Introduction
The presence of substantial quantities of plastic
debris in the environment has been documented since the 1960s
(1). However, it was not until 2004
that the term ‘microplastic’ (MP) was introduced in a report to
characterize microscopic plastic debris (2), signifying the commencement of research
focused on MPs. The production of plastic has surged markedly in
recent decades. As of 2015, ~4.9 billion tons of plastic waste had
accumulated in landfills or the natural environment. Projections
indicate that this figure could rise to ~12 billion tons by 2050
(3). The nature of global MP
pollution is of notable concern.
Compared with other suspended particulate matter,
MPs are distinguished by their extensive size distribution, diverse
shapes, low densities-often approximating that of water-and high
persistence (4). Plastics that
accumulate in substantial quantities in the environment undergo
degradation due to several environmental factors, including
ultraviolet radiation from sunlight, precipitation, water flow,
biological oxidation and mechanical weathering. The degradation
products exhibit a wide range of forms such as fragments, fibers,
spheres, pellets, lines, sheets, flakes and foams, with fragments
being the most prevalent (5). These
products can be further classified by size into nanoplastics (NPs;
≤0.1 µm), MPs (≤5 mm), medium plastics (0.5–5 cm), megaplastics
(5–50 cm) and macroplastics (≥50 cm) (6). The densities of plastics vary
markedly, ranging from 50 kg/m3 for extruded polystyrene
foam to 1,400 kg/m3 for polyvinyl chloride (PVC);
however, the densities of numerous plastics are similar to that of
water (4).
MPs can also be categorized into primary and
secondary types based on their origin. Primary MPs are small
plastic particles that are either intentionally manufactured or
generated as by-products of industrial processes (7). They are commonly present in products
such as exfoliating beads in facial cleansers (8). Secondary MPs originate from the
fragmentation or degradation of larger plastic materials (9), with the majority of environmental MPs
considered to be secondary in nature (10). Furthermore, the substantial
persistence of MPs enables their prolonged presence in waste
streams and environmental contexts. Research suggests that these
particles can accumulate and persist in natural environments for
decades (11). These
characteristics notably influence their environmental mobility,
distribution patterns, modes of human exposure and potential hazard
levels.
A particularly concerning facet of pervasive MP
pollution is their potential implications for human health.
Research indicates that MPs can enter the human body via ingestion,
inhalation or dermal contact, thereby posing diverse health risks
(12). Among these, ingestion is
identified as the predominant exposure pathway, with frequent
vectors including marine crustaceans, fish (13), sea salt, lake salt (14), tap water, spring water and bottled
water (15). Schwabl et al
(16) reported the presence of MPs
in human feces, indicating that following ingestion, MPs can
traverse the esophagus, stomach, small intestine and large
intestine. This pathway may lead to their accumulation in specific
target organs, thus posing potential health risks. Research
performed on mice and zebrafish has reported that ingested MPs can
accumulate in the intestines, potentially causing intestinal damage
or dysbiosis of the gut microbiota (17,18).
Furthermore, prolonged accumulation of MPs may contribute to
carcinogenesis (19). MPs are also
present in atmospheric deposition as well as in both indoor and
outdoor environments (20). These
particles may originate from urban dust, synthetic textiles,
material abrasion (such car tires or buildings) and the
resuspension of surface MPs (21).
Plastic particles measuring <5 µm in length and <3 µm in
diameter are readily inhalable (22), with certain particles accumulating
in lung tissue due to their inherent durability. This accumulation
has the potential to contribute to pulmonary inflammation, fibrosis
or cancer (23,24). Moreover, occupational exposure,
particularly amongst workers in the textile or mining industries,
has been associated with higher incidences of respiratory
irritation (25). Previous studies
have also highlighted dermal contact as a potential route for MPs
to enter the body, with personal care products such as cosmetics,
toothpaste and soaps being major sources (26,27).
Wu et al (27) reported that
the dermal barrier could be crossed by NPs (<100 nm), synthetic
fibers (<25 µm), the monomers, as well as the additives due to
the skin pores ranging from 40 to 8 µm. Furthermore, plastic
particles may also gain entry through wounds, sweat glands or hair
follicles (28).
In previous years, the relationship between MPs and
tumorigenesis has emerged as a key focus in MP and human health
research. Owing to their extensive surface area and high adsorption
capacity, MPs serve as vectors for several environmental toxicants,
including polycyclic aromatic hydrocarbons (29,30),
heavy metals (31) and
organochlorine pesticides (32),
all of which are recognized as potent carcinogens. These substances
can enter the human body alongside MPs and, under certain
conditions, may be either released or retained, thus increasing the
risk of carcinogenesis. Moreover, research indicates that MPs with
diameters ranging from 0.25–10 µm can penetrate cell membranes and
accumulate within cells, indicating their potential for prolonged
persistence within the body (33,34).
MPs may interact with cellular components through mechanisms
(35–37) such as: i) Elevating intracellular
reactive oxygen species (ROS) levels, which can lead to oxidative
stress, lipid peroxidation, protein oxidation and DNA damage; ii)
promoting cytokine release upon cellular contact, activating
specific pathways and triggering inflammatory responses; and iii)
disrupting immune surveillance by interacting with immune cells,
activating innate immune receptors [such as toll-like receptors
(TLR)] and perpetuating chronic inflammation. These mechanisms
collectively create a conducive environment for tumor growth and
influence the tumor microenvironment (TME).
The TME is a complex network consisting of tumor
cells, several stromal cells (such as fibroblasts, lymphocytes,
macrophages and endothelial cells) and extracellular components
[such as cytokines, inflammatory cells, signaling molecules and
extracellular matrix (ECM)] (38).
It serves a crucial role in cancer development and progression.
Recent in vitro experiments have demonstrated that exposure
to polystyrene (PS)-NPs can promote the progression of ovarian
cancer in murine models by modifying the TME (39), thereby providing further evidence of
the connection between MPs and the TME.
Given that direct research on the TME in the context
of MPs remains in its nascent stages, the present review aimed to
analyze the interactions between MPs and the TME. By evaluating
previous studies on the interactions of MPs with tumor cells,
macrophages, fibroblasts, endothelial cells and inflammatory
processes, the present study aimed to consolidate the latest
evidence from research on both cancerous and normal tissue cells.
Furthermore, the present study aimed to elucidate the potential
links between MPs, the TME and tumorigenesis.
MPs and the TME
MPs can influence the TME by affecting several cell
types. Fig. 1 illustrates a brief
overview of the role of MPs in the TME.
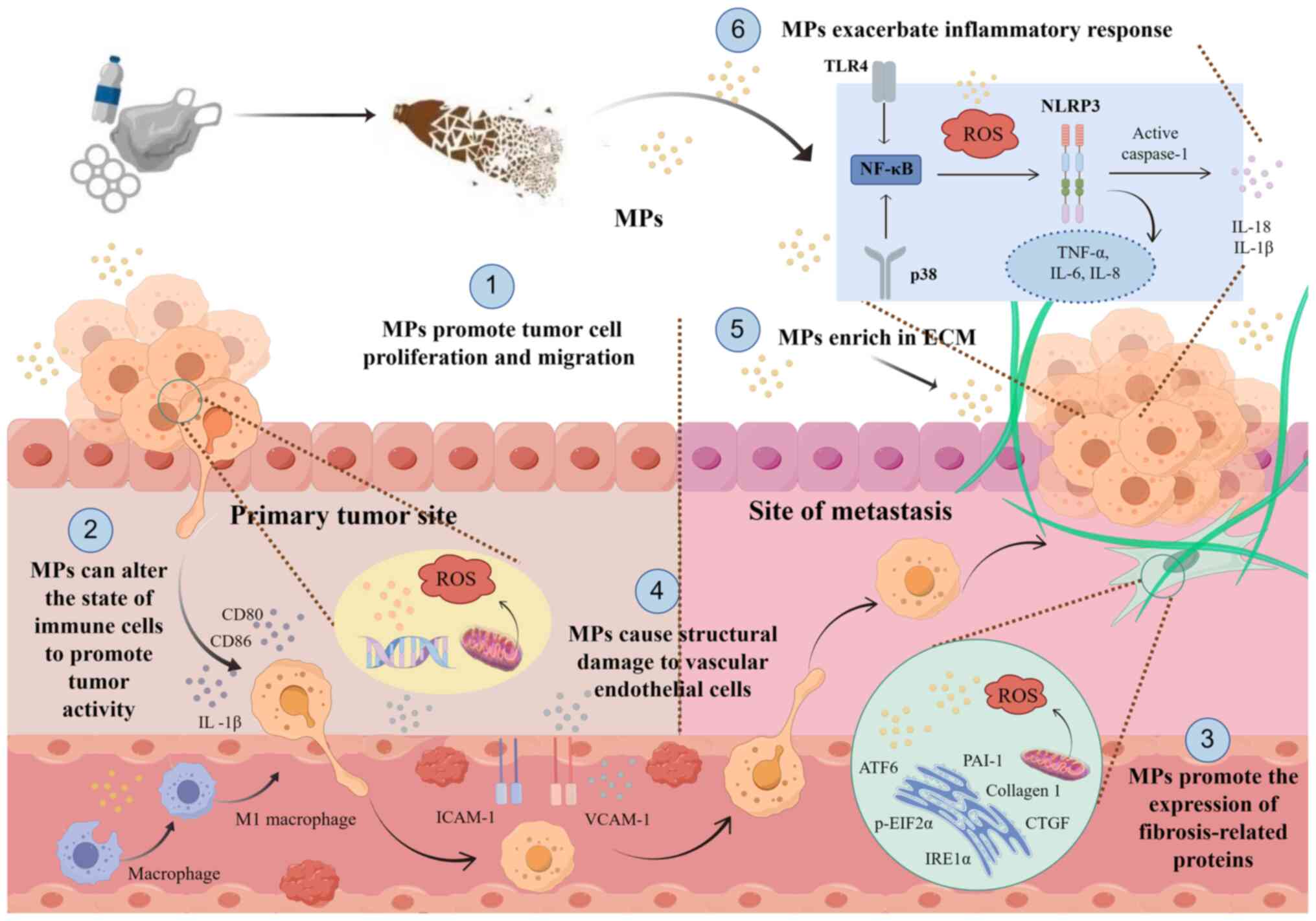 | Figure 1.Schematic representation of the
proposed mechanism illustrating the regulation of MPs on cells in
the TME, created using Figdraw2.0 (www.figdraw.com). As plastic products degrade, they
generate numerous MP particles, which can influence several
components of the TME upon contact with the tumor. MPs affect tumor
cell proliferation and migration within tissues and blood vessels
and alter immune cell states to enhance tumor activity, causing
structural damage to endothelial cells, promoting fibroblast
activation and the expression of related proteins and directly
impacting the ECM and inflammatory factors. MP, microplastic; TME,
tumor microenvironment; ECM, extracellular matrix; TLR4, toll-like
receptor 4; ROS, reactive oxygen species; ICAM-1, intercellular
adhesion molecule 1; VCAM-1, vascular cell adhesion protein 1;
NLRP3, NLR family pyrin domain containing 3; ATF6, activating
transcription factor 6; PAI-1, plasminogen activator inhibitor-1;
p-EIF2α, phosphorylated eukaryotic translation initiation factor
2α; IRE1α, inositol requiring enzyme 1; CTGF, connective tissue
growth factor. |
MPs and tumor cells
Tumor cells, which represent a substantial component
of the TME, engage in a reciprocal relationship with their
environment, mutually influencing their behaviors and progression.
As articulated by Paget (40) in
their ‘seed and soil’ hypothesis over a century ago, the TME serves
a critical role in tumor development. Modifications in the TME can
notably affect the growth and proliferation of tumor cells. For
example, research by Chen et al (39) demonstrated that the administration
of water containing PS-NPs to mice resulted in an increased growth
rate of epithelial ovarian cancer tumors. The analysis indicated
that exposure to PS-NPs markedly disrupted immune responses and
pathways within the TME (39).
Similarly, research utilizing human colonic organoids and the
Caco-2 colonic cell line demonstrated that MPs could compromise
intestinal barrier function, thereby altering the cellular
microenvironment and affecting the proliferation and wound healing
of Caco-2 cancer cells (41). Upon
colonization of host tissues, tumor cells induce substantial
molecular, cellular and physical alterations that, to varying
extents, promote the development of the TME (42). Several researchers contend that MPs
have the potential to augment tumor cell proliferation, modify
metabolic processes and facilitate metastasis, thereby impacting
the dynamics of the TME (29,39,43).
For instance, in breast cancer cells, Park et al (44) demonstrated that exposure to 16.4 µm
fragment-type polypropylene (PP)-MPs in breast cancer cells,
specifically MDA-MB-231 and MCF-7 lines, resulted in increased
expression of genes associated with the cell cycle and elevated
secretion of interleukin (IL)-6, without inducing cytotoxic
effects. This exposure consequently enhanced the metastatic
potential of these cancer cells (44). A separate study examined the effects
of MPs on human breast epithelial and breast cancer cells,
reporting that 1.0 µm PS particles increased the proliferation rate
of MDA-MB-231 cells and facilitated tumor cell migration (45). Moreover, experiments performed by
Wang et al (43)
demonstrated that MPs could be internalized by skin squamous cell
carcinoma lines in a time- and dose-dependent manner. This
internalization resulted in elevated mitochondrial ROS, activation
of the NLR family pyrin domain containing 3 (NLRP3) inflammasome
and ultimately promoted the proliferation of skin cancer cells
(43).
Contrary to these findings, Goodman et al
(46) observed that the viability
of cultured human alveolar A549 cells remained relatively stable
when exposed to 1 and 10 µm PS-MPs across different concentrations.
However, both metabolic activity and proliferation rates exhibited
marked reductions (46). Similarly,
Da Silva Brito et al (47)
performed experiments on A549 cells under controlled conditions,
simulating environmental scenarios to generate secondary PP-MPs via
laser ablation. After exposing human alveolar A549 cells and HaCaT
cells to PP-MPs and a positive control group, amine-modified PS-MPs
(PS-NH2-MPs), it was observed that PP-MPs only enhanced
the metabolic activity of A549 cells at elevated concentrations,
whereas HaCaT cells remained unaffected. Conversely, the
PS-NH2-MPs control group exhibited reduced metabolic
activity and notably increased cell death (47). The study concluded that
laser-ablated PP-MPs did not exhibit cytotoxic effects nor did they
influence metabolic proliferation during acute in vitro
exposure.
In addition to the direct effects of MPs, certain
compounds adsorbed onto transported by MPs may also impact tumor
cells and the broader TME. Böckers et al (48,49)
investigated two typical plasticizers-bisphenol compounds and
tri-o-cresyl phosphate, commonly used in plastic products, and
reported that both compounds could interact with estrogen receptor
α in MCF-7 breast cancer cells. This interaction resulted in 14
upregulated genes (ADORA1, DDIT4, CELSR2, etc.) and three
downregulated genes (BCAS3, PHF19, PRKCD), which are
associated with cell growth, invasion, migration, apoptosis and
cancer development. Consequently, the impact of MPs on tumor cells
is multifaceted and cannot be universally characterized. Variables
such as the type of tumor cells, the surrounding microenvironment,
the type, shape and size of MPs, the duration of exposure and the
presence of adsorbed substances all potentially influence these
interactions (Table I).
 | Table I.Summary of information on the studies
of microplastics and cancer cells. |
Table I.
Summary of information on the studies
of microplastics and cancer cells.
| First author/s,
year | Particle type | Size | Cell/animal
model | Aim | Conclusion | (Refs.) |
|---|
| Chen et al,
2024 | PS-NPs | 100 nm | Human EOC HEY cells
and mouse EOC cells | To investigate the
potential effects and molecular mechanisms of PS-NPs on EOC. | PS-NPs exposure
markedly accelerates EOC tumor growth in mice and reduces EOC cell
viability in a dose-dependent manner by altering the TME. | (39) |
| Ling et al,
2020 | MPs | N/A | Human colorectal
adenocarcinoma Caco-2 cells | To investigate the
effect of MPs on Caco-2 cells. | MPs impact the
growth of Caco-2 cancer cells, impair wound healing and disrupt
cellular metabolism. | (41) |
| Park et al,
2023 | PP-MPs | 16.4 µm | Breast cancer
MDA-MB-231 and MCF-7 cells | To investigate the
effect of 16.4 µm fragmentary PP-MPs on breast cancer. | PP-MPs upregulate
the expression of genes associated with metastasis and increase
cytokine production in breast cancer cells. | (44) |
| Schnee et
al, 2024 | PS-microbeads | 0.5, 1.0 and 4.5
µm | Breast cancer
HS-578-T-DSP1-7 and MDA-MB-231-DSP1-7 cells | To investigate the
influence of MPs on human mammary epithelial cells and breast
cancer cells. | MPs are taken up in
a dose- and particle size-dependent manner by human breast cancer
cells and have an impact on cell proliferation and migration. | (45) |
| Wang et al,
2023 | PE-MPs | 1 µm | Skin squamous cell
carcinoma SCL-1 and A431 cells | To investigate the
effect of MPs on skin cancer. | MPs promote tumor
cell proliferation while causing damage to normal skin cells
through NLRP3-mediated inflammation and scorch death. | (43) |
| Goodman et
al, 2021 | PS-MPs | 1 and 10 µm | Human alveolar A549
cells | To examine the
potential toxicological effects of MPs on human cells | A549 cells exposed
to PS-MPs shows a population-level decrease in metabolic activity
paralleled by a marked decrease in proliferation rate. | (46) |
| Da Silva Brito
et al, 2022 | PP-MPs and
PS-NH2-MPs | PP-MPs (200 nm) and
PS-NH2-MPs (50 nm) | Human alveolar A549
cells | To investigate the
biological response of laser-ablated MPs in human cell lines | Laser-ablated
PP-MPs do not show cytotoxic effects or affect metabolic
proliferation after effects acute exposure in vitro. | (47) |
| Böckers et
al, 2020 | TOCP | N/A | Breast cancer MCF-7
cells | To define the
overall endocrine potential of TOCP and its underlying molecular
mechanisms. | TOCP interacts with
estrogen receptor-α in MCF-7 breast cancer cells, alters gene
expression and promotes tumor cell growth and proliferation. | (49) |
| Böckers et
al, 2020 | Bisphenolic
compounds | N/A | Breast cancer MCF-7
cells | To define the
overall endocrine potential of bisphenolic compounds and their
underlying molecular mechanisms. | Bisphenolic
compounds bind to estrogen receptor-α in MCF-7 breast cancer cells,
changing gene expression and enhancing tumor growth. | (48) |
MPs and stromal cells
Immune cells
Tumor-associated macrophages, which are the
predominant infiltrating immune cells within the TME and have been
extensively studied for their roles in promoting tumor progression
(50). Macrophages, as the
principal phagocytic cells in mucosal environments such as the
gastrointestinal tract and pulmonary system, exhibit modified
functionality and viability upon exposure to MPs, a mechanism
potentially impacting the TME and tumor progression. In a study by
Yang et al (51), it was
demonstrated using a murine model that oral administration of
polyethylene (PE)-NPs or PS-NPs perturbed the gut microenvironment,
thereby modulating the adaptive immune response in a manner that
promoted the proliferation of pre-existing colorectal tumors. This
phenomenon was attributed to the induction of IL-1β-producing
macrophages in the colon, instigated by NP-induced lysosomal
damage, which subsequently facilitated the differentiation of Tregs
and Th17 cells. This process is associated with T cell exhaustion,
thus fostering a pro-tumorigenic environment (51).
Moreover, MPs have been reported to exert
pro-inflammatory effects on macrophages, which may contribute to
chronic inflammation that promotes tumor growth. A study performed
using RAW264.7 mouse macrophage cells demonstrated that the
production of ROS and nitric oxide, in conjunction with the
secretion of inflammatory cytokines, were key mechanisms through
which PS-NPs and PS-MPs elicited cytotoxicity and inflammation in
macrophages (52). Furthermore,
Brammatti et al (53)
investigated the effects of NPs of different sizes (25, 50 and 100
nm) and concentrations (25–500 µg/ml) on HT29 and U937 cell lines,
utilizing a Transwell system to assess macrophage activation and
the subsequent progression of the inflammatory response in
intestinal cells. The findings indicated that NPs of varying sizes
displayed differential permeability in intestinal cells, resulting
in alterations in macrophage infiltration and the activation of
HT29 cells. This activation led to the upregulation of IL-1β and
inducible nitric oxide synthase levels, thereby fostering an
inflammatory milieu conducive to the development of a
tumor-supportive microenvironment (53).
In terms of cell viability, Merkley et al
(54) demonstrated that
macrophages, upon phagocytosing MPs in a murine model, underwent a
metabolic shift towards glycolysis accompanied by a concomitant
reduction in mitochondrial respiration. This metabolic alteration
was associated with increased expression of the co-stimulatory
molecules, CD86 and CD80, on the cell surface, as well as the
upregulation of pro-inflammatory cytokine genes (54). These findings are consistent with
those reported by Ling et al (41) and Collin-Faure et al
(55). Additionally, Koner et
al (56) reported that exposure
to PS-NPs at concentrations of 50–500 µg/ml markedly reduced the
viability of human macrophages. This exposure also induced
oxidative stress, inhibited cellular proliferation, decreased
mitochondrial membrane potential and resulted in DNA damage
(56). Collectively, these findings
suggest that MPs disrupt the microenvironment, modulate macrophage
infiltration, induce oxidative stress and impair both lysosomal and
mitochondrial functions, along with several surface markers
integral to immune responses. Consequently, this diminishes
macrophage efficiency and disrupts the equilibrium of the innate
immune system, thereby facilitating tumor development and the
formation of the TME.
Beyond macrophages, Wolff et al (57) performed isolation and
differentiation or activation of human T cells and dendritic cells,
reporting that T lymphocytes exhibited minimal susceptibility to
cytotoxic effects induced by MPs. Conversely, phagocytic dendritic
cells and macrophages derived from isolated monocytes demonstrated
a high sensitivity to raw MPs. Following 24-h MP exposure, marker
expression indicated a downregulation of the M2 macrophage-induced
inflammatory phenotype and an upregulation of M1 macrophage
markers. This shift may compromise the innate immune defense of the
host, potentially promoting tumorigenesis and the development of
the TME (57). In a related study,
Weber et al (58) primarily
assessed the effects of NP exposure on primary human monocytes and
monocyte-derived dendritic cells, reporting that NP exposure
induced the secretion of both pro- and anti-inflammatory cytokines
in these cells (58). Notably, the
results of these two experiments exhibited certain inconsistencies,
which may be attributed to Weber's utilization of specific
polymers, such as PVC and polymethyl methacrylate or the employment
of irregularly shaped particles.
Fibroblasts
Fibroblasts are integral to maintaining tissue
homeostasis, as they are involved in the synthesis, degradation and
preservation of the ECM. They also serve a role in leukocyte
recruitment, angiogenesis and the promotion of chronic inflammation
within tissues (59). In the TME,
fibroblasts contribute to cancer progression through intricate
interactions with several cell types. They influence tumor
angiogenesis and metabolism by secreting factors and metabolic
products, processes frequently modulated by epigenetic alterations
(60). Research on the interaction
between MPs and fibroblasts frequently discusses inflammation and
stress responses. Wang et al (61) reported that fibroblasts are capable
of internalizing PS-MPs. When fibroblasts were cultured in a medium
conditioned with PS-MPs, there was a notable increase in the
production of ROS and proteins associated with endoplasmic
reticulum stress, such as activating transcription factor 6,
phosphorylated eukaryotic translation initiation factor 2α and
inositol requiring enzyme 1. Additionally, there was an
upregulation in the expression of proteins related to fibrosis,
including plasminogen activator inhibitor-1, collagen type I and
connective tissue growth factor (61). Martin et al (62) reported that dermal fibroblasts
co-cultured with NPs exhibited enhanced uptake of these particles,
alongside an upregulation of α-smooth muscle actin and pro-collagen
Iα. This suggests a notable differentiation of fibroblasts into
myofibroblasts, potentially initiating inflammatory or immune
responses (62). Furthermore,
several studies propose that MPs may disrupt the homeostasis of the
ECM by affecting fibroblast protein expression, which could have a
substantial impact on the TME. Eom et al (63) elucidated that exposure to PS-MPs in
human dermal fibroblasts led to the upregulation of matrix
metalloproteinase-1 (64), a
collagenase known for degrading type I collagen, and several other
ECM proteins. This process of exposure to PS-MPs markedly
diminished the expression of adhesion and ECM-related genes (ELN,
LAMA, LAMB, LAMC, etc.), thereby weakening the mechanical linkages
between the intracellular and extracellular environments and the
ECM. Moreover, a downregulation of integrin β subunits (65) was also observed in Eom's study,
which are cell surface receptors that mediate cell-matrix adhesion,
along with downstream focal adhesion kinase expression. This
downregulation subsequently activates the PI3K/AKT signaling
pathway, reducing cell migration and inducing apoptosis (63).
Endothelial cells
Endothelial cells, which constitute a thin layer of
cells lining blood vessels, are integral to the regulation of
connective tissue cell growth and development, as well as the
facilitation of angiogenesis. The formation of new blood vessels is
vital for delivering nutrients and oxygen, thus sustaining tumor
growth and progression. Therefore, angiogenesis mediated by
endothelial cells is a fundamental process in tumor development
(66). The influence of MPs on
endothelial cells may represent a pivotal mechanism connecting MPs
to tumor progression and alterations in the TME. Vlacil et
al (67) assessed the effects
of carboxylated PS microparticles (1 µm) on murine endothelial
cells and reported that these PS particles had the capacity to
activate endothelial cells, inducing the expression of adhesion
molecules. This activation subsequently enhanced leukocyte adhesion
and stimulated monocytes to secrete pro-inflammatory cytokines
(67). Mobayen et al
(68) evaluated the effects of
irregular PS-MPs, exposing human umbilical vein endothelial cells
to PS-MPs for a duration of 24 h. The results demonstrated a marked
upregulation in the expression of intercellular cell adhesion
molecule-1 and vascular cell adhesion molecule-1, thereby
corroborating the activating influence of MPs on endothelial cells.
Furthermore, it was reported that MPs induced alterations in
endothelial cell phenotypes and shortened the lag time for fibrin
formation, consequently heightening the risk of vascular
inflammation or thrombosis (68).
Mice models were used to assess the relationship between MPs and
vascular damage and inflammation. According to a previous study, NP
exposure caused structural damage to vascular endothelial cells,
triggered inflammatory responses and weakened coagulation,
resulting from the activation of the Janus kinase 1/STAT3/tissue
factor signaling pathway and inflammatory mediators, such as IL-6
(69).
MPs and the ECM
The ECM functions as an essential scaffold that
preserves cellular homeostasis by facilitating cell-matrix adhesion
and mediating direct interactions between cells and their
extracellular milieu (70). Within
the context of tumors, the ECM constitutes a notable component,
fulfilling crucial roles, including the provision of mechanical
support, the regulation of the microenvironment and serving as a
reservoir for signaling molecules (71). Although specific research directly
demonstrating the impact of MPs on ECM modulation in relation to
tumor growth is currently lacking, Huang et al (72) performed a Kyoto Encyclopedia of
Genes and Genomes analysis on differentially expressed genes in
mice exposed to MPs. The findings identified an enrichment of MPs
in the cell adhesion molecule pathway, indicating that MPs can
directly influence the ECM. The excessive accumulation of MPs may
not only interfere with cell signaling pathways and inhibit the
activation of immune responses, but also impact a range of
biological processes. These processes include the viability of
tissue stem cells, cell differentiation, the regulation of growth
factors and potentially the development of cancer (72). These observations underscore
important directions for future exploration (Table II).
 | Table II.Summary of information on the studies
of MPs on stromal cells and ECM. |
Table II.
Summary of information on the studies
of MPs on stromal cells and ECM.
| First author/s,
year | Particle type | Size | Cell/animal
model | Aim | Conclusion | (Refs.) |
|---|
| Yang et al,
2023 | PE-NPs | 500 nm | Murine macrophage
RAW 264.7 cells | To explore the
effects of PE-NPs on the intestinal microenvironment of murine
colorectal cancer. | NPs cause lysosome
damage in colon macrophages, prompting IL-1β production. This leads
to Treg and Th17 differentiation and T cell exhaustion, fostering a
tumor-friendly environment in the colon. | (51) |
| Wang et al,
2023 | PS-NPs and
PS-MPs | 80 nm and 3 µm | Murine macrophage
RAW264.7 cells | To investigate the
effects of 80 nm PS-NPs and 3 µm PS-MPs on murine macrophages
RAW264.7 cells. | Exposure to PS-NPs
or PS-MPs enhance the secretion of inflammatory cytokines, cause
cytotoxicity and pro-inflammatory effect on macrophages and lead to
intestinal inflammation. | (52) |
| Brammatti et
al, 2023 | NPs | 25, 50 and 100
nm | Human U937 cells
and Caco-2 cells | To assess the
effect of different sizes of NPs at the activation of macrophages
in a co-culture model with intestinal cells. | Macrophages undergo
infiltrative changes. HT29 cells up-regulate IL1-β and inducible
nitric oxide synthase levels and the levels of monocyte
chemoattractant protein 1 are altered when cells are exposed to
NPs. | (53) |
| Merkley et
al, 2022 | PS-MPs | 10 µm | Primary murine
macrophages | To examine the
metabolic response in macrophages to MP particles. | Macrophage
phagocytosis of MPs triggers a metabolic shift to glycolysis, and
increases CD80/CD86 markers and glycolysis-related cytokine gene
expression. | (54) |
| Collin-Faure et
al, 2023 | PS-NPs and
PS-MPs | 100 nm-6 µm | Murine macrophage
J774A.1 cells | To investigate how
macrophages respond to the ingestion of plastic particles. | Alterations are
observed in oxidative stress, lysosomal and mitochondrial
functions, along with alterations in immune response surface marker
expression. | (55) |
| Ling et al,
2020 | MPs | N/A | Murine
macrophages | To investigate the
effect of MP on macrophage. | Macrophage
engulfment of MPs triggers a glycolytic metabolic shift and alters
metabolite expression. | (41) |
| Koner et al,
2023 | PS-NPs | 450 nm | Human
macrophages | To examine the
differential toxic effects of PS-NPs on human macrophages. | PS-NPs exposure
markedly decreases human macrophage viability, triggers oxidative
stress, hinders cell proliferation and damages DNA. | (56) |
| Wolff et al,
2023 | PS, PMMA and
PS-NH2-MPs | 50-1, 100 nm | Human peripheral
blood mononuclear cells | To examine the
direct effect on immune cells after exposure to MPs sized 50-1,100
nm. | M2 macrophage
induction is indicated by reduced inflammatory phenotypes after 24
h of MPs exposure. | (57) |
| Weber et al,
2022 | PS, PMMA and PVC
NPs | 50-310 nm | Human peripheral
blood mononuclear cells | To investigate
whether NPs exposure induces inflammatory processes in primary
human monocytes cells. | NPs exposure can
provoke human immune cells to secrete cytokines as key initiators
of inflammation. This response is specific to certain PVC and
particle shapes. | (58) |
| Wang et al,
2024 | PS-MPs | N/A | Human kidney HK-2
cells | To evaluate how
PS-MPs affected tubular cells and fibroblasts. | PS-MP-induced
extracellular vesicles lead to ER stress-related proteins, ROS
production and fibrosis-related proteins in tubular cells and
fibroblasts. | (61) |
| Martin et
al, 2024 | PS-NPs | 100-500 nm | Human dermal
fibroblast cells | To investigate the
entry of NPs into a human skin system modeling skin with
compromised barrier functions. | Trans-epidermal NPs
trigger a marked shift from fibroblast to myofibroblast cells,
boosting α-smooth muscle actin and pro-collagen Ia production. | (62) |
| Eom et al,
2024 | PS particles | N/A | Human dermal
fibroblast cells | To examine the 3D
behavior of skin-derived cells exposed to PS particles. | MPs affect gene
expression linked to ECM and integrin-mediated adhesion. | (63) |
| Vlacil et
al, 2022 | PS-MPs | 1 µm | Murine myocardial
endothelial cells | To investigate how
carboxylated PS particles affect murine endothelial and immune
cells involved in vascular inflammation. | PS-MPs trigger
adhesion molecule expression in endothelial cells, leading to
leukocyte adhesion in both static and flow conditions and increase
pro-inflammatory cytokine expression. | (67) |
| Mobayen et
al, 2023 | PS-MPs | <5 µm | Human umbilical
vein endothelial cells | To determine the
effects of MPs exposure on endothelial cells and thrombus
formation. | MPs possess the
ability to activate the endothelium, reduce the lag time to fibrin
formation and maximal turbidity, indicating denser clot
formation. | (68) |
| Wang et al,
2023 | PS,
PS-NH2 and PS-COOH NPs | 80 nm | 6-8-week-old male
BALB/c mice | To investigate the
adverse cardio-vascular impacts of PS, PS-NH2 and
PS-COOH NPs on mice. | NPs can induce
injury and dysfunction through the activation of Janus kinase
1/STAT3/tissue factor pathway. | (69) |
| Huang et al,
2023 | MPs | 5 µm | 4-week-old male
C57BL/6 mice | TO examine the
innate immune response of mice exposed to 5 µm MPs. | MPs disrupt immune
receptors and impair cell signaling in the liver and spleen,
hindering serum immune signal activation. | (72) |
MPs and inflammation
Hanahan et al (73) proposed that inflammation notably
contributes to the initiation and progression of tumors. Recognized
as a hallmark of cancer, inflammation is implicated in every phase
of tumorigenesis, encompassing development, malignancy, invasion
and metastasis, whilst also interacting intricately with the TME.
MPs intensify inflammatory responses by releasing cytokines,
triggering inflammatory signaling pathways, inducing oxidative
stress and establishing a microenvironment conducive to cancer
initiation and progression (Table
III) (37,74).
 | Table III.Summary of information on the studies
of MPs and inflammation. |
Table III.
Summary of information on the studies
of MPs and inflammation.
| First author/s,
year | Particle type | Size | Cell/animal
model | Aim | Conclusion | (Refs.) |
|---|
| He et al,
2022 | PS-NPs | 100 nm | 8-week-old male
C57BL/6 mice | To investigate the
inflammatory relationship and molecular mechanisms between PS-NPs
and intestinal injury. | PS-NPs exacerbate
lipopolysaccharide-induced inflammation and increase duodenal
permeability in mice via the ROS-driven NF-κB/NLRP3 pathway. | (75) |
| Wen et al,
2024 | PS-NPs | 20 nm | 6-week-old male
mice and AML-12 hepatocytes | To explore the
mechanisms of hepatotoxicity and inflammation induced by
PS-NPs. | The NRF2-NLRP3
pathway contributes to hepatotoxicity and inflammation induced by
PS-NPs. | (81) |
| Zhang et al,
2022 | PS-MPs | 5 µm | 1-day-old
chicks | To investigate the
mechanism of MPs-induced heart injury in chickens. | Alterations in
NF-κB-NLRP3-gasdermin D and AMP-activated protein kinase-PPARG
coactivator 1α pathways, driven by PS-MPs and ROS overload, lead to
oxidative stress, myocardial pyroptosis, inflammation and
mitochondrial and energy metabolism dysfunction. | (82) |
| Li et al,
2023 | PS-MPs | 5 µm | 1-day-old high land
broilers | To assess the role
of MPs on immune organs (chicken thymus). | Exposure to PS-MPs
activates the Nrf2/NF-κB, Bcl-2/Bax and AKT pathways in the thymus,
leading to increased downstream signaling that causes inflammation,
apoptosis and autophagy. | (83) |
| Antunes et
al, 2021 | PS-NPs | 25, 50 and 100
nm | Human colon
adenocarcinoma HT29 cells | To evaluate NP
toxicity and their potential to interfere with inflammation-related
pathways in HT29 cells. | Increased p50 and
p38 expression after PS-NP exposure, indicating activation of the
NF-κB pathway. | (84) |
| Woo et al,
2023 | PP-NPs | 0.66± 0.27 µm | Human alveolar A549
cells and 7-week-old male ICR mice | To study of
inhalation toxicity and inflammatory effects of PP particles on the
lungs. | PP stimulation
elevates phosphorylated-p38 and phosphorylated-NF-κB protein levels
in vivo and in vitro, while PP-induced cytotoxicity
in A549 cells is controlled by p38 and ROS inhibition. | (88) |
| Danso et al,
2024 | PS-MPs, PP-MPs and
PE-MPs | <20 µm | 7-week-old male
C57BL/6 mice | To examine the
toxicity of PP, PS and PE-MP fragments in the pulmonary system of
C57BL/6 mice. | PS fragments
trigger lung inflammation by activating NF-ĸB and NLRP3
inflammasomes via the TLR4 pathway. | (89) |
| Han et al,
2024 | MPs | Nano-sized | Human- and mouse-
derived skin cells | To explore the
toxicological effects of nano-sized MPs on the skin. | Nano-sized MPs
cause higher inflammation in skin cells, increase absent in
melanoma 2 expression based on concentration, trigger IL-1β
release, and start an inflammatory response. | (90) |
| Zeng et al,
2024 | PS-MPs | 0.1, 1 and 5
µm | Human colorectal
adenocarcinoma Caco-2 cells and 6-week-old male C57BL/6 mice | To investigate the
molecular mechanisms contributing to MP-induced intestinal barrier
dysfunction. | PS-MPs cause
intestinal inflammation and barrier dysfunction through the
ROS-dependent NF-κB/NLRP3/IL-1β/MLCK signaling pathway. | (91) |
Inflammatory factors are critical in modulating
inflammatory and immune responses and notably impact tumor
progression within the TME through several mechanisms. These
factors establish signaling pathways that mediate the
pro-inflammatory effects of MPs and modify the microenvironment.
Recent research has demonstrated that PS-NPs exacerbate
lipopolysaccharide-induced duodenal inflammation in murine models
via ROS-driven activation of NF-κB and NLRP3 (75). NF-κB is a pivotal regulator of
pro-inflammatory mediator expression and serves a central role in
the pathogenesis of inflammatory diseases. Notably, NF-κB operates
in a cell-type-specific manner, facilitating the activation of
genes that promote inflammation within the TME (76). The NLRP3 inflammasome contributes to
the regulation of inflammatory responses by activating caspase-1,
which in turn promotes the secretion of pro-inflammatory cytokines,
IL-18 and IL-1β and induces pyroptosis (77). In vitro experiments have
demonstrated that PS-MPs suppress cell viability via a
mitochondria-dependent pathway, leading to increased production of
ROS (78). Excessive ROS not only
activates NF-κB but also serves as a critical mechanism for the
activation of the NLRP3 inflammasome in response to exogenous
stimuli (77,79,80).
Consequently, the ROS-NF-κB-NLRP3 signaling pathway has emerged as
a central focus in research concerning MPs and inflammation.
Despite ethical considerations, a notable number of these
experiments are performed using animal models. Wen et al
(81) evaluated the relationship
between exposure to PS-NPs and liver inflammation in mice, and
reported that PS-NPs exposure markedly increased the expression
levels of NLRP3, IL-1β and caspase-1, in addition to activating
NF-κB. These results further corroborate the hypothesis that the
ROS-NF-κB-NLRP3 signaling pathway is a mechanism through which
PS-NPs induce inflammatory damage (81). Similarly, in a study by Zhang et
al (82), the effects of
varying concentrations of PS-MPs on chicken hearts and primary
cardiomyocytes were assessed. The findings indicated that PS-MPs
induced myocardial pyroptosis, inflammatory cell infiltration and
mitochondrial damage via the NF-κB-NLRP3-gasdermin D signaling
pathway. This process resulted in the upregulation of factors such
as NLRP3, caspase-1, IL-1β, IL-18 and IL-6, thereby exacerbating
myocardial inflammation (82). A
comparable conclusion was drawn in a previous study evaluating the
effects of PS-MPs on thymic inflammation in chickens (83). The study reported that PS-MPs
induced oxidative stress in the thymus and activated the nuclear
factor erythroid 2-related factor 2/NF-κB, Bcl-2/Bax and AKT
signaling pathways. This activation subsequently enhanced the
expression of downstream molecules such as IL-1β, caspase-3 and
Beclin1, culminating in thymic inflammation, apoptosis and
autophagy (83).
In addition to the ROS-NF-κB-NLRP3 signaling
pathway, other studies have suggested that toll-like receptor 4
(TLR4), p38 and p50 may also serve a role in mediating the
pro-inflammatory effects of MPs. Antunes et al (84) performed an experiment in which the
human colorectal cancer HT29 cell line was exposed to PS-NPs. The
results demonstrated that HT29 cells exhibited an upregulation of
p50 and p38 expression, along with an increase in TLR4 expression.
Notably, p38, a member of the MAPK family, is implicated in several
signaling cascades, including those related to inflammatory
responses, and is responsive to environmental stressors (85). TLR4 functions as a membrane protein
in the pattern recognition receptor (PRR) family. The activation of
TLR4 can lead to the synthesis of pro-inflammatory cytokines and
chemokines (86), as well as the
activation of the classical NF-κB pathway and p38, thereby
promoting inflammatory responses (87). Consistent results were reported in a
study by Woo et al (88),
where exposure of mouse lungs and A549 cells to PP-NPs resulted in
a marked increase in the number of inflammatory cells, ROS
production and levels of inflammatory cytokines and chemokines both
in vivo and in vitro. This was accompanied by
elevated levels of phosphorylated p38 and NF-κB proteins. In A549
cells, the inflammation induced by PP exposure was regulated by
inhibitors targeting p38 and ROS. These results suggest that PP-NPs
can promote inflammation through p38-mediated NF-κB signaling
pathways (88). Additionally, Danso
et al (89) administered 5
mg/kg MP fragments intratracheally to mice over a period of 14 days
to assess the pulmonary toxicity and inflammatory effects
associated with MPs. Compared with the control group, the lung
tissues of mice administered with PS-MP fragments exhibited an
increased presence of inflammasome components, including NLRP3,
apoptosis-associated speck-like protein containing a caspase
recruitment domain and caspase-1. This observation supports the
conclusion that lung inflammation induced by PS microplastic
fragments is mediated via TLR4 activation of the NF-ĸB and NLRP3
inflammasome pathways (89).
Although the pro-inflammatory pathways triggered by
MPs in numerous experimental studies exhibit variability, they
consistently implicate the NLRP3 inflammasome, underscoring its
critical role in MP-induced inflammation. Contrarily, in the study
by Han et al (90) on
nano-sized MPs and their induction of inflammatory responses in
skin cells, activation of the NLRP3 inflammasome was not detected.
Instead, the study reported that nano-MPs upregulated the ‘absent
in melanoma 2’ PRR in a concentration-dependent manner, thereby
promoting the release of IL-1β and initiating the inflammatory
response (90). This enhances the
comprehension of inflammation induced by MPs.
On the other hand, beyond their pro-inflammatory
effects, inflammatory factors have the potential to disrupt
cellular junctions, alter the microenvironment and potentially
contribute to malignant transformation. Zeng et al (91) reported that exposure of the human
colorectal cancer Caco-2 cell line to PS-MPs resulted in increased
permeability of tight junction proteins within the cultured Caco-2
monolayer. This effect is likely attributable to the induction of
oxidative stress and the activation of NF-κB and the NLRP3
inflammasome by PS-MPs in Caco-2 cells, which subsequently elevated
the expression of inflammatory factors, such as IL-6, IL-8, TNF-α
and IL-1β (91). Notably, IL-1β is
known to activate the NF-κB/myosin light chain kinase (MLCK)
signaling pathway (92), whereas
TNF-α stimulates IL-8 secretion, thereby promoting intestinal
inflammation and MLCK expression (93). Consequently, PS-MPs elicit
inflammatory responses and compromise colonic epithelial tight
junctions through the ROS-dependent NF-κB/NLRP3/IL-1β/MLCK
signaling pathway. This process enhances mucosal barrier
permeability and perturbs the intestinal microenvironment (91).
Conclusions
MPs have become deeply embedded within human
society, establishing themselves as a major global pollutant. Their
persistence, mobility, high production volume and wide range of
applications have led to their ubiquitous presence in the natural
environment. Consequently, MPs are increasingly encountered by the
human body, posing potential health risks and potentially elevating
the likelihood of disease and cancer (94). Research has reported that under
conditions characterized by high concentration, extended exposure
and heightened individual susceptibility, MPs may exert cytotoxic
effects through mechanisms such as chronic inflammation, oxidative
stress, DNA damage, immunotoxicity and the delivery of toxic
substances. It potentially culminates in malignant transformation
(95). Furthermore, previous
studies have broadened the scope of understanding regarding the
effects of MPs, demonstrating their influence not only on tumor
initiation but also on tumor progression by modulating the TME
(39). Despite the nascent stage of
research in this field and the limited scope of existing
literature, the present review offers a systematic analysis of
contemporary studies examining the interactions between MPs and
several components of the TME. These elements include tumor cells,
immune cells (with a focus on macrophages), endothelial cells,
fibroblasts, ECM and inflammatory factors. The primary conclusion
is that MPs within the TME markedly contribute to the proliferation
and metastasis of tumor cells. They achieve this by modulating the
immune cell status to enhance tumor activity, inducing structural
changes in vascular endothelial cells, facilitating the activation
of fibroblasts and the expression of associated proteins and
directly influencing the ECM and inflammatory mediators. We
hypothesize that these theoretical foundations will enhance the
comprehension of the role of MPs in carcinogenesis and offer novel
research trajectories for future scholars. Furthermore, they may
identify new diagnostic and therapeutic targets for individuals
whose health is compromised by MPs, including patients with
cancer.
Similarly, carbon nanomaterials (CNMs) exhibit
distinctive physical and chemical properties due to their nanoscale
dimensions. CNMs present novel opportunities for cancer therapy by
specifically targeting cancer cells and components of the TME
(96). The present review serves as
an impetus for the investigation into the application of MPs within
the context of the TME. Nonetheless, the current understanding of
the interaction between MPs and the TME represents merely the tip
of the iceberg. Consequently, further research is imperative to
elucidate the intricate relationship between MPs and cancer
development, establishing it as a crucial area of study.
It is important to recognize that the present review
revealed considerable variability in experimental outcomes,
attributable to disparities in research methodologies, sample
types, the physical properties of samples as well as exposure
concentrations and durations. These inconsistencies present
challenges in comparing and categorizing study findings. Therefore,
future research on MPs and the TME should incorporate several MP
types, concentrations and exposure durations, in both in
vitro and in vivo settings, alongside rigorously
designed control groups. Whilst this approach may increase the
complexity of experiments, it will enhance the reliability and
accuracy of the data and conclusions.
In summary, the study of MPs, tumors and the TME
necessitates sustained research investment and the adoption of
multidisciplinary methodologies. As the present review
progressively unravels the complex web of interactions among these
elements, the resulting insights will contribute to the development
of more efficacious preventive measures, facilitate earlier
detection and enhance therapeutic strategies for individuals
affected by MPs.
Acknowledgements
Not applicable.
Funding
The present research was supported by the educational research
project of Nanjing Medical University (Nanjing, China; grant no.
2023YJS-LX011 and 2023YJS-LX012), the National Natural Science
Foundation of China (grant nos. 82203216 and 82102073) and the
Natural Science Foundation of Jiangsu Province (grant no.
BK20220724).
Availability of data and materials
Not applicable.
Authors' contributions
YC drafted and revised the manuscript. ZZ, KJ, QZ,
LQ and CY collected the relevant papers and helped to revise the
manuscript. ZZ and QZ designed the tables and charts. CY and LQ
reviewed the article. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryan PG and Moloney CL: Marine litter
keeps increasing. Nature. 361:231993. View
Article : Google Scholar
|
|
2
|
Thompson RC, Olsen Y, Mitchell RP, Davis
A, Rowland SJ, John AW, McGonigle D and Russell AE: Lost at sea:
Where is all the plastic? Science. 304:8382004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geyer R, Jambeck JR and Law KL:
Production, use, and fate of all plastics ever made. Sci Adv.
3:e17007822017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kooi M, Besseling E, Kroeze C, van Wezel
AP and Koelmans AA: Modeling the fate and transport of plastic
debris in freshwaters: Review and guidance. Freshwater
Microplastics: Emerging Environmental Contaminants? Wagner M and
Lambert S: Springer International Publishing; Cham: pp. 125–152.
2018, View Article : Google Scholar
|
|
5
|
Faure F, Demars C, Wieser O, Kunz M and de
Alencastro L: Plastic pollution in Swiss surface waters: Nature and
concentrations, interaction with pollutants. Environmental
Chemistry. 12:582–591. 2015. View
Article : Google Scholar
|
|
6
|
Lebreton L, Slat B, Ferrari F, Sainte-Rose
B, Aitken J, Marthouse R, Hajbane S, Cunsolo S, Schwarz A, Levivier
A, et al: Evidence that the great pacific garbage patch is rapidly
accumulating plastic. Sci Rep. 8:46662018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laskar N and Kumar U: Plastics and
microplastics: A threat to environment. Environmental Technology
& Innovation. 14:1003522019. View Article : Google Scholar
|
|
8
|
Fendall LS and Sewell MA: Contributing to
marine pollution by washing your face: Microplastics in facial
cleansers. Mar Pollut Bull. 58:1225–1228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aueviriyavit S, Phummiratch D and
Maniratanachote R: Mechanistic study on the biological effects of
silver and gold nanoparticles in Caco-2 cells-induction of the
Nrf2/HO-1 pathway by high concentrations of silver nanoparticles.
Toxicol Lett. 224:73–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Efimova I, Bagaeva M, Bagaev A, Kileso A
and Chubarenko IP: Secondary microplastics generation in the sea
swash zone with coarse bottom sediments: Laboratory experiments.
Front Mar Sci. 5:3132018. View Article : Google Scholar
|
|
11
|
Xiang Y, Jiang L, Zhou Y, Luo Z, Zhi D,
Yang J and Lam SS: Microplastics and environmental pollutants: Key
interaction and toxicology in aquatic and soil environments. J
Hazard Mater. 422:1268432022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domenech J and Marcos R: Pathways of human
exposure to microplastics, and estimation of the total burden. Curr
Opin Food Sci. 39:144–151. 2021. View Article : Google Scholar
|
|
13
|
Santillo D, Miller K and Johnston P:
Microplastics as contaminants in commercially important seafood
species. Integr Environ Assess Manag. 13:516–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karami A, Golieskardi A, Choo CK, Larat V,
Galloway TS and Salamatinia B: The presence of microplastics in
commercial salts from different countries. Sci Rep. 7:461732017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosuth M, Mason SA and Wattenberg EV:
Anthropogenic contamination of tap water, beer, and sea salt. PLoS
One. 13:e01949702018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwabl P, Köppel S, Königshofer P,
Bucsics T, Trauner M, Reiberger T and Liebmann B: Detection of
various microplastics in human stool: A prospective case series.
Ann Intern Med. 171:453–457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Y, Zhang Y, Lemos B and Ren H: Tissue
accumulation of microplastics in mice and biomarker responses
suggest widespread health risks of exposure. Sci Rep. 7:466872017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu
Q, Ren H and Zhang Y: Accumulation of different shapes of
microplastics initiates intestinal injury and gut microbiota
dysbiosis in the gut of zebrafish. Chemosphere. 236:1243342019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar R, Manna C, Padha S, Verma A, Sharma
P, Dhar A, Ghosh A and Bhattacharya P: Micro(nano)plastics
pollution and human health: How plastics can induce carcinogenesis
to humans? Chemosphere. 298:1342672022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Brien S, Okoffo ED, O'Brien JW, Ribeiro
F, Wang X, Wright SL, Samanipour S, Rauert C, Toapanta TYA,
Albarracin R and Thomas KV: Airborne emissions of microplastic
fibres from domestic laundry dryers. Sci Total Environ.
747:1411752020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prata JC: Airborne microplastics:
Consequences to human health? Environ Pollut. 234:115–126. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gasperi J, Wright SL, Dris R, Collard F,
Mandin C, Guerrouache M, Langlois V, Kelly FJ and Tassin B:
Microplastics in air: Are we breathing it in? Curr Opin Environ Sci
Health. 1:1–5. 2018. View Article : Google Scholar
|
|
23
|
Zhang J, Du J, Liu D, Zhuo J, Chu L, Li Y,
Gao L, Xu M, Chen W, Huang W, et al: Polystyrene microplastics
induce pulmonary fibrosis by promoting alveolar epithelial cell
ferroptosis through cGAS/STING signaling. Ecotoxicol Environ Saf.
277:1163572024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milillo C, Aruffo E, Di Carlo P, Patruno
A, Gatta M, Bruno A, Dovizio M, Marinelli L, Dimmito MP, Giacomo
VD, et al: Polystyrene nanoplastics mediate oxidative stress,
senescence, and apoptosis in a human alveolar epithelial cell line.
Front Public Health. 12:13853872024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warheit DB, Hart GA, Hesterberg TW,
Collins JJ, Dyer WM, Swaen GM, Castranova V, Soiefer AI and Kennedy
GL Jr: Potential pulmonary effects of man-made organic fiber (MMOF)
dusts. Crit Rev Toxicol. 31:697–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu P, Lin S, Cao G, Wu J, Jin H, Wang C,
Wong MH, Yang Z and Cai Z: Absorption, distribution, metabolism,
excretion and toxicity of microplastics in the human body and
health implications. J Hazard Mater. 437:1293612022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu P, Zhang H, Singh N, Tang Y and Cai Z:
Intertidal zone effects on occurrence, fate and potential risks of
microplastics with perspectives under COVID-19 pandemic. Chemical
Engineering J. 429:1323512022. View Article : Google Scholar
|
|
28
|
Schneider M, Stracke F, Hansen S and
Schaefer UF: Nanoparticles and their interactions with the dermal
barrier. Dermatoendocrinol. 1:197–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira M, Ribeiro A, Hylland K and
Guilhermino L: Single and combined effects of microplastics and
pyrene on juveniles (0+ group) of the common goby Pomatoschistus
microps (Teleostei, Gobiidae). Ecological Indicators. 34:641–647.
2013. View Article : Google Scholar
|
|
30
|
Kleinteich J, Seidensticker S, Marggrander
N and Zarfl C: Microplastics reduce short-term effects of
environmental contaminants. Part II: Polyethylene particles
decrease the effect of polycyclic aromatic hydrocarbons on
microorganisms. Int J Environ Res Public Health. 15:2872018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou Y, Liu X and Wang J: Characterization
of microplastics and the association of heavy metals with
microplastics in suburban soil of central China. Sci Total Environ.
694:1337982019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogata Y, Takada H, Mizukawa K, Hirai H,
Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A, et al:
International pellet watch: Global monitoring of persistent organic
pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs,
DDTs, and HCHs. Mar Pollut Bull. 58:1437–1446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brynzak-Schreiber E, Schögl E, Bapp C,
Cseh K, Kopatz V, Jakupec MA, Weber A, Lange T, Toca-Herrera JL,
Favero GD, et al: Microplastics role in cell migration and
distribution during cancer cell division. Chemosphere.
353:1414632024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shahzadi C, Di Serafino A, Aruffo E,
Mascitelli A and Di Carlo P: A549 as an in vitro model to evaluate
the impact of microplastics in the air. Biology (Basel).
12:12432023.PubMed/NCBI
|
|
35
|
Prata JC, da Costa JP, Lopes I, Duarte AC
and Rocha-Santos T: Environmental exposure to microplastics: An
overview on possible human health effects. Sci Total Environ.
702:1344552020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rahman A, Sarkar A, Yadav OP, Achari G and
Slobodnik J: Potential human health risks due to environmental
exposure to nano- and microplastics and knowledge gaps: A scoping
review. Sci Total Environ. 757:1438722021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang W, Jannatun N, Zeng Y, Liu T, Zhang
G, Chen C and Li Y: Impacts of microplastics on immunity. Front
Toxicol. 4:9568852022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arneth B: Tumor microenvironment. Medicina
(Kaunas). 56:152019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen G, Shan H, Xiong S, Zhao Y, van
Gestel CAM, Qiu H and Wang Y: Polystyrene nanoparticle exposure
accelerates ovarian cancer development in mice by altering the
tumor microenvironment. Sci Total Environ. 906:1675922024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
41
|
Ling C, Meyer-Hagen J and Castillo EF:
Ingested microplastics pose a potentially serious risk to the
gastrointestinal microenvironment. J Immunol. 204:83.21.
2020.PubMed/NCBI
|
|
42
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Xu X and Jiang G: Microplastics
exposure promotes the proliferation of skin cancer cells but
inhibits the growth of normal skin cells by regulating the
inflammatory process. Ecotoxicol Environ Saf. 267:1156362023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park JH, Hong S, Kim OH, Kim CH, Kim J,
Kim JW, Hong S and Lee HJ: Polypropylene microplastics promote
metastatic features in human breast cancer. Sci Rep. 13:62522023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schnee M and Dittmar T: Impact of
microplastic on mammary epithelial cells, breast-cancer-cells and
cell fusion. Oncology Research and Treatment. 47:402024.
|
|
46
|
Goodman KE, Hare JT, Khamis ZI, Hua T and
Sang QXA: Exposure of human lung cells to polystyrene microplastics
significantly retards cell proliferation and triggers morphological
changes. Chem Res Toxicol. 34:1069–1081. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Da Silva Brito WA, Singer D, Honnorat B,
Saadati F, Wende K and Bekeschus S: Laser-ablated polypropylene
microplastics and their biological responses in human cell lines.
Toxicol Lett. 368:S2982022. View Article : Google Scholar
|
|
48
|
Böckers M, Paul NW and Efferth T:
Bisphenolic compounds alter gene expression in MCF-7 cells through
interaction with estrogen receptor α. Toxicol Appl Pharmacol.
399:1150302020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Böckers M, Paul NW and Efferth T:
Organophosphate ester tri-o-cresyl phosphate interacts with
estrogen receptor α in MCF-7 breast cancer cells promoting cancer
growth. Toxicol Appl Pharmacol. 395:1149772020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang Q, Dai H, Wang B, Xu J, Zhang Y, Chen
Y, Ma Q, Xu F, Cheng H, Sun D and Wang C: Nanoplastics shape
adaptive anticancer immunity in the colon in mice. Nano Lett.
23:3516–3523. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang X, Ren XM, He H, Li F, Liu K, Zhao F,
Hu H, Zhang P, Huang B and Pan X: Cytotoxicity and pro-inflammatory
effect of polystyrene nano-plastic and micro-plastic on RAW264.7
cells. Toxicology. 484:1533912023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brammatti I, Antunes J, Carvalho C,
Martins M and Branco V: P17-25: Nanoplastics effect over
co-cultures of intestinal and immune cells. Toxicol Lett.
384:S2122023. View Article : Google Scholar
|
|
54
|
Merkley SD, Moss HC, Goodfellow SM, Ling
CL, Meyer-Hagen JL, Weaver J, Campen MJ and Castillo EF:
Polystyrene microplastics induce an immunometabolic active state in
macrophages. Cell Biol Toxicol. 38:31–41. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Collin-Faure V, Vitipon M, Torres A,
Tanyeres O, Dalzon B and Rabilloud T: The internal dose makes the
poison: Higher internalization of polystyrene particles induce
increased perturbation of macrophages. Front Immunol.
14:10927432023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koner S, Florance I, Mukherjee A and
Chandrasekaran N: Cellular response of THP-1 macrophages to
polystyrene microplastics exposure. Toxicology. 483:1533852023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wolff CM, Singer D, Schmidt A and
Bekeschus S: Immune and inflammatory responses of human
macrophages, dendritic cells, and T-cells in presence of micro- and
nanoplastic of different types and sizes. J Hazard Mater.
459:1321942023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weber A, Schwiebs A, Solhaug H, Stenvik J,
Nilsen AM, Wagner M, Relja B and Radeke HH: Nanoplastics affect the
inflammatory cytokine release by primary human monocytes and
dendritic cells. Environ Int. 163:1071732022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wei K, Nguyen HN and Brenner MB:
Fibroblast pathology in inflammatory diseases. J Clin Invest.
131:e1495382021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang YL, Huang CC, Zheng CM, Liu WC, Lee
YH and Chiu HW: Polystyrene microplastic-induced extracellular
vesicles cause kidney-related effects in the crosstalk between
tubular cells and fibroblasts. Ecotoxicol Environ Saf.
273:1160982024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Martin L, Simpson K, Brzezinski M, Watt J
and Xu W: Cellular response of keratinocytes to the entry and
accumulation of nanoplastic particles. Part Fibre Toxicol.
21:222024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eom S, Shim W and Choi I:
Microplastic-induced inhibition of cell adhesion and toxicity
evaluation using human dermal fibroblast-derived spheroids. J
Hazard Mater. 465:1333592024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van Doren SR: Matrix metalloproteinase
interactions with collagen and elastin. Matrix Biol. 44-46:224–231.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bökel C and Brown NH: Integrins in
development: Moving on, responding to, and sticking to the
extracellular matrix. Dev Cell. 3:311–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sobierajska K, Ciszewski WM,
Sacewicz-Hofman I and Niewiarowska J: Endothelial cells in the
tumor microenvironment. Adv Exp Med Biol. 1234:71–86. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vlacil AK, Trippel N, Bänfer S, Jacob R,
Schieffer B and Grote K: Microplastic particles induce endothelial
activation. Atherosclerosis. 355:5–6. 2022. View Article : Google Scholar
|
|
68
|
Mobayen G, Auyang E, Mitchell W,
Arachchillage D, Wright S and McKinnon T: The effects of
polystyrene microplastics on thrombosis. Research and Practice in
Thrombosis and Haemostasis. 7:1008272023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang X, Jia Z, Zhou X, Su L, Wang M, Wang
T and Zhang H: Nanoplastic-induced vascular endothelial injury and
coagulation dysfunction in mice. Sci Total Environ. 865:1612712023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang J, Zhang L, Wan D, Zhou L, Zheng S,
Lin S and Qiao Y: Extracellular matrix and its therapeutic
potential for cancer treatment. Signal Transduct Target Ther.
6:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang H, Hou J, Liao Y, Wei F and Xing B:
Polyethylene microplastics impede the innate immune response by
disrupting the extracellular matrix and signaling transduction.
iScience. 26:1073902023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tanveer M, Mansha N, Nimra A, Khawar MB,
Afzal A, Afzal H, Farooq M, Ehsan S, Rana R and Shahzaman S:
Microplastics: Unraveling the signaling pathways involved in
reproductive health. Environ Sci Pollut Res Int. 30:95077–95085.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
He Y, Li Z, Xu T, Luo D, Chi Q, Zhang Y
and Li S: Polystyrene nanoplastics deteriorate LPS-modulated
duodenal permeability and inflammation in mice via ROS
drived-NF-κB/NLRP3 pathway. Chemosphere. 307:1356622022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Antunes J, Sobral P, Martins M and Branco
V: Nanoplastics activate a TLR4/p38-mediated pro-inflammatory
response in human intestinal and mouse microglia cells. Environ
Toxicol Pharmacol. 104:1042982023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun R, Liu M, Xiong F, Xu K, Huang J, Liu
J, Wang D and Pu Y: Polystyrene micro- and nanoplastics induce
gastric toxicity through ROS mediated oxidative stress and
P62/Keap1/Nrf2 pathway. Sci Total Environ. 912:1692282024.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Dominic A, Le NT and Takahashi M: Loop
between nlrp3 inflammasome and reactive oxygen species. Antioxid
Redox Signal. 36:784–796. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gloire G and Piette J: Redox regulation of
nuclear post-translational modifications during NF-kappaB
activation. Antioxid Redox Signal. 11:2209–2222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wen Y, Deng S, Wang B, Zhang F, Luo T,
Kuang H, Kuang X, Yuan Y, Huang J and Zhang D: Exposure to
polystyrene nanoplastics induces hepatotoxicity involving
NRF2-NLRP3 signaling pathway in mice. Ecotoxicol Environ Saf.
278:1164392024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang Y, Yin K, Wang D, Wang Y, Lu H, Zhao
H and Xing M: Polystyrene microplastics-induced cardiotoxicity in
chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes.
Sci Total Environ. 840:1567272022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li J, Yin K, Hou L, Zhang Y, Lu H, Ma C
and Xing M: Polystyrene microplastics mediate inflammatory
responses in the chicken thymus by Nrf2/NF-κB pathway and trigger
autophagy and apoptosis. Environ Toxicol Pharmacol. 100:1041362023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Antunes JC, Branco V, Sobral P and Martins
M: P20-32 polystyrene nanoparticles interference in human colon
adenocarcinoma cell line HT29. Toxicol Lett. 350:S195–S196. 2021.
View Article : Google Scholar
|
|
85
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochemical J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang W, Weng J, Yu L, Huang Q, Jiang Y and
Guo X: Role of TLR4-p38 MAPK-Hsp27 signal pathway in LPS-induced
pulmonary epithelial hyperpermeability. BMC Pulm Med. 18:1782018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Woo JH, Seo HJ, Lee JY, Lee I, Jeon K, Kim
B and Lee K: Polypropylene nanoplastic exposure leads to lung
inflammation through p38-mediated NF-κB pathway due to
mitochondrial damage. Part Fibre Toxicol. 20:22023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Danso IK, Woo JH, Baek SH, Kim K and Lee
K: Pulmonary toxicity assessment of polypropylene, polystyrene, and
polyethylene microplastic fragments in mice. Toxicol Res.
40:313–323. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Han W, Cui J, Sun G, Miao X, Pufang Z and
Nannan L: Nano-sized microplastics exposure induces skin cell
senescence via triggering the mitochondrial localization of GSDMD.
Environ Pollut. 349:1238742024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zeng G, Li J, Wang Y, Su J, Lu Z, Zhang F
and Ding W: Polystyrene microplastic-induced oxidative stress
triggers intestinal barrier dysfunction via the
NF-κB/NLRP3/IL-1β/MCLK pathway. Environ Pollut. 345:1234732024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang J, Zhao H, Lv K, Zhao W, Zhang N,
Yang F, Wen X, Jiang X, Tian J, Li X, et al: Pterostilbene
Ameliorates DSS-Induced intestinal epithelial barrier loss in mice
via suppression of the NF-κB-Mediated MLCK-MLC signaling pathway. J
Agric Food Chem. 69:3871–3878. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shen Y, Zhou M, Yan J, Gong Z, Xiao Y,
Zhang C, Du P and Chen Y: miR-200b inhibits TNF-α-induced IL-8
secretion and tight junction disruption of intestinal epithelial
cells in vitro. Am J Physiol Gastrointest Liver Physiol.
312:G123–G132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vethaak AD and Legler J: Microplastics and
human health. Science. 371:672–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kumar N, Lamba M, Pachar AK, Yadav S and
Acharya A: Microplastics-A growing concern as carcinogens in cancer
etiology: Emphasis on biochemical and molecular mechanisms. Cell
Biochem Biophys. 82:3109–3121. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Asil SM, Guerrero ED, Bugarini G, Cayme J,
De Avila N, Garcia J, Hernandez A, Mecado J, Madero Y, Moncayo F,
et al: Theranostic applications of multifunctional carbon
nanomaterials. View (Beijing). 4:202200562023.PubMed/NCBI
|















