Introduction
Cancer is a significant public health concern
globally, affecting millions of individuals and causing
considerable mortality and social burden. Among the numerous types
of cancer, colorectal cancer (CRC) is one of the most prevalent
malignant tumors, with an estimated 5-year prevalence of >5.2
million individuals worldwide. Furthermore, CRC accounts for the
second-highest cancer mortality rate (9.4%) worldwide (1). The high morbidity and mortality rates
suggest that current diagnostic and therapeutic approaches have
their limitations. Despite the considerable progress made in the
diagnosis and treatment of CRC in recent years, particularly in
early screening techniques, minimally invasive surgical approaches,
chemotherapy and targeted therapies, the incidence of this disease
continues to rise (2) and the
overall prognosis of patients remains unsatisfactory. A significant
proportion of patients are diagnosed at an advanced stage of the
disease, which limits the therapeutic benefits that can be offered
and results in relatively low survival rates. This situation
emphasizes the urgent need for more accurate and validated
prognostic tools to guide treatment decisions and improve patient
survival in clinical practice.
Chronic inflammation significantly contributes to
tumor development, which may be triggered by several factors,
including infections, abnormal immune responses or environmental
factors (such as smoking, inhalation of pollutants or dietary
factors) (3). Inflammation and
immune response play an important role in the mechanisms underlying
CRC (4,5). A previous study found that
inflammatory processes substantially affect the postoperative
recovery and prognosis of patients with CRC (6).
Immune-inflammatory biomarkers that can influence
the prognosis of CRC have a more favorable predictive effect than
traditional tumor-related biomarkers. It is expected that
biomarkers based on inflammatory processes will prove to be
significant predictors of surgical outcomes and long-term prognosis
(7). Some peripheral blood
parameters, including the neutrophil/lymphocyte ratio (NLR),
monocyte/lymphocyte ratio (MLR) and platelet count-to-lymphocyte
count ratio (PLR), have been identified as potential prognostic
markers for a range of malignant tumors, such as lung, gastric and
breast cancer (8,9).
In recent years, there has been a growing interest
in using pan-immune-inflammatory values (PIVs) derived from
peripheral blood parameters to thoroughly assess the
immune-inflammatory response. The PIV is a new biomarker that
integrates peripheral neutrophils, platelets, monocytes and
lymphocytes (neutrophils × platelets × monocytes/lymphocytes)
(10,11), and has demonstrated a good
predictive role in the prognosis of numerous cancer types,
including colorectal, liver cand esophageal cancer (12–14).
Nevertheless, additional evidence is needed to
support the use of PIVs in predicting the prognosis of patients
with CRC. In this regard, the present study aimed to examine the
potential value of PIV in forecasting outcomes for CRC and to
establish a new reference point for clinical decision-making.
Patients and methods
Patients
The present study retrospectively analyzed 470
patients with non-metastatic CRC who underwent radical surgery at
the Cancer Hospital of Xinjiang Medical University (Urumqi, China)
between January 2016 and December 2017. The mean age of the cohort
was 58.3 years, with an age range of 20–87 years. The sample size
was determined based on a priori power analysis to ensure
sufficient statistical power to detect clinically meaningful
differences. With a significance level set at α=0.05, the sample
size provided >80% statistical power to identify significant
differences between groups. This calculation was based on previous
studies (15–18). Inclusion criteria were as follows:
i) Age >18 years old; ii) postoperative pathological diagnosis
of clear CRC; and iii) complete and reliable clinical and follow-up
data. Exclusion criteria were as follows: i) Patients with other
malignant tumors; ii) previous history of blood disease, autoimmune
disease or chronic inflammation; iii) previous history of blood
transfusion; iv) inability to undergo radical surgery; v)
preoperative neoadjuvant chemotherapy; and vi) distant metastasis.
The sample size was determined based on the number of eligible
patients meeting the inclusion and exclusion criteria during the
specified time frame. A post hoc sample size calculation, assuming
a hazard ratio (HR) of 2.0, an event rate of 30% and a power of 80%
at a significance level of 0.05, indicated that 354 patients were
required. The actual sample size of 470 patients exceeded this
requirement, ensuring sufficient statistical power for the analyses
performed. The study set March 2023 as the follow-up cutoff to
ensure that all patients had at least 5 years of follow-up data,
which is critical for analyzing long-term survival outcomes in CRC.
The Ethics Committee of Xinjiang Medical University Cancer Hospital
approved the ethical review of this study after reviewing the study
for compliance with ethical principles (approval no. K-2024056).
Written informed consent was obtained from all participants.
Follow-up
The patients were strictly monitored during
follow-up. The primary endpoint of the study was overall survival
(OS). OS time was defined as the time from surgery to all-cause
death or last follow-up. Follow-up continued until death or March
2023.
Data collection
All patient data were obtained from the electronic
information system of Xinjiang Medical University Cancer Hospital.
The following data variables were collected: Age, sex, body mass
index (BMI), smoking history, alcohol consumption, carcinoembryonic
antigen (CEA) level, preoperative blood counts (lymphocytes,
monocytes, neutrophils and platelets), T stage, N stage,
Tumor-Node-Metastasis (TNM) stage, differentiated degree, nerve
invasion, intravascular tumor emboli and follow-up information
(survival outcome and survival time). Tumor staging was performed
according to the seventh edition of the Union for International
Cancer Control-American Joint Committee on cancer classification
for CRC (19). The calculation
formulae were as follows: PIV=[neutrophil count (109/l)]
× [monocyte count (109/l)] × [platelet count
(109/l)]/[lymphocyte count (109/l)] (16); NLR=[neutrophil count
(109/l)]/[lymphocyte count (109/l)] (20); MLR=[monocyte count
(109/l)]/[lymphocyte count (109/l)] (21); and PLR=[platelet count
(109/l)]/[lymphocyte count (109/l)] (22).
Statistical analysis
Continuous variables are expressed as the median and
interquartile range. Categorical variables are expressed as
frequencies and percentages. In the univariate analysis,
categorical variables were analyzed using the χ2 test or
Fisher's exact test, while continuous variables were assessed using
Student's t-test for unpaired data or the rank-sum test for
non-normally distributed variables. The optimal cutoff values for
continuous variables were determined using maximally selected rank
statistics, which stratified patients into the Low-PIV and High-PIV
groups based on baseline PIV. Survival curves were constructed
using the Kaplan-Meier method and differences between groups were
compared with the log-rank test. Univariate analysis was further
performed using Cox proportional hazards regression. Variables that
were identified as significant (P<0.05) in the univariate
analysis were included in the multivariate analysis to determine
the independent risk factors associated with OS. Nomograms were
constructed to predict OS rate at 1-, 3-, and 5-year
postoperatively based on independent risk factors. The performance
of the nomograms was assessed using the consistency index (C-index)
and receiver operating characteristic curve (ROC). Calibration
curves were used to assess the agreement between predicted and
observed survival. P<0.05 was considered to indicate a
statistically significant difference. All analyses were conducted
using SPSS (version 26.0; IBM Corp.) and R software (version 4.2.3;
http://cran.r-project.org).
Results
Association between preoperative PIV
levels and clinical characteristics
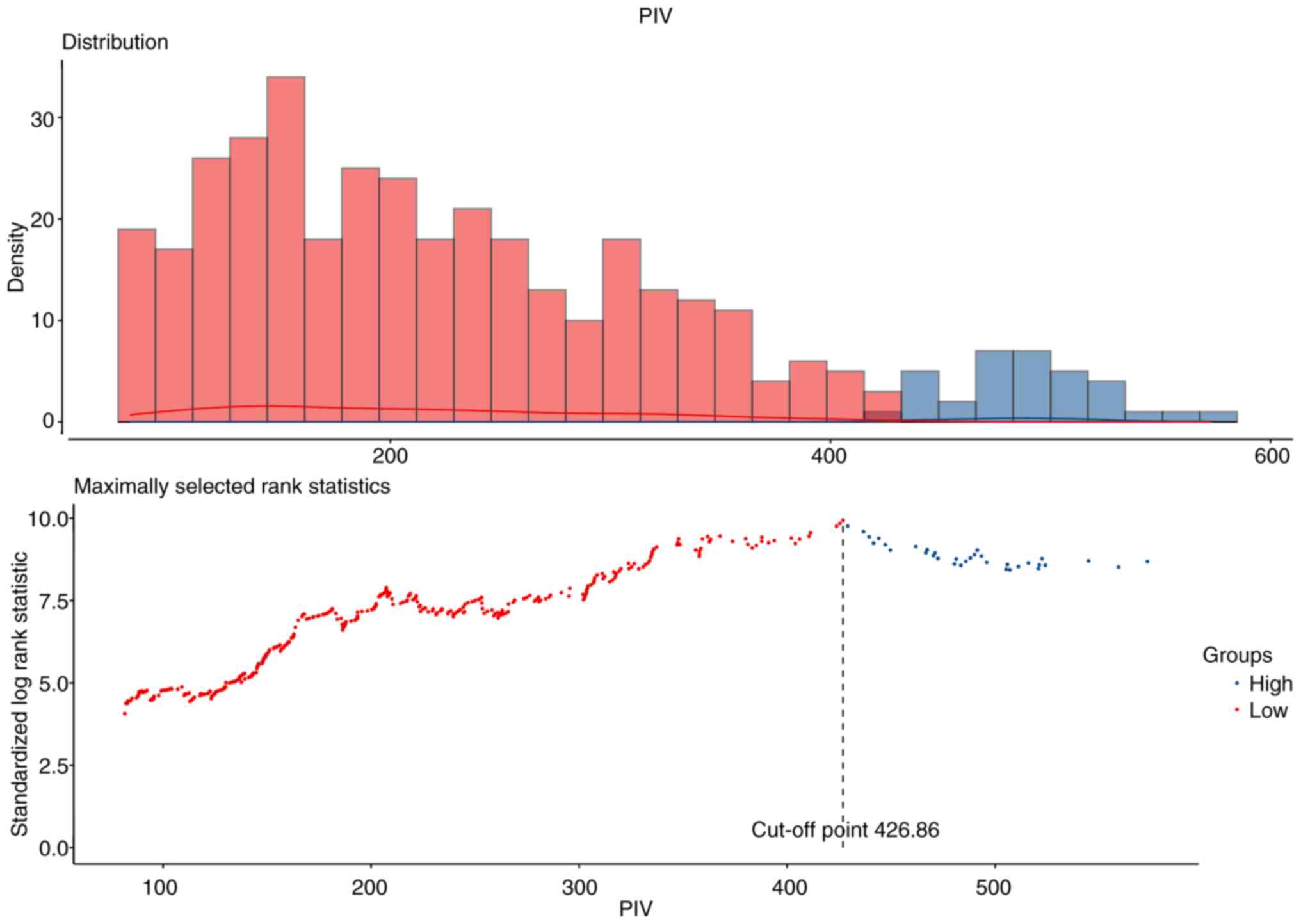
Using maximally selected rank statistics, the
optimal cut-off value for PIV in the entire cohort was 426.86
(Fig. 1). The same methodology was
employed to ascertain the optimal cut-off values for NLR (3.08),
MLR (0.38) and PLR (166.48) (Fig.
S1). A total of 470 patients were included in the study. The
cohort was divided according to the optimal cut-off value,
resulting in 388 patients in the preoperative Low-PIV group and 82
patients in the preoperative High-PIV group. The association
between clinical characteristics and PIV throughout the study is
represented in Table I. There were
no statistically significant differences between the two groups in
terms of age distribution, sex and BMI (P=0.176, P=0.867 and
P=0.375, respectively). Regarding lifestyle factors, history of
smoking (P=0.965) and alcohol consumption (P=0.636) were also not
statistically significantly different between the two groups. Among
the pathological features, T stage, TNM stage, differentiated
degree and nerve invasion were significantly different between the
two groups (all P<0.05). However, N stage, intravascular tumor
emboli and CEA level did not exhibit a significant difference
between the two groups (all P>0.05). Preoperative platelet
count, neutrophil count, monocyte count and lymphocyte count were
239×109/l, 3.62×109/l, 0.45×109/l
and 1.79×109/l, respectively (Table I). Overall, these findings provide
insights into the baseline characteristics of the study population,
underscoring potential areas of discrepancy that may influence
predictive outcomes.
 | Table I.Association between clinical
characteristics and PIV in patients with colorectal cancer. |
Table I.
Association between clinical
characteristics and PIV in patients with colorectal cancer.
|
Characteristics | Overall patients
(n=470) | Low-PIV group
(n=388) | High-PIV group
(n=82) | P-value |
|---|
| Age, n (%) |
|
|
| 0.176 |
| ≥60
years | 209 (44.47) | 167 (43.04) | 42 (51.22) |
|
| <60
years | 261 (55.53) | 221 (56.96) | 40 (48.78) |
|
| Sex, n (%) |
|
|
| 0.867 |
|
Male | 279 (59.36) | 231 (59.54) | 48 (58.54) |
|
|
Female | 191 (40.64) | 157 (40.46) | 34 (41.46) |
|
| BMI, n (%) |
|
|
| 0.375 |
|
<18.5 kg/m2 | 14 (2.98) | 10 (2.58) | 4 (4.88) |
|
| 18.5–24
kg/m2 | 203 (43.19) | 165 (42.53) | 38 (46.34) |
|
| 24–28
kg/m2 | 189 (40.21) | 162 (41.75) | 27 (32.93) |
|
| ≥28
kg/m2 | 64 (13.62) | 51 (13.14) | 13 (15.85) |
|
| Smoking, n (%) |
|
|
| 0.965 |
|
Yes | 150 (31.91) | 124 (31.96) | 26 (31.71) |
|
| No | 320 (68.09) | 264 (68.04) | 56 (68.29) |
|
| Alcohol, n (%) |
|
|
| 0.636 |
|
Yes | 89 (18.94) | 75 (19.33) | 14 (17.07) |
|
| No | 381 (81.06) | 313 (80.67) | 68 (82.93) |
|
| T stage, n (%) |
|
|
| 0.001 |
| T1 | 33 (7.02) | 31 (7.99) | 2 (2.44) |
|
| T2 | 71 (15.11) | 66 (17.01) | 5 (6.10) |
|
| T3 | 335 (71.28) | 271 (69.85) | 64 (78.05) |
|
| T4 | 31 (6.60) | 20 (5.15) | 11 (13.41) |
|
| N stage, n (%) |
|
|
| 0.713 |
| N0 | 282 (60.00) | 236 (60.82) | 46 (56.10) |
|
| N1 | 112 (23.83) | 90 (23.20) | 22 (26.83) |
|
| N2 | 76 (16.17) | 62 (15.98) | 14 (17.07) |
|
| TNM stage, n
(%) |
|
|
| 0.014 |
| I | 94 (20.00) | 87 (22.42) | 7 (8.54) |
|
| II | 190 (40.43) | 150 (38.66) | 40 (48.78) |
|
|
III | 186 (39.57) | 151 (38.92) | 35 (42.68) |
|
| Differentiated
degree, n (%) |
|
|
| 0.005 |
|
Poorly | 73 (15.53) | 52 (13.40) | 21 (25.61) |
|
|
Moderately | 385 (81.91) | 328 (84.54) | 57 (69.51) |
|
|
Well | 12 (2.55) | 8 (2.06) | 4 (4.88) |
|
| Nerve invasion, n
(%) |
|
|
| 0.044 |
|
Positive | 84 (17.87) | 63 (16.24) | 21 (25.61) |
|
|
Negative | 386 (82.13) | 325 (83.76) | 61 (74.39) |
|
| Intravascular tumor
emboli, n (%) |
|
|
| 0.377 |
|
Positive | 87 (18.51) | 69 (17.78) | 18 (21.95) |
|
|
Negative | 383 (81.49) | 319 (82.22) | 64 (78.05) |
|
| CEA, n (%) |
|
|
| 0.192 |
|
High | 171 (36.38) | 136 (35.05) | 35 (42.68) |
|
|
Normal | 299 (63.62) | 252 (64.95) | 47 (57.32) |
|
| PLT
(×109/l)a | 239.00 (198.00,
290.00) | 231.00 (191.00,
268.00) | 316.50 (250.25,
374.50) |
|
| NE
(×109/l)a | 3.62 (2.92,
4.52) | 3.38 (2.84,
4.03) | 5.60 (4.65,
6.62) |
|
| MONO
(×109/l)a | 0.45 (0.35,
0.56) | 0.42 (0.33,
0.51) | 0.64 (0.56,
0.78) |
|
| LY
(×109/l)a | 1.79 (1.45,
2.19) | 1.84 (1.50,
2.20) | 1.56 (1.30,
2.10) |
|
Survival analysis
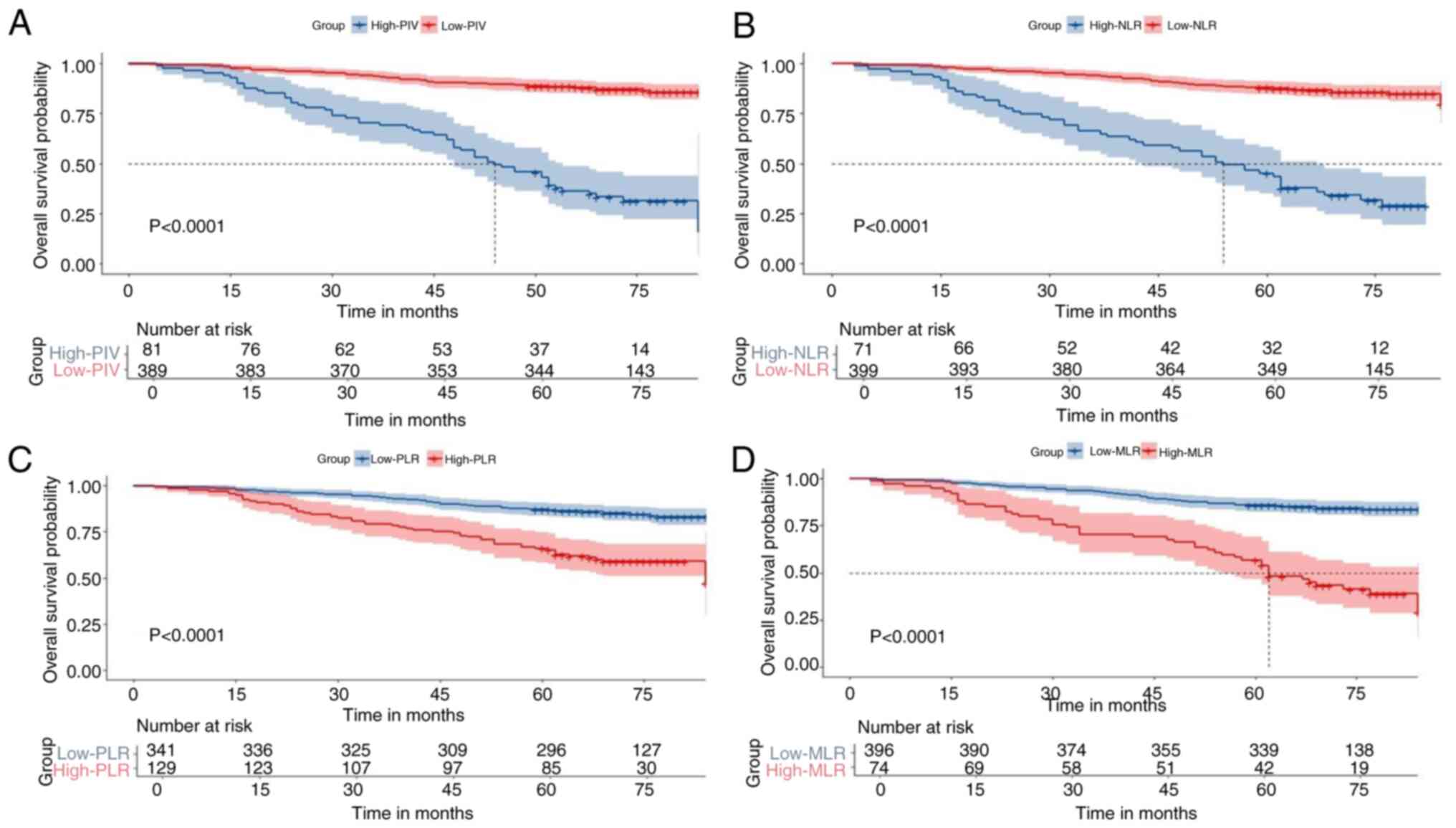
The cohort was divided into High-PIV and Low-PIV
groups based on the optimal cut-off value. A 5-year OS analysis
demonstrated that patients in the Low-PIV group exhibited a
significantly higher OS rate than those in the High-PIV group (88.7
vs. 46.3%; P<0.001; Fig. 2A). In
addition, patients were grouped according to the best cut-off
values of other peripheral blood inflammation indicators. In terms
of survival outcomes, patients in the Low-NLR group (NLR <3.08)
had a significantly higher 5-year OS rate than patients in the
High-NLR group (NLR ≥3.08) (87.6 vs. 45.1%; P<0.001; Fig. 2B), and patients in the Low-PLR group
(PLR <166.48) had a significantly higher 5-year OS rate than
patients in the High-PLR group (PLR ≥166.48) (87.1 vs. 65.9%;
P<0.001; Fig. 2C). Similarly, in
the MLR subgroup, patients in the Low-MLR group (MLR <0.38) had
a significantly higher 5-year OS rate than patients in the High-MLR
group (MLR ≥0.038) (85.9 vs. 56.8%; P<0.001; Fig. 2D). In addition, a subgroup survival
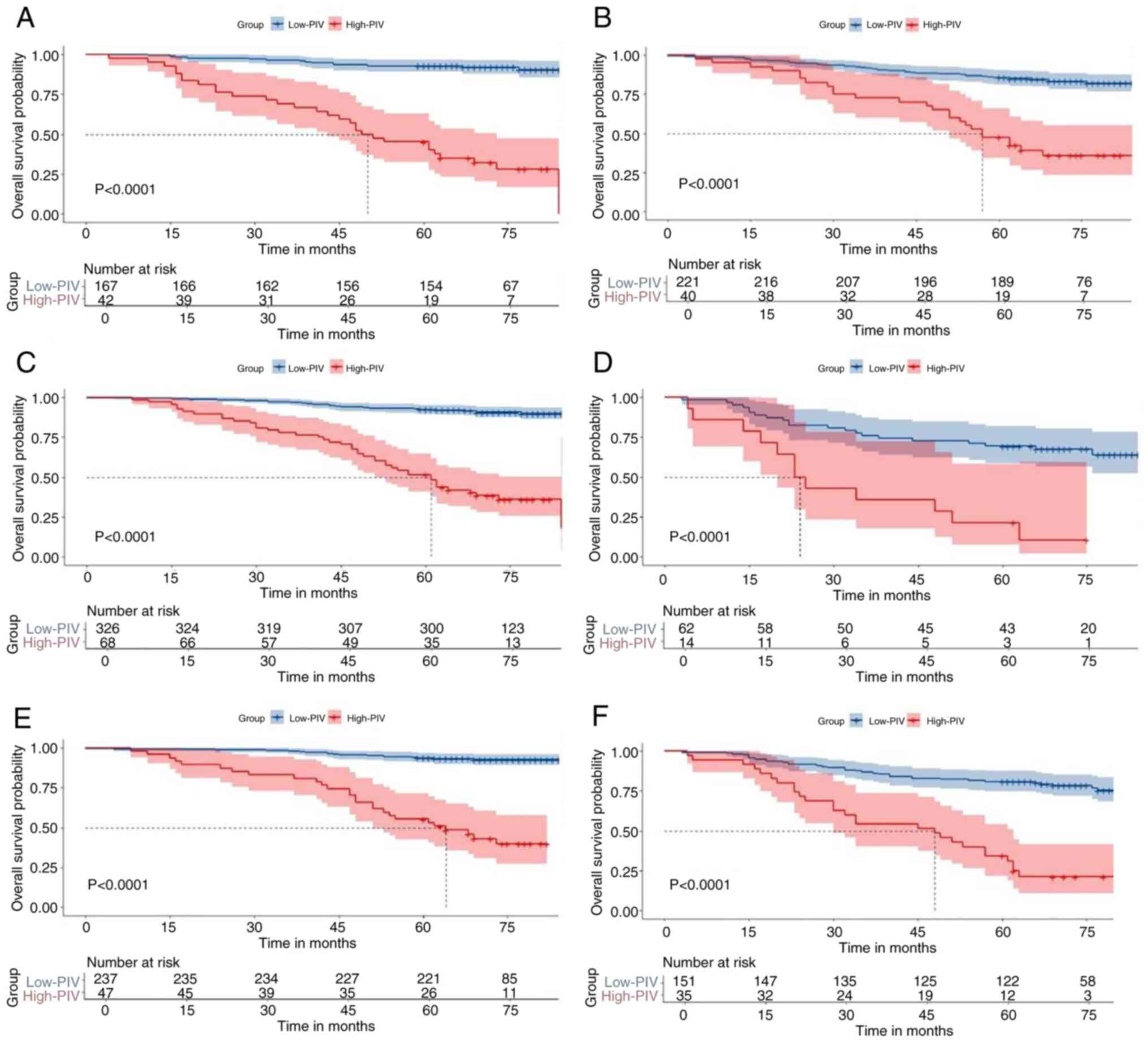
analysis was performed to assess the prognostic value of PIV.
Patients were categorized into elderly (age ≥60 years) and
non-elderly (age <60 years) subgroups according to their age.
Both in the non-elderly group (84.0 vs. 46.9%, P<0.001; Fig. 3A) and the elderly group (89.7 vs.
43.9%, P<0.001; Fig. 3B), the
5-year OS was significantly higher in the Low-PIV group than that
in the High-PIV group.
Additionally, patients were divided into N0/1 and N2
subgroups according to their N stage. The 5-year OS was
significantly higher in the Low-PIV group compared with the
High-PIV group in both the N01 group (90.3 vs. 51.5%, P<0.001;
Fig. 3C) and the N2 group (70 vs.
20%, P<0.001; Fig. 3D). Patients
were categorized into stage I/II and stage III subgroups according
to their Tumor stage. In the stage I/II group (51.2 vs. 88.8%,
P<0.001; Fig. 3E) and the stage
III group (34.9 vs. 81%, P<0.001; Fig. 3F), the 5-year OS was significantly
worse in the High-PIV group compared with that in the Low-PIV
group. Other subgroup analyses provided similar results. In
multiple subgroups, including sex, age, BMI and various tumor
characteristics (T stage, N stage and TNM stage), high PIV was
consistently associated with poorer overall survival (Fig. 4).
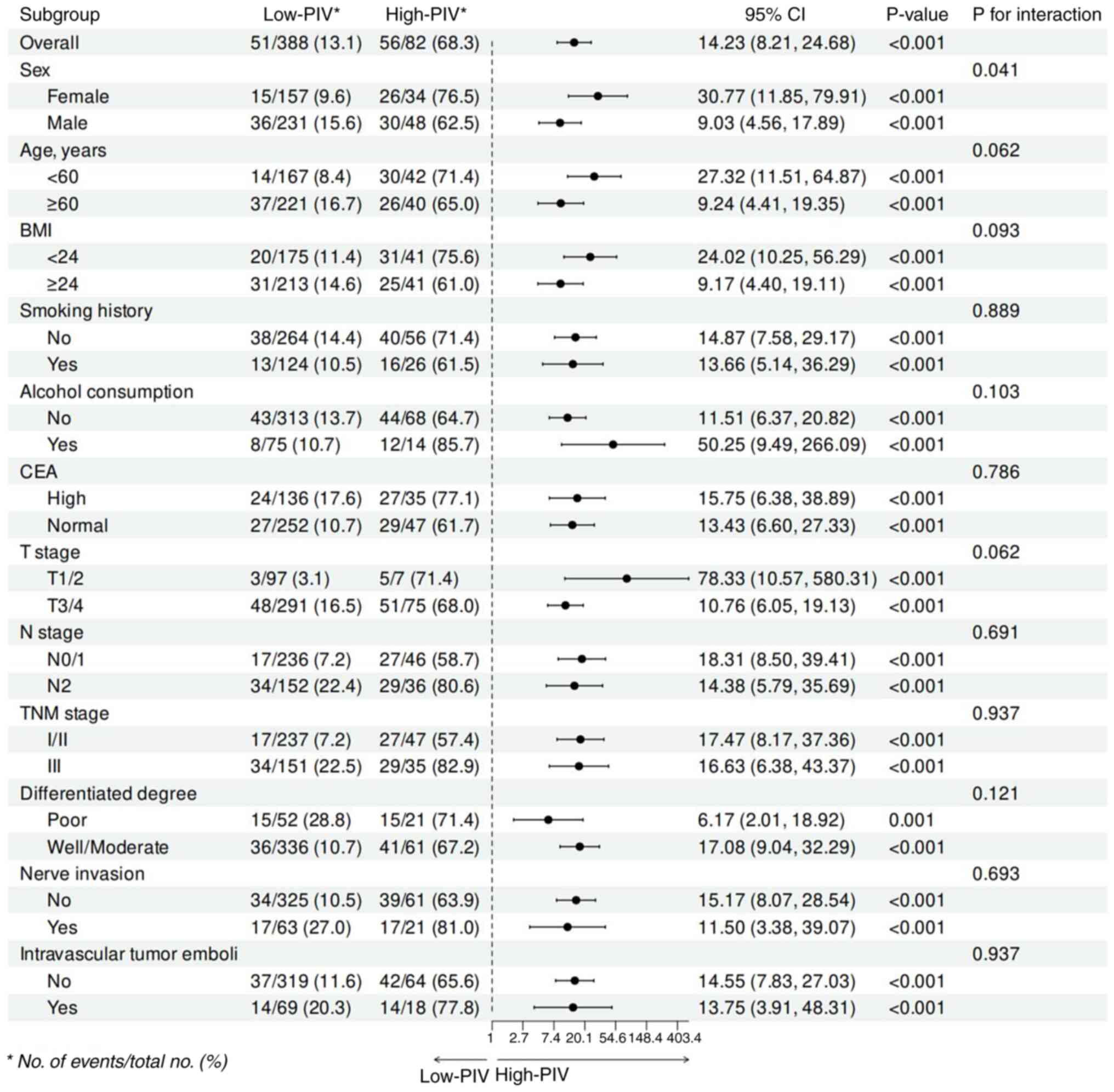 | Figure 4.Subgroup analyses of the association
between the PIV and overall survival risk. Subgroup analysis
adjusted for age, sex, BMI, smoking history and alcohol
consumption, CEA, T stage, N stage, TNM stage, differentiated
degree, nerve invasion and intravascular tumor emboli. BMI, body
mass index; CEA, carcinoembryonic antigen; TNM,
Tumor-Node-Metastasis; PIV, pan-immune-inflammatory value. |
Univariate and multivariate Cox
regression analyses
Table II shows the
results of the univariate and multivariate Cox regression analyses.
In the univariate analysis of variance, T stage (HR, 3.65; 95% CI,
1.58-8.43; P<0.001), N stage (HR, 3.13; 95% CI, 1.92-5.11;
P<0.001), TNM stage (HR, 1.96; 95% CI, 1.23-3.12; P=0.005),
differentiated degree (HR, 3.24; 95% CI, 1.98-5.29; P<0.001),
Nerve invasion (HR, 3.20; 95% CI, 1.98-5.18; P<0.001),
Intravascular tumor emboli (HR, 1.92; 95% CI, 1.16-3.20; P=0.012),
CEA level (HR, 1.66; 95% CI, 1.04-2.64; P=0.033), NLR (HR, 8.39;
95% CI, 5.24-13.43; P<0.001), PLR (HR, 3.19; 95% CI, 2.00-5.06;
P<0.001), MLR (HR, 5.68; 95% CI, 3.55-9.08; P<0.001) and PIV
(HR, 8.74; 95% CI, 5.47-13.97; P<0.001) exhibited significant
differences between the High-PIV and Low-PIV groups. Poor
differentiation was observed in 13.40% of the High-PIV group
compared with 25.61% of the Low-PIV group (P=0.014), while nerve
invasion was present in 16.24% of the High-PIV group compared with
25.61% of the Low-PIV group (P=0.005). These findings suggest that
these variables may influence survival outcomes and interact with
PIV in predicting prognosis. Subsequently, variables that
demonstrated significant prognostic value in the univariate
analysis were included in a multifactorial regression framework to
identify independent risk factors. The results of the
multifactorial analysis showed that N stage (HR, 2.00; 95% CI,
1.04-3.84; P=0.039), differentiated degree (HR, 1.98; 95% CI,
1.16-3.38; P=0.012), NLR (HR, 4.00; 95% CI, 2.19-7.29; P<0.001)
and PIV (HR, 4.12; 95% CI, 2.04-8.32; P<0.001) were independent
predictors of OS.
 | Table II.One-way multifactor regression
analyses were performed using the Cox proportional risk model. |
Table II.
One-way multifactor regression
analyses were performed using the Cox proportional risk model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥48 vs. <48
years) | 1.25
(0.78-2.00) | 0.354 | - | - |
| Sex (male vs.
female) | 0.74
(0.46-1.20) | 0.221 | - | - |
| BMI (<18.5 vs.
≥18.5 kg/m2) | 0.85
(0.53-1.34) | 0.480 | - | - |
| Smoking history
(yes vs. no) | 1.33
(0.78-2.27) | 0.290 | - | - |
| Alcohol consumption
(yes vs. no) | 0.93
(0.52-1.67) | 0.811 | - | - |
| T stage (T3/4 vs.
T1/2) | 3.65
(1.58-8.43) | <0.001 | 1.14
(0.46-2.86) | 0.775 |
| N stage (N2 vs.
N0/1) | 3.13
(1.92-5.11) | <0.001 | 2.00
(1.04-3.84) | 0.039 |
| TNM stage (I/II vs.
III) | 1.96
(1.23-3.12) | 0.005 | 1.15
(0.63-2.10) | 0.644 |
| Differentiated
degree (well/moderate vs. poor) | 3.24
(1.98-5.29) | <0.001 | 1.98
(1.16-3.38) | 0.012 |
| Nerve invasion (yes
vs. no) | 3.20
(1.98-5.18) | <0.001 | 1.66
(0.95-2.90) | 0.076 |
| Intravascular tumor
emboli (yes vs. no) | 1.92
(1.16-3.20) | 0.012 | 1.14
(0.63-2.05) | 0.671 |
| CEA (high vs.
normal) | 1.66
(1.04-2.64) | 0.033 | 1.45
(0.85-2.49) | 0.175 |
| NLR group (≥ 3.08
vs. <3.08) | 8.39
(5.24-13.43) | <0.001 | 4.00
(2.19-7.29) | <0.001 |
| PLR group (≥166.48
vs. <166.48) | 3.19
(2.00-5.06) | <0.001 | 1.10
(0.63-1.93) | 0.745 |
| MLR group (≥0.38
vs. <0.38) | 5.68
(3.55-9.08) | <0.001 | 1.02
(0.48-2.17) | 0.955 |
| PIV group (≥426.86
vs. <426.86) | 8.74
(5.47-13.97) | <0.001 | 4.12
(2.04-8.32) | <0.001 |
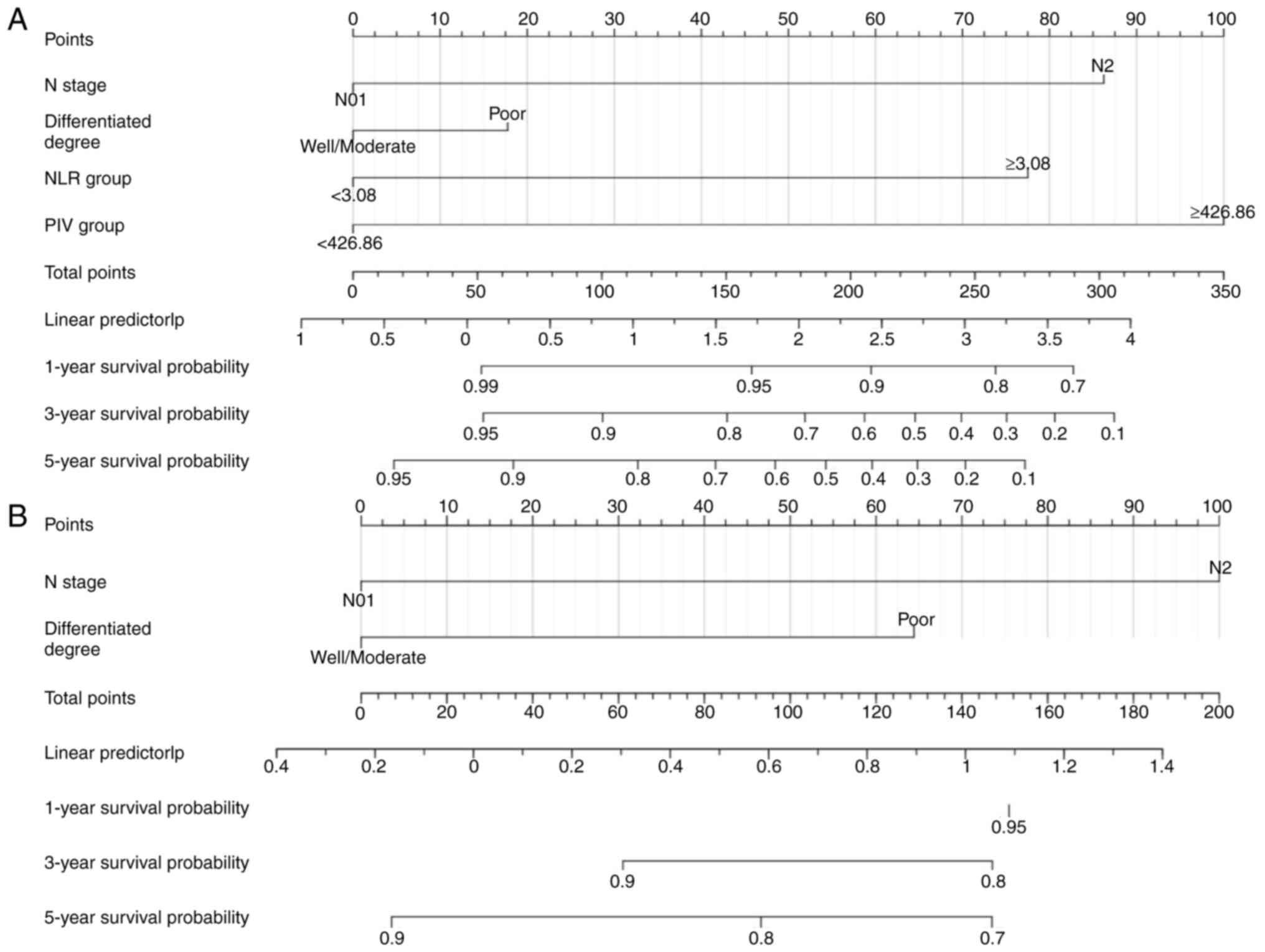
Construction and validation of
nomogram model
Nomogram models were constructed to predict 1-, 3-
and 5-year OS based on independent risk factors identified through
multifactorial Cox proportional risk regression (Fig. 5A and B). The C-index of the
conventional nomogram, which included the N stage and
differentiated degree, was 0.642. The C-index of the nomogram based
on inflammatory indicators, including N staging, differentiated
degree, NLR and PIV, was 0.789 (95% CI, 0.746-0.832). Adding an
inflammation index to the conventional model improved the
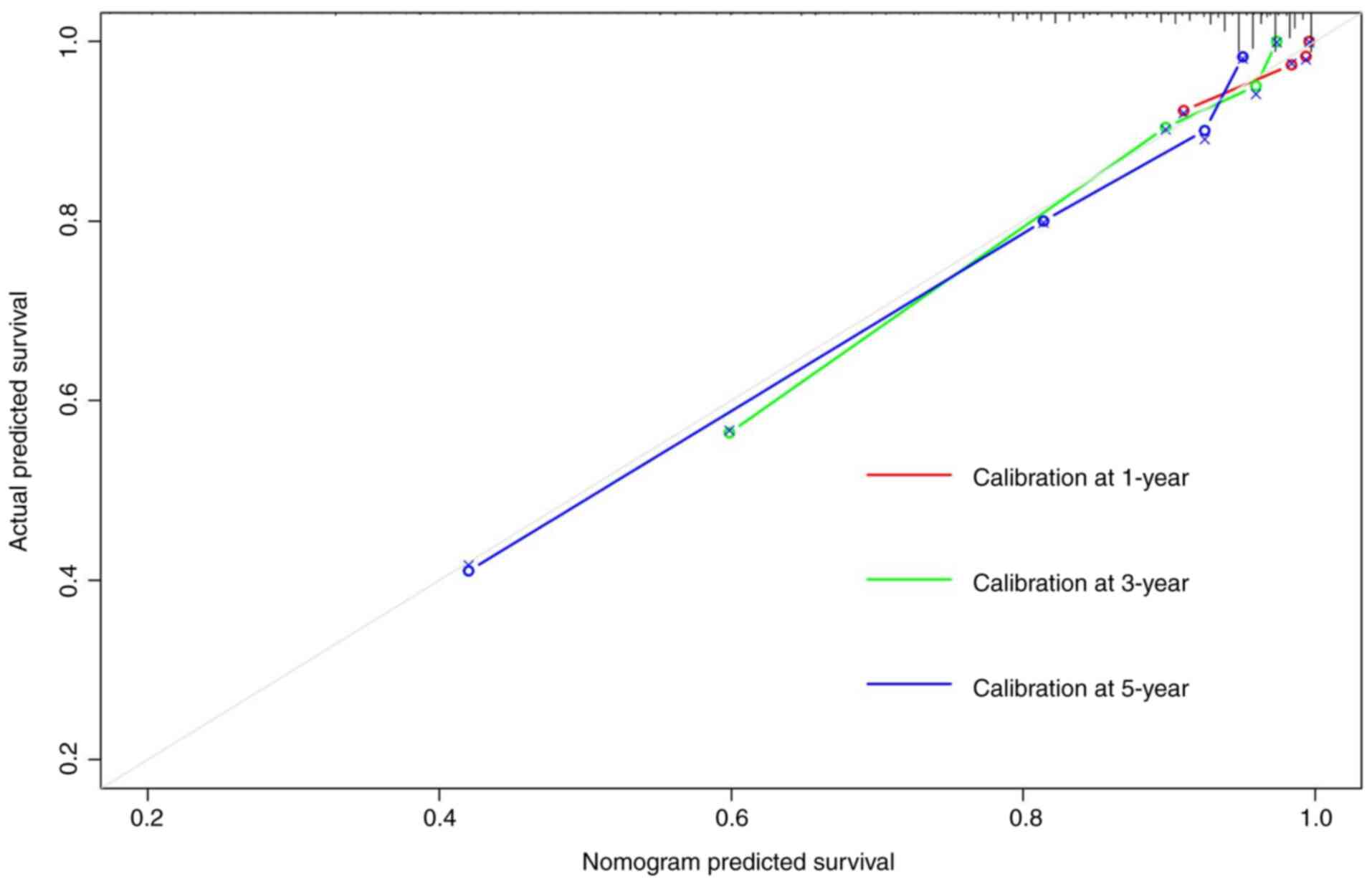
prediction of 5-year OS. The calibration curves of the 1-, 3- and
5-year survival rates of the nomogram prediction model based on
inflammation indicators were close to the ideal curve, suggesting
that the predicted survival probability of the whole cohort had a
good calibration relationship with the actual survival probability
(Fig. 6). The ROC analysis showed
that the area under the curve (AUC) values of the nomogram model
for predicting the 1-, 3- and 5-year OS were 0.823, 0.845 and
0.845, respectively, demonstrating that the model had a much better
predictive ability than TNM staging or PIV alone (Fig. S2; Table III).
 | Table III.Evaluation of predictive models for
OS. |
Table III.
Evaluation of predictive models for
OS.
| AUC | Nomogram | TNM | PIV |
|---|
| 1-year OS | 0.823 | 0.582 | 0.772 |
| 3-year OS | 0.845 | 0.753 | 0.761 |
| 5-year OS | 0.845 | 0.580 | 0.751 |
Discussion
CRC is a gastrointestinal tumor that is becoming
increasingly prevalent worldwide. Determining the prognosis of
patients with CRC remains a significant challenge, and the
identification of optimal prognostic markers requires further
investigation and validation. To the best of our knowledge, the
present study is the first to discuss the impact of PIV and
survival status in patients with non-metastatic CRC. This
retrospective study collected baseline blood parameters and
clinical information from 470 patients and analyzed them to examine
the association between PIV and clinical prognosis. In the present
study, significant differences were found in key clinical
characteristics, such as the degree of differentiation and nerve
invasion, between the High-PIV and Low-PIV groups. These variables
may themselves be independent determinants of disease prognosis,
suggesting that the between-group differences may have had a
confounding effect on the predictive ability of PIV as a prognostic
indicator. To minimize this effect, adjustments for these
potentially confounding variables were made in multivariate
analyses, which showed that PIV still had significant independent
predictive value. The results of the survival analysis indicated
that patients in the High-PIV group were significantly associated
with a poor prognosis. Furthermore, the nomogram model was
constructed based on multivariate Cox regression analysis to
predict the prognosis of patients with CRC. Subsequently, a
comprehensive assessment was conducted on the predictive
performance of the nomogram model, which demonstrated robust
discriminatory and predictive capabilities. This indicates that PIV
may serve as a potential marker for identifying patients with
potential adverse clinical outcomes.
The significant association observed between PIV and
survival outcomes may stem from the intricate interplay between
inflammation and cancer progression. Inflammation has an important
role in the development and progression of CRC (23). As a comprehensive biomarker, PIV
contains various components of the systemic inflammatory response,
including neutrophils, monocytes, platelets and lymphocytes.
Neutrophils exhibit a dual role in tumor biology
(24). The regulation of the tumor
microenvironment and the production of cytokines, chemokines and
growth factors by neutrophils can facilitate the removal of tumor
cells under certain conditions (25). However, this same process can also
directly promote tumor progression, metastasis and angiogenesis
(26). Additionally, neutrophils
are capable of producing interleukins (ILs) and other
tumor-associated factors that are involved in tumor invasion and
metastasis (27). For instance,
IL-1β is involved in cell proliferation, differentiation and
apoptosis, while also promoting the production of angiogenic
factors by stromal cells within the tumor microenvironment (TME).
These factors induce tumor angiogenesis, endothelial cell
activation and the development of immunosuppressive cells.
Secondly, some neutrophils also promote epithelial-mesenchymal
transition through the TGF-β/Smad signaling pathway (28). This is also considered to be a key
factor in tumor development and progression (29).
Monocytes are the origin of tumor-associated
macrophages, dendritic cells and myeloid-derived suppressor cells,
which control the immune response and cancer cell biology in the
TME (30). Monocytes contribute to
both cancer development and progression, with various
subpopulations exhibiting functions such as phagocytosis, secretion
of tumor-killing mediators, promotion of angiogenesis, remodeling
of the extracellular matrix, recruitment of lymphocytes, and
differentiation into tumor-associated macrophages and dendritic
cells (31,32). Monocytes can generate antitumor
responses and activate antigen-presenting cells to exert antitumor
effects (33).
Several mechanisms have been identified whereby
platelets are involved in the development and progression of cancer
(34). It seems that platelet
activation is a crucial factor in the growth of tumors and the
successful establishment of metastatic colonies. The activation of
platelets releases a multitude of factors that regulate the tumor
microenvironment, including vascular endothelial growth factor and
fibroblast growth factor, lipids and extracellular vesicles rich in
genetic material (35). These
substances induce phenotypic alterations in target cells, including
immune, stromal and tumor cells, thereby facilitating the formation
of cancerous lesions and metastases (36). There is a significant degree of
interaction between platelets and cancer cells during the
progression of cancer. Activation of platelets results in the
modulation of the migration of hematopoietic and immune cells
towards the tumor site, thereby exacerbating cancer-associated
inflammation (37). Furthermore,
the activation of platelets enables cancer cells to utilize them as
a physical barrier against blood shear forces and natural killer
cells (38). Evidence suggests that
inhibiting platelet function may impede tumor growth, thereby
enhancing overall patient survival (39–41).
Lymphocytes associated with the inflammatory
response are involved in the formation of the association between
tumor cells and the surrounding microenvironment. Lymphocytes also
serve a dual function in the progression of cancer (42). On the one hand, lymphocytes can
induce apoptosis in tumor cells by triggering an antitumor response
within the immune system (43). On
the other hand, activated lymphocytes inhibit the proliferation of
CD4+CD25− and CD8+CD25+
T cells, leading to immunosuppression and thus inhibiting the
immune attack on tumor cells (44,45).
As a peripheral blood cell-based biomarker, the PIV
integrates different peripheral blood immune cell subsets. A
meta-analysis of the prognostic value of PIV in patients with CRC,
comprising 1,879 subjects across six studies, revealed that
patients in the high baseline PIV group exhibited inferior OS rates
and progression-free survival (PFS) rates compared with those in
the low baseline PIV group (14).
Furthermore, in a retrospective study by Zhao et al
(46), PIV was found to be
significantly different in patients with different pathological N
and TNM stages (P<0.05), which may aid in assessing tumor
staging based on preoperative PIV. The lack of significant
prognostic value for T staging in the present dataset may be
attributed to the relatively homogenous distribution of T staging
among patients, with a predominance of T3 cases. This imbalance
could reduce the ability to detect meaningful differences.
Additionally, the sample size in early (T1-T2) and advanced (T4)
subgroups may have been insufficient to achieve statistical power.
Future studies with larger and more balanced cohorts are needed to
confirm the prognostic role of T staging in non-metastatic CRC.
Notably, PIV also has a potential role in monitoring disease
progression in patients with metastatic CRC receiving first-line
chemotherapy (47). One study found
that PIV not only serves as an independent prognostic factor for
OS, but that it can also predict the occurrence of postoperative
complications in CRC (48). The
current study aligns with previous findings that the High-PIV group
exhibited a higher tumor stage and poorer survival outcomes
compared with the Low-PIV group.
The present study has several strengths. First, it
provides a novel predictive model based on the PIV, which
integrates multiple inflammatory components into a single
biomarker. Compared with traditional tumor markers, the PIV offers
a more comprehensive assessment of tumor-host interactions. Second,
the study included a relatively large cohort of patients with
non-metastatic CRC, ensuring robust statistical power and reliable
conclusions. Third, the nomogram model demonstrated excellent
predictive performance, as indicated by its C-index and AUC values.
Compared with prior research (49),
which primarily focused on individual inflammatory markers such as
NLR, PLR and MLR, the present study highlights the superior
predictive value of PIV, a composite biomarker. The significant
associations between the risk scores of the nomogram model and the
clinicopathological factors, such as differentiation and nerve
invasion, highlight its clinical value. By reflecting established
prognostic variables, the model demonstrates its applicability in
real-world clinical settings. Future studies should validate its
use across diverse cohorts to ensure generalizability. The
association of the PIV with prognosis has been reported in other
cancer types, including lung (49),
breast (50), and esophageal
(15) cancer, further supporting
its potential utility in oncology. Future research should validate
the nomogram model using larger, multicenter cohorts to improve
generalizability. Investigating the biological mechanisms
underlying the prognostic value of the PIV, including its role in
immune escape and metastasis, will provide deeper insights.
Additionally, dynamic monitoring of the PIV during treatment may
enhance its applicability in clinical practice.
The present study does, however, have a few
limitations. Firstly, this is a single-center, retrospective study
with a small sample size and there may be selective bias. While the
present study included 470 patients, which exceeds the required
sample size based on statistical power calculations, there are
limitations to consider. As a single-center, retrospective study,
the generalizability of the findings may be constrained by the
homogeneity of the study population. The patient cohort represents
a specific geographic and institutional context, which might limit
the applicability of these results to broader, more diverse
populations. Moreover, although the sample size was sufficient to
meet statistical requirements, a larger, multicenter cohort would
provide greater external validation and strengthen the reliability
of the findings. Future prospective studies involving multiple
centers and larger sample sizes are warranted to confirm the
prognostic value of PIV in non-metastatic CRC and further validate
the constructed nomogram model. These efforts would not only
enhance the robustness of the findings, but also facilitate the
development of widely applicable, individualized prognostic tools
to guide clinical decision-making. Prospective studies with larger
sample sizes are needed to validate the current results. Second,
the patients were only from one institution, and external
validation was not possible. It would have been better if external
validation could have been performed to verify the general
applicability of the present findings. Thirdly, this study only
analyzed patients with non-metastatic CRC and could not be
replicated in a wider population. This limits the generalizability
of the findings to metastatic CRC and other tumor types. Therefore,
there is an urgent need for future studies to conduct multicenter,
large-scale prospective studies supplemented by external validation
to strongly improve the reliability and scientific validity of the
findings. In future studies, the present authors plan to
incorporate molecular biomarkers, expand the sample size, and
validate the performance of the model at longer follow-up times and
in a broader population. Fourthly, the present study combined the
N0 and N1 groups into one category for the purpose of statistical
analysis. We acknowledge that N stage is a critical factor
influencing the prognosis of CRC, and that N0 and N1 stages usually
have distinct prognostic implications. However, due to the limited
sample size in the cohort, separating these two stages for analysis
could have resulted in insufficient statistical power, particularly
in subgroup analyses. This could have introduced additional
variability and uncertainty into the results. Preliminary analyses
indicated that the survival outcomes of the patients in the N0 and
N1 groups were relatively similar within the cohort. This
observation led to the merging of these groups to enhance the
robustness of the statistical analyses and to maintain a meaningful
comparison between groups. Additionally, this grouping strategy
allowed better control for potential confounding factors in the
models. Despite these considerations, we recognize that this
grouping strategy could obscure finer differences between the N0
and N1 stages, which may affect the precision of the conclusions.
The lack of a sufficient sample size to analyze these groups
individually is a limitation of the current study. Future studies
with larger sample sizes should explore the individual prognostic
significance of N0 and N1 stages in greater detail to confirm
whether the observed similarities in survival outcomes are
generalizable. In addition, the integration of PIV into routine
clinical practice still requires further clinical validation and
standardization work to ensure its reliable application in
different diseases and populations. Nevertheless, the present study
further demonstrated that PIV can be an independent prognostic
factor for the prognosis of patients with CRC. Since elevated
preoperative PIV is a poor prognostic factor and is independently
associated with an increased risk of a poor prognosis, identifying
the inflammatory status of patients preoperatively can help predict
their survival and provide timely prophylaxis to patients. In
addition, it may help healthcare professionals make informed
treatment choices and follow-up strategies.
In conclusion, in the present study, the PIV was
found to be an independent predictor of prognosis in patients with
non-metastatic colorectal. In addition, a new nomogram model was
developed, which showed good calibration and the ability to
distinguish outcomes. Therefore, the model can be used as an
effective tool to identify patients at high risk for adverse
outcomes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Natural Science
Foundation of Xinjiang Uygur Autonomous Region (project no.
2022D01C297).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KJL and ZLZ contributed to study concept and design.
ZYZ collected clinical data. YC, YPP, ZMW, KW and XYZ contributed
to analyze the data. KJL contributed to the preparation of the
manuscript. ZLZ provided critical feedback on methods and
supervised the study. ZLZ and YC confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The guidelines of the Declaration of Helsinki were
followed during the investigation. The study was approved by the
Ethics Committee of the Affiliated Cancer Hospital, Xinjiang
Medical University (Urumqi, China; approval no. K-2024056). All
methods were performed in accordance with relevant guidelines and
regulations. Written informed consent was obtained from each
patient or their guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. Cancer J Clinicians. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zauber AG, van Ballegooijen M and Schapiro
M: Colonoscopic polypectomy and Long-term prevention of
Colorectal-cancer deaths. N Engl J Med. 366:687–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmitt M and Greten FR: The inflammatory
pathogenesis of colorectal cancer. Nat Rev Immunol. 21:653–667.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Chen Y, Wang F, Lin J, Tan X, Chen
C, Wu LL, Zhang X, Wang Y, Shi Y, et al: Caveolin-1 promotes glioma
progression and maintains its mitochondrial inhibition resistance.
Discov Oncol. 14:1612023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto T, Kawada K and Obama K:
Inflammation-related biomarkers for the prediction of prognosis in
colorectal cancer patients. Int J Mol Sci. 22:80022021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Shi Y, Ying J, Chen Y, Guo R,
Zhao X, Jia L, Xiong J and Jiang F: A bibliometric and visualized
research on global trends of immune checkpoint inhibitors related
complications in melanoma, 2011–2021. Front Endocrinol.
14:11646922023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L and Lv Y: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis: Neutrophil-to-Lymphocyte Ratio
in Colorectal Cancer. Int J Cancer. 134:2403–2413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu L, Li H, Chen L, Ma X, Li X, Gao Y,
Zhang Y, Xie Y and Zhang X: Prognostic role of lymphocyte to
monocyte ratio for patients with cancer: Evidence from a systematic
review and meta-analysis. Oncotarget. 7:319262016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turan YB: The prognostic importance of the
pan-immune-inflammation value in patients with septic shock. BMC
Infect Dis. 24:692024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Z, Luo Y, Zhang Y, Wang Y, Chen Y,
Xu Y, Peng H and Zhang G: A novel NAP1L4/NUTM1 fusion arising from
translocation t(11;15)(p15;q12) in a myeloid neoplasm with
eosinophilia and rearrangement of PDGFRA highlights an unusual
clinical feature and therapeutic reaction. Ann Hematol.
99:1561–1564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fucà G, Guarini V, Antoniotti C, Morano F,
Moretto R, Corallo S, Marmorino F, Lonardi S, Rimassa L,
Sartore-Bianchi A, et al: The pan-immune-inflammation value is a
new prognostic biomarker in metastatic colorectal cancer: Results
from a pooled-analysis of the Valentino and TRIBE first-line
trials. Br J Cancer. 123:403–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corti F, Lonardi S, Intini R, Salati M,
Fenocchio E, Belli C, Borelli B, Brambilla M, Prete AA, Quarà V, et
al: The pan-immune-inflammation value in microsatellite
instability-high metastatic colorectal cancer patients treated with
immune checkpoint inhibitors. Eur J Cancer. 150:155–167. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XC, Liu H, Liu DC, Tong C, Liang XW
and Chen RH: Prognostic value of pan-immune-inflammation value in
colorectal cancer patients: A systematic review and meta-analysis.
Front Oncol. 12:10368902022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baba Y, Nakagawa S, Toihata T, Harada K,
Iwatsuki M, Hayashi H, Miyamoto Y, Yoshida N and Baba H:
Pan-immune-inflammation value and prognosis in patients with
esophageal cancer. Ann Surg Open. 3:e1132022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Şahin AB, Cubukcu E, Ocak B, Deligonul A,
Oyucu Orhan S, Tolunay S, Gokgoz MS, Cetintas S, Yarbas G, Senol K,
et al: Low pan-immune-inflammation-value predicts better
chemotherapy response and survival in breast cancer patients
treated with neoadjuvant chemotherapy. Sci Rep. 11:146622021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Liu C, Yang N and Qiu C:
Pan-immune-inflammation value: A new prognostic index in operative
laryngeal and pharyngeal carcinomas. Clin Transl Oncol. 27:151–159.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ligorio F, Fucà G, Zattarin E, Lobefaro R,
Zambelli L, Leporati R, Rea C, Mariani G, Bianchi GV, Capri G, et
al: The Pan-immune-inflammation-value predicts the survival of
patients with human epidermal growth factor receptor 2
(HER2)-positive advanced breast cancer treated with first-line
Taxane-trastuzumab-pertuzumab. Cancers (Basel). 13:19642021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th Edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buonacera A, Stancanelli B, Colaci M and
Malatino L: Neutrophil to lymphocyte Ratio: An emerging marker of
the relationships between the immune system and diseases. Int J Mol
Sci. 23:36362022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tao BF, Zhu HQ, Qi LN, Zhong JH, Mai R and
Ma L: Preoperative monocyte-to-lymphocyte ratio as a prognosis
predictor after curative hepatectomy for intrahepatic
cholangiocarcinoma. BMC Cancer. 24:11792024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gasparyan AY, Ayvazyan L, Mukanova U,
Yessirkepov M and Kitas GD: The Platelet-to-Lymphocyte ratio as an
inflammatory marker in rheumatic diseases. Ann Lab Med. 39:3452019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wesselink E, Balvers MGJ, Kok DE, Winkels
RM, van Zutphen M, Schrauwen RWM, Keulen ETP, Kouwenhoven EA,
Breukink SO, Witkamp RF, et al: Levels of inflammation markers are
associated with the risk of recurrence and All-cause mortality in
patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev.
30:1089–1099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Zhang Y, Wang Z, Wang Y, Luo Y,
Sun N, Zheng S, Yan W, Xiao X, Liu S, et al: CHST15 gene germline
mutation is associated with the development of familial
myeloproliferative neoplasms and higher transformation risk. Cell
Death Dis. 13:5862022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Zhao S, Chen Y, Ma W, Lu S, He L,
Chen J, Chen X, Zhang X, Shi Y, et al: Vimentin promotes glioma
progression and maintains glioma cell resistance to oxidative
phosphorylation inhibition. Cell Oncol. 46:1791–1806. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quail DF, Amulic B, Aziz M, Barnes BJ,
Eruslanov E, Fridlender ZG, Goodridge HS, Granot Z, Hidalgo A,
Huttenlocher A, et al: Neutrophil phenotypes and functions in
cancer: A consensus statement. J Exp Med. 219:e202200112022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Apte RN, Dotan S, Elkabets M, White MR,
Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y and Voronov E: The
involvement of IL-1 in tumorigenesis, tumor invasiveness,
metastasis and tumor-host interactions. Cancer Metastasis Rev.
25:387–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HJ, Wang HY, Zhang HT, Su JM, Zhu J,
Wang HB, Zhou WY, Zhang H, Zhao MC, Zhang L and Chen XF:
Transforming growth factor-β1 promotes lung adenocarcinoma invasion
and metastasis by epithelial-to-mesenchymal transition. Mol Cell
Biochem. 355:309–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shapaer T, Chen Y, Pan Y, Wu Z, Tang T,
Zhao Z and Zeng X: Elevated BEAN1 expression correlates with poor
prognosis, immune evasion, and chemotherapy resistance in rectal
adenocarcinoma. Discov Oncol. 15:4462024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engblom C, Pfirschke C and Pittet MJ: The
role of myeloid cells in cancer therapies. Nat Rev Cancer.
16:447–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olingy CE, Dinh HQ and Hedrick CC:
Monocyte heterogeneity and functions in cancer. J Leukoc Biol.
106:309–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Z, Chen Y, Yu G and Ma Y: Research
trends and hotspots in surgical treatment of recurrent
nasopharyngeal carcinoma: A bibliometric analysis from 2000 to
2023. Asian J Surg. 47:2939–2941. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ugel S, Canè S, De Sanctis F and Bronte V:
Monocytes in the tumor microenvironment. Annu Rev Pathol.
16:93–122. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Wu Z, Pan Y, Chen Y, Chu J, Cong Y
and Fang Q: GNL3L exhibits pro-tumor activities via NF-κB pathway
as a poor prognostic factor in acute myeloid leukemia. J Cancer.
15:4072–4080. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin L, Xie S, Chen Y, Li W, Jiang X, Li H,
Li J, Wu Z, Xiao X, Zhang G, et al: Novel germline mutation KMT2A
G3131S confers genetic susceptibility to familial
myeloproliferative neoplasms. Ann Hematol. 100:2229–2240. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Contursi A, Grande R, Dovizio M, Bruno A,
Fullone R and Patrignani P: Platelets in cancer development and
diagnosis. Biochem Soc Trans. 46:1517–1527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Wu Z, Sun S, Fu Y, Chen Y and Liu
J: POEMS syndrome in the 21st century: A bibliometric analysis.
Heliyon. 9:e206122023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palacios-Acedo AL, Mège D, Crescence L,
Dignat-George F, Dubois C and Panicot-Dubois L: Platelets,
Thrombo-inflammation, and cancer: Collaborating with the enemy.
Front Immunol. 10:18052019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rothwell PM, Wilson M, Price JF, Belch
JFF, Meade TW and Mehta Z: Effect of daily aspirin on risk of
cancer metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Algra AM and Rothwell PM: Effects of
regular aspirin on Long-term cancer incidence and metastasis: A
systematic comparison of evidence from observational studies versus
randomised trials. Lancet Oncol. 13:518–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Burn J, Gerdes AM, Macrae F, Mecklin JP,
Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L,
et al: Long-term effect of aspirin on cancer risk in carriers of
hereditary colorectal cancer: An analysis from the CAPP2 randomised
controlled trial. Lancet. 378:2081–2087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutrition J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rosenberg SA: Progress in human tumour
immunology and immunotherapy. Nature. 411:380–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi
N, Itoh M, Iwata M, Shimizu J and Sakaguchi S: Immunologic
self-tolerance maintained by CD25+CD4+ naturally anergic and
suppressive T cells: Induction of autoimmune disease by breaking
their anergic/suppressive state. Int Immunol. 10:1969–1980. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shayimu P, Awula M, Wang CY, Jiapaer R,
Pan YP, Wu ZM, Chen Y and Zhao ZL: Serum nutritional predictive
biomarkers and risk assessment for anastomotic leakage after
laparoscopic surgery in rectal cancer patients. World J
Gastrointest Surg. 16:3142–3154. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao H, Chen X, Zhang W, Cheng D, Lu Y,
Wang C, Li J, You L, Yu J, Guo W, et al: Pan-immune-inflammation
value is associated with the clinical stage of colorectal cancer.
Front Surg. 9:9968442022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pérez-Martelo M, González-García A,
Vidal-Ínsua Y, Blanco-Freire C, Brozos-Vázquez EM,
Abdulkader-Nallib I, Álvarez-Fernández J, Lázare-Iglesias H,
García-Martínez C, Betancor YZ, et al: Clinical significance of
baseline Pan-immune-inflammation value and its dynamics in
metastatic colorectal cancer patients under first-line
chemotherapy. Sci Rep. 12:68932022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Seo YJ, Kim KE, Jeong WK, Baek SK and Bae
SU: Effect of preoperative pan-immune-inflammation value on
clinical and oncologic outcomes after colorectal cancer surgery: A
retrospective study. Ann Surg Treat Res. 106:169–177. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Yan N, Feng Y, Wu Y, Sun Y, Gao
X, Gu C, Ma X, Gao F, Zhang H and Zhou J: Inflammatory markers
predict efficacy of immunotherapy in advanced non-small cell lung
cancer: A preliminary exploratory study. Discov Oncol. 16:82025.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin F, Zhang LP, Xie SY, Huang HY, Chen
XY, Jiang TC, Guo L and Lin HX: Pan-immune-inflammation value: A
new prognostic index in operative breast cancer. Front Oncol.
12:8301382022. View Article : Google Scholar : PubMed/NCBI
|