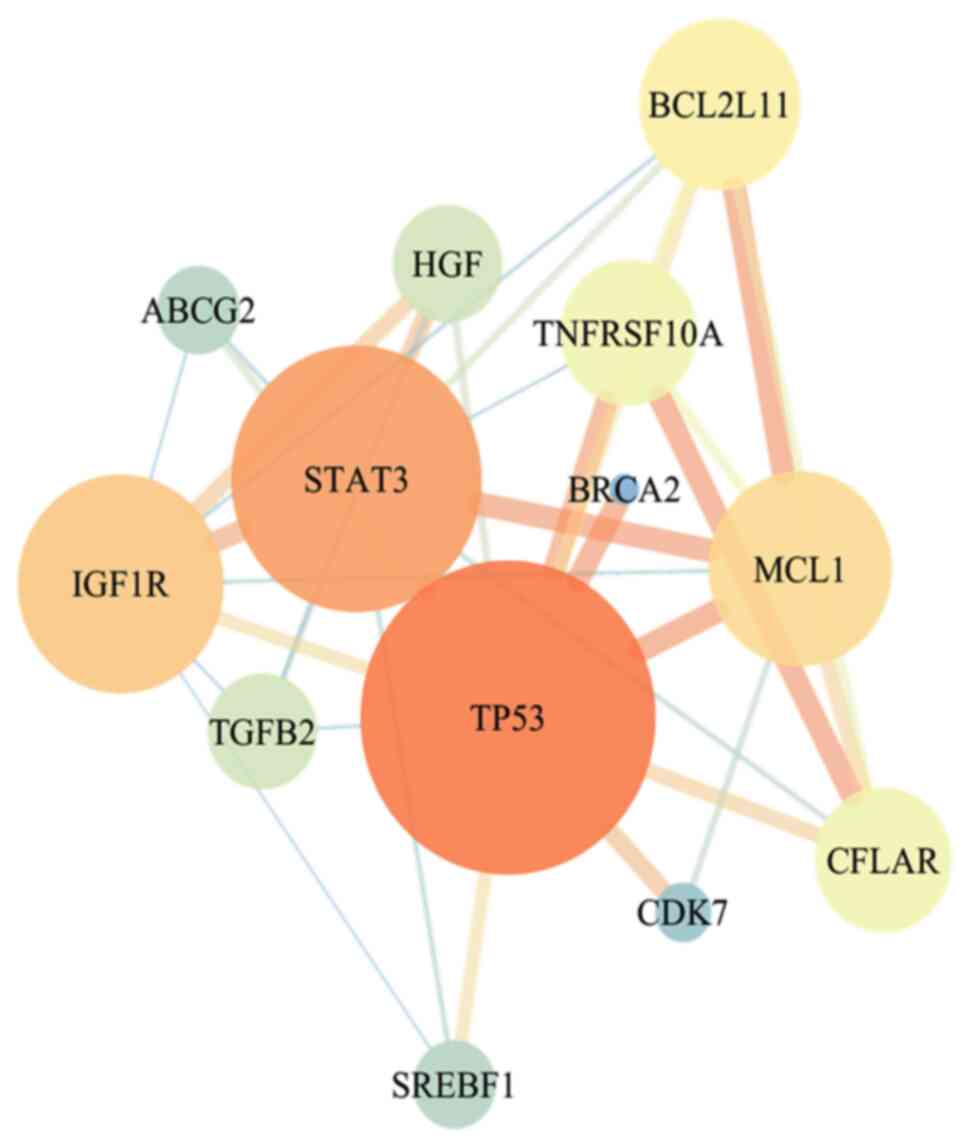
with the disease-related targets. A PPI network was then built to
identify the key targets (Fig. 2).
The 5 targets with degree values >5 were screened as the key
therapeutic targets, from 1 to 5 in the order of TP53, STAT3,
IGF1R, MCL1 and BCL2L11.
Pathway enrichment analysis
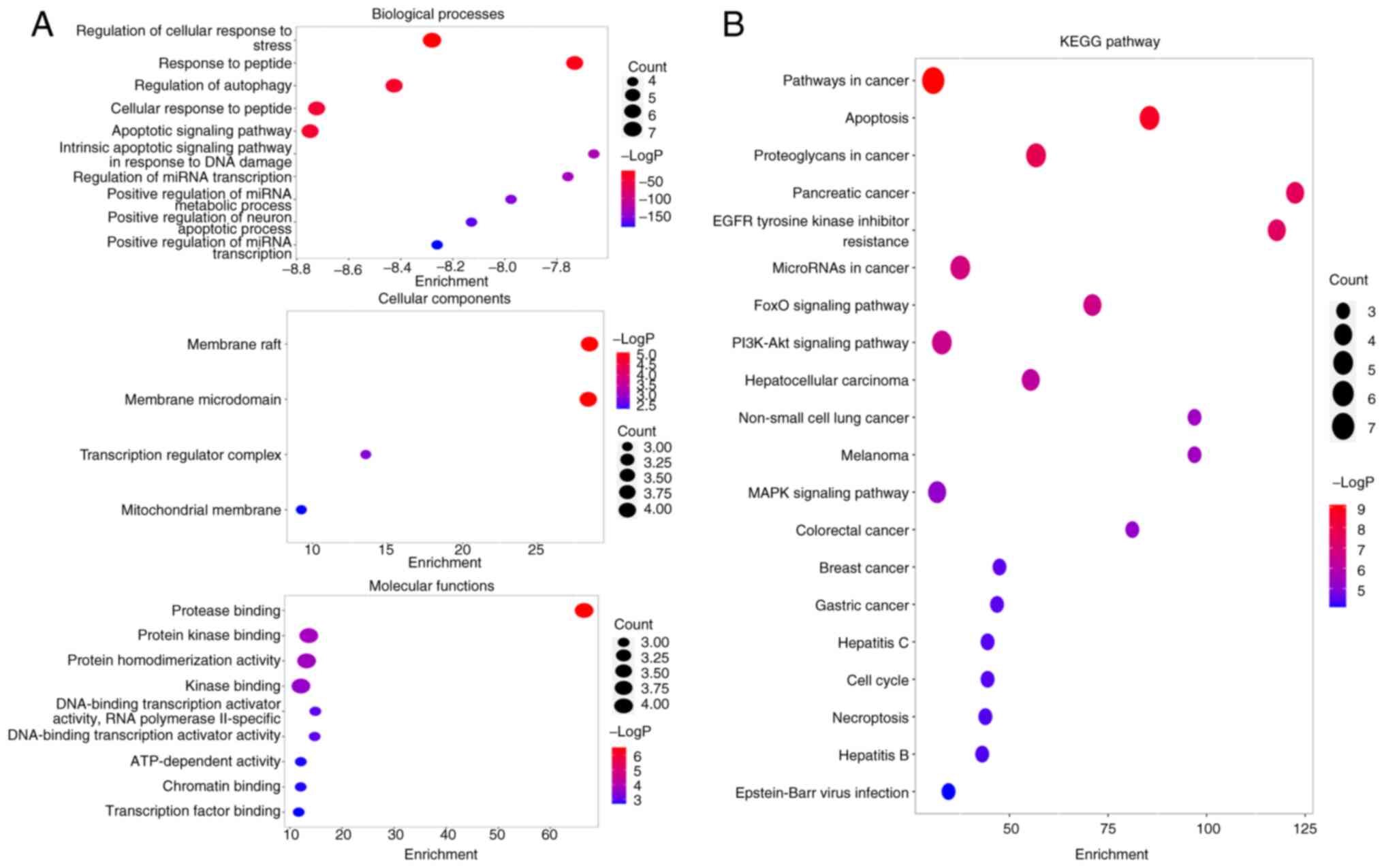
To explore the potential mechanisms by which
resveratrol delays osimertinib resistance, GO and KEGG analyses of
the 13 genes derived from the PPI network were conducted (Fig. 3). The identified biological process
terms included ‘apoptotic signaling pathway’ and ‘cellular response
to peptide’. The molecular function terms included ‘protease
binding’, ‘protein kinase binding’ and ‘kinase binding’. The
cellular component terms included ‘membrane raft’ and ‘membrane
microdomain’. Moreover, the top 20 pathways were related to KEGG
pathways including ‘Pathways in cancer’, ‘Apoptosis’ and ‘EGFR
tyrosine kinase inhibitor resistance’ (Fig. 3B). Consequently, the apoptosis
pathway was selected as a pivotal focus for subsequent
investigation on the basis of this pathway enrichment.
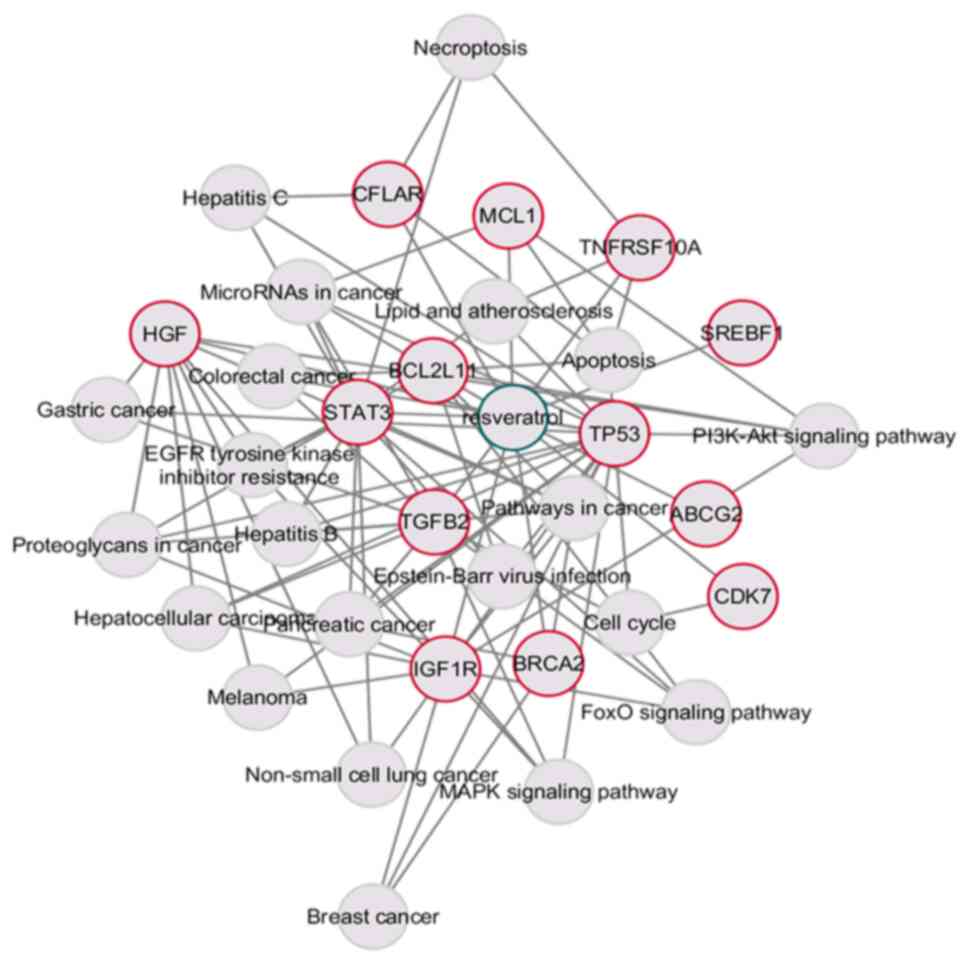
Compound-target-pathway network
construction
To visualize and elucidate the pharmacological
potential of resveratrol for modulating osimertinib resistance,
Cytoscape was utilized to construct a network of resveratrol-common
target genes and KEGG pathways with 35 nodes and 93 edges (Fig. 4). Among the core target genes, TP53
(19 edges), STAT3 (13 edges), IGF1R (10 edges), MCL1 (4 edges),
BCL2L11 (9 edges) and the apoptosis pathway (5 edges) had a high
degree of connectivity, suggesting their involvement in the
development of osimertinib resistance.
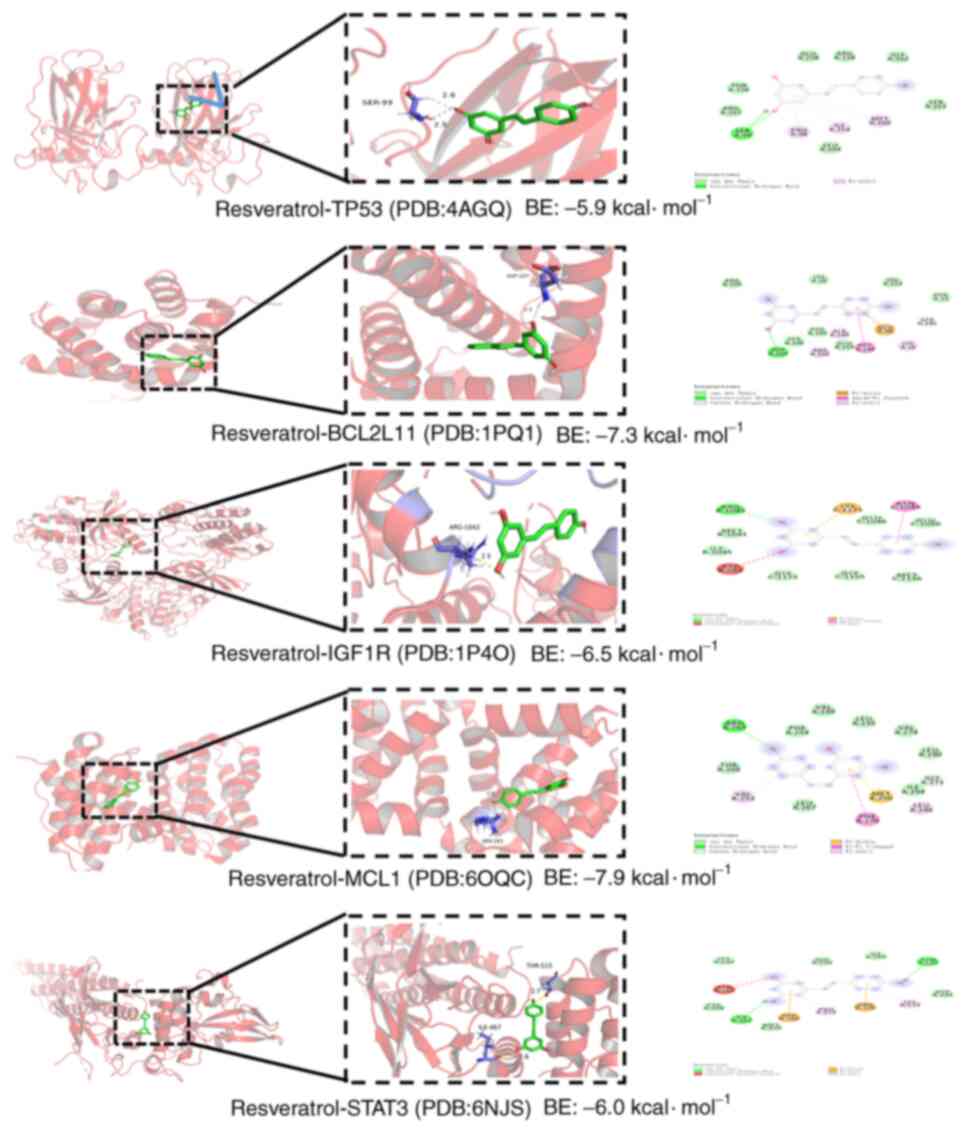
Molecular docking
Molecular docking simulations were performed with
the 5 identified targets (TP53, STAT3, IGF1R, MCL1 and BCL2L11) and
resveratrol. It was found that resveratrol was predicted to bind to
TP53, STAT3, IGF1R, MCL1 and BCL2L11 with energies of −5.9, −6.0,
−6.5, −7.9 and −7.3 kJ/mol, respectively (Fig. 5). The smaller the binding energy is,
the better the binding activity between the ligand and receptor.
When the binding energy is <-7 kcal/mol, this signifies a strong
binding affinity (40). Therefore,
resveratrol may have strong binding affinities with MCL1 and
BCL2L11, indicating that the mechanism by which resveratrol delays
osimertinib resistance may be closely linked to binding to these
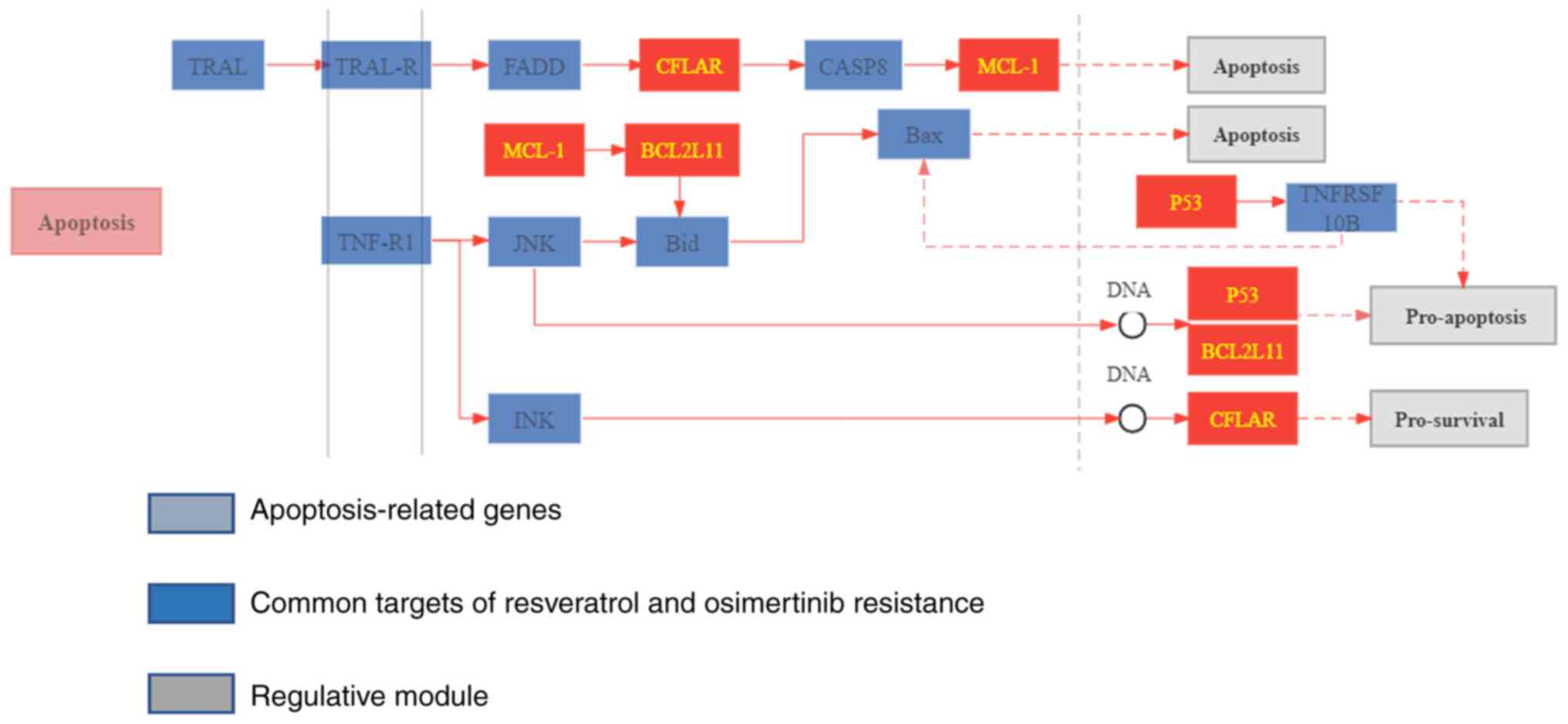
targets. Furthermore, the distribution of the targets of
resveratrol in the apoptosis pathway was mapped, and it was found
that resveratrol can regulate the apoptosis pathway by targeting
MCL1 and BCL2L11 (Fig. 6).
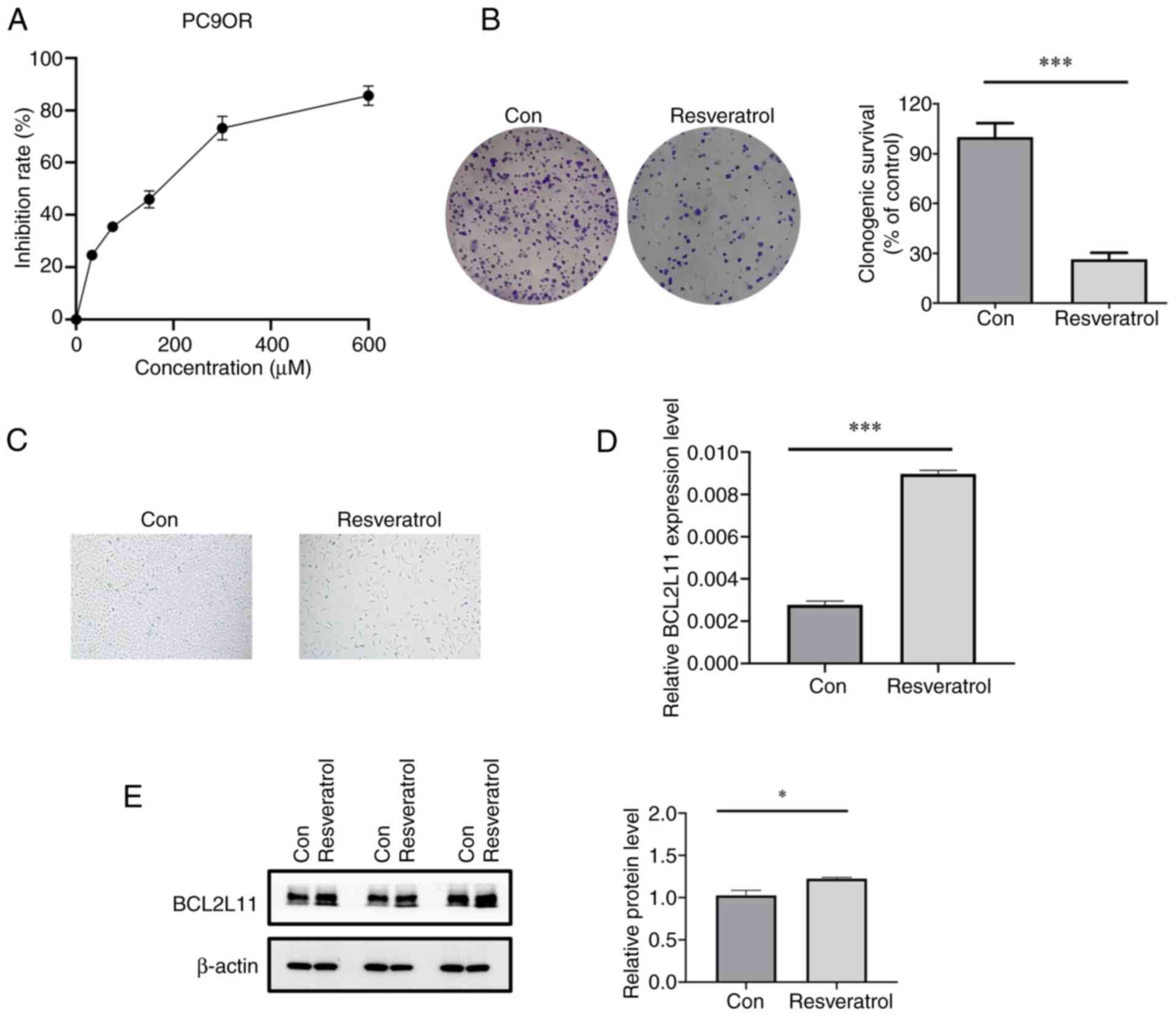
Resveratrol inhibits the proliferation
of osimertinib-resistant lung cancer cells
To verify the results of the network pharmacological
analysis, in vitro experiments were conducted. Various
concentrations of resveratrol (0–160 µM) were used to treat PC9OR
cells for 48 h and cell viability was determined via a CCK-8 assay.
The results showed that resveratrol significantly reduced the cell
viability of PC9OR cells, with an IC50 of ~60 µM
(Fig. 7A). Furthermore, resveratrol
inhibited the long-term proliferation of PC9OR cells (Fig. 7B) and led to a notable alteration in
the morphology of PC9OR cells, resulting in a flattened appearance
(Fig. 7C).
Resveratrol upregulates the expression
of BCL2L11
RT-qPCR was performed to confirm whether resveratrol
delays osimertinib resistance through the targets identified via
network pharmacology (MCL1 and BCL2L11). Resveratrol increased the
mRNA expression levels of BCL2L11 in PC9OR cells (P<0.001)
(Fig. 7D). Notably, resveratrol
decreased the expression levels of MCL1, but there was no
significant difference (P>0.05). Concurrently, resveratrol
reduced the mRNA expression levels of Bcl-2 and increased the
expression levels of Bax (P<0.001) (Fig. S1). Furthermore, resveratrol
increased the protein expression levels of BCL2L11 in PC9OR cells
(Fig. 7E). These findings
demonstrated that resveratrol might promote apoptosis to delay
osimertinib resistance.
Discussion
Globally, osimertinib resistance has been a
long-term challenge. Despite the implementation of drug combination
therapy, immunotherapy and individualized treatment approaches in
clinical practice, significant breakthroughs in addressing drug
resistance have not been achieved (41). Thus, exploring novel strategies to
overcome osimertinib resistance is crucial. To overcome this
difficulty, complementary and alternative therapies, including the
use of herbal compounds, natural compounds or medicinal plant
monomers to delay drug resistance, have become the focus of
research. Resveratrol inhibits tumor cell proliferation and
viability through diverse mechanisms (42). Resveratrol has also been reported to
demonstrate anti-oxidizing and anti-inflammatory properties
(43,44). Moreover, resveratrol can modulate
multiple signaling pathways involved in cell cycle regulation,
apoptosis and other key cellular processes to impact the biological
behavior of tumor cells (45).
Furthermore, resveratrol has been shown to enhance the sensitivity
of cells to chemotherapy drugs, and resveratrol-based nanoparticles
can reverse multidrug resistance (46–48).
However, the effects of resveratrol on osimertinib resistance in
lung cancer and the underlying mechanisms have not been evaluated.
In the present study, it was observed that resveratrol inhibited
the viability of osimertinib-resistant lung cancer cells,
potentially by inducing apoptosis.
Apoptosis, a subtle and energy-dependent form of
cell death, represents the primary mechanism by which most cells in
the body naturally perish, and does not cause an inflammatory
response (49). Apoptosis plays a
crucial role in the development and occurrence of lung cancer
(50). However, during the
progression of lung cancer, tumor cells frequently evade apoptosis,
leading to tumor growth and metastasis (51). Moreover, abnormal apoptosis is
closely associated with drug resistance in cancer therapies
(52,53). Various therapeutic approaches,
including chemotherapy, targeted therapy and immunotherapy, aim to
inhibit tumor growth by promoting apoptosis. Nevertheless, lung
cancer cells employ diverse mechanisms to evade apoptosis,
resulting in the development of drug resistance (54). These mechanisms include the
regulation of apoptosis-related protein expression, the modulation
of apoptosis signaling pathways and the upregulation of cell
survival signals (55). In the
present study, network pharmacology and molecular docking were
employed to predict the involvement of resveratrol in regulating
osimertinib resistance by promoting apoptosis. Furthermore, PC9OR
cells were utilized as an in vitro model of osimertinib
resistance in lung cancer. The present study demonstrated that
resveratrol significantly suppressed the proliferation of PC9OR
cells, upregulated the expression of BCL2L11 and promoted cellular
apoptosis.
BCL2L11 has potent proapoptotic properties and is
categorized within the BCL2 protein family (56). BCL2L11 plays a crucial role in
maintaining the balance of apoptosis in T-cell and B-cell
homeostasis (57). Impairment of
the EGFR-TKI-mediated apoptosis pathway (BCL2L11 deletion
polymorphism) can lead to resistance to EGFR-TKIs (58). Research has revealed that
sensitivity to gefitinib can be restored by upregulating the
expression of BCL2L11 (59).
Therefore, these studies suggest that upregulating BCL2L11 delays
the development of drug resistance. Additionally, these findings
indicate that, through the examination of gene expression patterns
in patients, it may be possible to identify patients with NSCLC who
are likely to benefit from resveratrol treatment, enabling
individualized treatment. Concurrently, these data can inform the
future direction of drug and molecular inhibitor development aimed
at combating resistance in lung cancer. Moreover, the present study
established resveratrol as a candidate for reversing drug
resistance, laying the groundwork for the design of clinical
trials. Resveratrol has the potential to prolong the duration of
the response to osimertinib, defer the emergence of resistance and
ultimately enhance the therapeutic outcomes for patients.
To summarize, the present study employed network
pharmacology and molecular docking to investigate the potential of
resveratrol to delay the development of osimertinib resistance by
modulating apoptosis. Following in vitro experiments, it was
confirmed that resveratrol increased the expression of BCL2L11 in
PC9OR cells, confirming its ability to delay osimertinib resistance
by inducing cell apoptosis.
This study has several notable advantages. First, a
network pharmacological analysis that leveraged various databases,
including TCMSP and GeneCards, was conducted. This cross-database
integration enhanced the thoroughness and precision of the
screening methodology, which was crucial for identifying a broader
range of potential targets. Moreover, the identified targets were
substantiated via molecular docking and in vitro assays.
This synergistic approach of computational analysis and
experimental validation bolsters the credibility of the results and
confirms that resveratrol was an inhibitor of osimertinib
resistance. To the best of our knowledge, the current study
represents a groundbreaking effort in the comprehensive
identification and validation of the administration of resveratrol
as a therapeutic approach to overcome osimertinib resistance. The
present study addresses a gap in the literature and offers
researchers a basis for further exploration.
However, the present study has certain limitations.
First, molecular docking serves merely as a predictive measure with
regard to the binding stability between small and large molecules.
Additionally, the bioavailability of resveratrol is influenced by a
multitude of factors, including drug metabolism, distribution and
excretion, which requires further exploration. Second, although the
tumor-inhibiting effect of resveratrol has been revealed, the safe
dosage remains uncertain. For this reason, the potential toxicity
of this drug needs to be carefully assessed. Furthermore, there is
limited clinical evidence supporting the efficacy of resveratrol
for combating resistance to osimertinib. Finally, the present study
did not include a comparison between resveratrol and osimertinib or
their combination. This will be a promising direction for future
research. Additionally, more in-depth studies will further clarify
the specific mechanisms by which resveratrol delays osimertinib
resistance, which is eagerly anticipated.
In conclusion, the present study revealed that
resveratrol could delay osimertinib resistance by targeting
BCL2L11, which was identified as a key target, and by inducing
apoptosis. The present study revealed the potential therapeutic
value of resveratrol for the treatment of osimertinib-resistant
lung cancer, laying a foundation for the clinical application of
resveratrol.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was sponsored by National Natural Science Foundation
of China (grant no. 82170033), the Natural Science Foundation of
Shanghai (grant no. 21ZR1479200), the Chang-Feng Talent Fund of
Changhai Hospital, Special Fund for ‘Research on Community Medicine
and Health Management’ in Shanghai (grant no. 2023SQ01), the
Medical Research Project of Health Commission of Shanghai Hongkou
District (grant no. HW2302-43) and the Basic Public Welfare
Research Project of Zhejiang Province (grant no. LQ22H010001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY, SLS and HLZ designed the study. XY, YY and HWZ
performed the experimental work. JTZ and NNZ conducted the data
analyses. SLS and HLZ revised the manuscript and confirmed the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Remon J, Steuer CE, Ramalingam SS and
Felip E: Osimertinib and other third-generation EGFR TKI in
EGFR-mutant NSCLC patients. Ann Oncol. 29 (Suppl_1):i20–i27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blaquier JB, Ortiz-Cuaran S, Ricciuti B,
Mezquita L, Cardona AF and Recondo G: Tackling osimertinib
resistance in EGFR-mutant Non-small cell lung cancer. Clin Cancer
Res. 29:3579–3591. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Yang S, Wang K and Sun SY: MET
inhibitors for targeted therapy of EGFR TKI-resistant lung cancer.
J Hematol Oncol. 12:632019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaft JE, Shyr Y, Sepesi B and Forde PM:
Preoperative and postoperative systemic therapy for operable
Non-small-Cell lung cancer. J Clin Oncol. 40:546–555. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomatou G, Syrigos N and Kotteas E:
Osimertinib resistance: Molecular mechanisms and emerging treatment
options. Cancers (Basel). 15:8412023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartmaier RJ, Markovets AA, Ahn MJ,
Sequist LV, Han JY, Cho BC, Yu HA, Kim SW, Yang JC, Lee JS, et al:
Osimertinib + Savolitinib to overcome acquired MET-mediated
resistance in epidermal growth factor Receptor-mutated,
MET-amplified Non-small cell lung cancer: TATTON. Cancer Discov.
13:98–113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Planchard D, Jänne PA, Cheng Y, Yang JC,
Yanagitani N, Kim SW, Kim SW, Sugawara S, Yu Y, Fan Y, et al:
Osimertinib with or without Chemotherapy in EGFR-mutated advanced
NSCLC. N Engl J Med. 389:1935–1948. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu L, Ke L, Zhang Z, Yu J and Meng X:
Development of EGFR TKIs and options to manage resistance of
Third-Generation EGFR TKI Osimertinib: Conventional ways and immune
checkpoint inhibitors. Front Oncol. 10:6027622020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vicencio JM, Evans R, Green R, An Z, Deng
J, Treacy C, Mustapha R, Monypenny J, Costoya C, Lawler K, et al:
Osimertinib and anti-HER3 combination therapy engages immune
dependent tumor toxicity via STING activation in trans. Cell Death
Dis. 13:2742022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai X, Miao J, Sun R, Wang S, Molina-Vila
MA, Chaib I, Rosell R and Cao P: Dihydroartemisinin overcomes the
resistance to osimertinib in EGFR-mutant non-small-cell lung
cancer. Pharmacol Res. 170:1057012021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Q, Lin S, Zeng Y, Yao M, Liu K, Yuan
H, Liu C and Jiang G: Ginsenoside Rg3 attenuates the osimertinib
resistance by reducing the stemness of Non-small cell lung cancer
cells. Environ Toxicol. 35:643–651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rauf A, Imran M, Butt MS, Nadeem M, Peters
DG and Mubarak MS: Resveratrol as an Anti-cancer agent: A review.
Crit Rev Food Sci Nutr. 58:1428–1447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galiniak S, Aebisher D and
Bartusik-Aebisher D: Health benefits of resveratrol administration.
Acta Biochim Pol. 66:13–21. 2019.PubMed/NCBI
|
|
17
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18:25892017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Li C, Li H, Li M and Shu X:
Resveratrol-mediated reversal of tumor Multi-drug resistance. Curr
Drug Metab. 15:703–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong F, Xie C, Zhao X, Zong X, Bu L, Zhang
B, Tian H and Ma S: Resveratrol regulates PINK1/Parkin-mediated
mitophagy via the lncRNA ZFAS1-miR-150-5p-PINK1 axis, and enhances
the antitumor activity of paclitaxel against non-small cell lung
cancer. Toxicol Res (Camb). 11:962–974. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan XX, Yao XJ, Xu SW, Wong VK, He JX,
Ding J, Xue WW, Mujtaba T, Michelangeli F, Huang M, et al:
(Z)3,4,5,4′-trans-tetramethoxystilbene, a new analogue of
resveratrol, inhibits gefitinb-resistant non-small cell lung cancer
via selectively elevating intracellular calcium level. Sci Rep.
5:163482015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hopkins AL: Network pharmacology: The next
paradigm in drug discovery. Nat Chem Biol. 4:682–690. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kibble M, Saarinen N, Tang J, Wennerberg
K, Mäkelä S and Aittokallio T: Network pharmacology applications to
map the unexplored target space and therapeutic potential of
natural products. Nat Prod Rep. 32:1249–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: Tcmsp: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Consortium U and Dogan T: Uniprot: A
worldwide hub of protein knowledge. Nucleic Acids Res.
47:D506–D515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in Genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a Biologist-oriented resource for the analysis of
Systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hadley W: Ggplot2: Elegant Graphics for
Data Analysis. Springer-Verlag; New York: 2016
|
|
30
|
Kim S, Chen J, Cheng T, Gindulyte A, He J,
He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, et al: PubChem 2023
update. Nucleic Acids Res. 51:D1373–D1380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilcken R, Liu X, Zimmermann MO,
Rutherford TJ, Fersht AR, Joerger AC and Boeckler FM:
Halogen-enriched fragment libraries as leads for drug rescue of
mutant P53. J Am Chem Soc. 134:6810–6818. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy
K, McEachern D, Chen J, Yang CY, Liu Z, Wang M, et al: A potent and
selective Small-molecule degrader of STAT3 achieves complete tumor
regression in vivo. Cancer Cell. 36:498–511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Munshi S, Hall DL, Kornienko M, Darke PL
and Kuo LC: Structure of apo, unactivated insulin-like growth
factor-1 receptor kinase at 1.5 A resolution. Acta Crystallogr D
Biol Crystallogr. 59:1725–1730. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caenepeel S, Brown SP, Belmontes B, Moody
G, Keegan KS, Chui D, Whittington DA, Huang X, Poppe L, Cheng AC,
et al: AMG 176, a selective MCl1 inhibitor, is effective in
hematologic cancer models alone and in combination with established
therapies. Cancer Discov. 8:1582–1597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Dai S, Zhu Y, Marrack P and Kappler
JW: The structure of a Bcl-xL/Bim fragment complex: Implications
for Bim function. Immunity. 19:341–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berman HM, Westbrook J, Feng Z, Gilliland
G, Bhat TN, Weissig H, Shindyalov IN and Bourne PE: The protein
data bank. Nucleic Acids Res. 28:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sang S, Sun C, Ding R, Jiang J, Han Y, Gan
S, Bi L and Gong Y: Feiyanning formula modulates the molecular
mechanism of osimertinib resistance in lung cancer by regulating
the Wnt/β-catenin pathway. Front Pharmacol. 13:10194512022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sununliganon L and Singhatanadgit W:
Highly osteogenic PDL stem cell clones specifically express
elevated levels of ICAM1, ITGB1 and TERT. Cytotechnology. 64:53–63.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang AY, Liu HL and Yang YF: Study on the
mechanism of action of Scutellaria barbata on hepatocellular
carcinoma based on network pharmacology and bioinformatics. Front
Pharmacol. 13:10725472022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu K, Xie F, Wang F and Fu L: Therapeutic
strategies for EGFR-mutated Non-small cell lung cancer patients
with osimertinib resistance. J Hematol Oncol. 15:1732022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xin R, Shen B, Huang ZY, Liu JB, Li S,
Jiang GX, Zhang J, Cao YH, Zou DZ, Li W, et al: Research progress
in elucidating the mechanisms underlying resveratrol action on lung
cancer. Curr Pharm Biotechnol. 24:427–437. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng T, Xiao D, Muhammed A, Deng J, Chen L
and He J: Anti-inflammatory action and mechanisms of resveratrol.
Molecules. 26:2292021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chupradit S, Bokov D, Zamanian MY, Heidari
M and Hakimizadeh E: Hepatoprotective and therapeutic effects of
resveratrol: A focus on Anti-inflammatory and antioxidative
activities. Fundam Clin Pharmacol. 36:468–485. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Delmas D, Solary E and Latruffe N:
Resveratrol, a phytochemical inducer of multiple cell death
pathways: Apoptosis, autophagy and mitotic catastrophe. Curr Med
Chem. 18:1100–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maleki DP, Sadoughi F, Asemi Z and Yousefi
B: The role of polyphenols in overcoming cancer drug resistance: A
comprehensive review. Cell Mol Biol Lett. 27:12022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Borska S, Pedziwiatr M, Danielewicz M,
Nowinska K, Pula B, Drag-Zalesinska M, Olbromski M, Gomulkiewicz A
and Dziegiel P: Classical and atypical resistance of cancer cells
as a target for resveratrol. Oncol Rep. 36:1562–1568. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liping Y, Jian H, Zhenchao T, Yan Z, Jing
Y, Yangyang Z, Jing G and Liting Q: GSH-responsive poly-resveratrol
based nanoparticles for effective drug delivery and reversing
multidrug resistance. Drug Deliv. 29:229–237. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hetts SW: To die or not to die: An
overview of apoptosis and its role in disease. JAMA. 279:300–307.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pore MM, Hiltermann TJ and Kruyt FA:
Targeting apoptosis pathways in lung cancer. Cancer Lett.
332:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han SW and Roman J: Targeting apoptotic
signaling pathways in human lung cancer. Curr Cancer Drug Targets.
10:566–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 (Suppl):S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hickman JA: Apoptosis and chemotherapy
resistance. Eur J Cancer. 32A:921–926. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, Lu Z and Zhao X: Targeting Bcl-2
for cancer therapy. Biochim Biophys Acta Rev Cancer.
1876:1885692021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gillings AS, Balmanno K, Wiggins CM,
Johnson M and Cook SJ: Apoptosis and autophagy: BIM as a mediator
of tumour cell death in response to oncogene-targeted therapeutics.
Febs J. 276:6050–6062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nieters A, Conde L, Slager SL,
Brooks-Wilson A, Morton L, Skibola DR, Novak AJ, Riby J, Ansell SM,
Halperin E, et al: PRRC2A and BCL2L11 gene variants influence risk
of non-Hodgkin lymphoma: Results from the InterLymph consortium.
Blood. 120:4645–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang L and Fu L: Mechanisms of resistance
to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 5:390–340.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li H, Zhou S, Li X, Wang D, Wang Y, Zhou C
and Schmid-Bindert G: Gefitinib-resistance is related to BIM
expression in non-small cell lung cancer cell lines. Cancer Biother
Radiopharm. 28:115–1123. 2013.PubMed/NCBI
|