Introduction
Intracranial tumors represent a highly heterogeneous
group of neoplastic diseases, exhibiting wide variability in
prognosis and treatment approaches. The most common intracranial
tumors are gliomas and meningiomas (1). Gliomas, particularly glioblastomas,
represent the most prevalent malignant intrinsic brain tumors in
adults, with a poor prognosis and a median 5-year survival rate of
<7% (1). By contrast,
meningiomas are mostly benign extra-axial brain tumors, with an
overall 10-year survival rate of >95% (2). These types of tumors differ markedly
in terms of prognosis, etiology and therapeutic approaches.
Glioblastoma, considered to originate from astrocytic cells, is
treated with surgery and radiochemotherapy (3), while meningioma arises from the
tumorous proliferation of arachnoid mater cells and is primarily
managed with surgery and, in some cases, radiotherapy (4).
Magnetic resonance imaging (MRI) is routinely
performed to diagnose and distinguish cranial tumorous lesions. In
cases of uncertainty, positron emission tomography (PET) is used to
enhance diagnostic accuracy and determine the underlying tumor
diagnosis. The use of [68Ga]-labeled
tetraazacyclododecanetetraacetic acid (DOTA) PET/MRI as a
somatostatin-receptor binding tracer is well established for the
diagnosis of neuroendocrine tumors (NETs) (5). However, similar to NETs, somatostatin
receptors (SSTRs) are upregulated in numerous intracranial tumors,
including meningioma (6).
Currently, [68Ga]-DOTA PET is primarily utilized to
identify meningiomas due to their high expression of SSTR type 2
(SSTR2) (7). However, a recent
report has highlighted that SSTR2 is not only expressed in
meningioma but also in glioma (8).
Consequently, the specificity of [68Ga]-DOTA PET/MRI for
diagnosing meningiomas should be discussed carefully.
The present study discusses the role of
[68Ga]-DOTA-conjugated SSTR-targeting peptide PET/MRI
imaging in distinguishing meningioma and glioma. A challenging case
is presented of a patient with two intracranial lesions, suggesting
the comorbidity of glioblastoma and meningioma. Moreover, a
systematic review of [68Ga]-DOTA-conjugated
SSTR-targeting peptide imaging is provided to evaluate its
diagnostic value in identifying glioma.
Case report
Methods
The case is reported according to the Case Report
(CARE) guidelines (9) and the
patient provided written informed consent for this publication. The
two tissue samples referenced in this report were obtained from the
same patient in March 2024 during a single surgical procedure. One
sample was collected from the lesion within the preexisting
resection cavity, while the other was taken from a distant site at
the planum sphenoidale. Following the surgery, the samples were
fixed in formalin and subsequently transferred to the local
biobank. The tissues were then made available for scientific
research and subjected to detailed neuropathological analysis. This
process included embedding the tissues in paraffin and preparing
thin sections on microscope slides. Histological staining was
conducted using hematoxylin and eosin (H&E) or hematoxylin
alone, alongside immunohistochemical staining for SSTR2 and glial
fibrillary acidic protein (GFAP), following standardized
protocols.
All tissue samples were fixed in 4% buffered
formalin at room temperature, embedded in paraffin, and sectioned
to a thickness of 1 µm. H&E and hematoxylin staining was
performed using the VENTANA© HE 600 System (Roche
Diagnostics GmbH). Immunohistochemical staining was carried out on
the BenchMark ULTRA Slide Staining System (Roche Diagnostics GmbH)
using an anti-GFAP polyclonal rabbit antibody (cat. no. Z0334;
Dako; Agilent Technologies, Inc.) at a 1:4,000 dilution, with an
incubation time of 16 min at 36°C. Staining for SSTR2 was performed
using a polyclonal rabbit antibody (cat. no. RBK046-05; Zytomed
Systems GmbH) at a 1:50 dilution, with antigen retrieval for 64 min
at 90°C in CC1 buffer (Roche Diagnostics GmbH), followed by
antibody incubation for 24 min at 36°C. All stained slides were
examined under a light microscope (Eclipse 80i; Nikon
Corporation).
Patient details
In September 2021, a 56-year-old male patient
attended University Hospital Essen (Essen, Germany) and was
diagnosed with an intracerebral, right temporal, ring-like
contrast-enhancing lesion. The patient underwent microsurgical
tumor removal, and a neuropathological assessment revealed the
diagnosis of a glioblastoma of Central Nervous System World Health
Organization (WHO) grade 4 (10).
The prior medical history included a posterior fossa neurinoma,
which was previously treated with stereotactic irradiation.
Postoperatively, concomitant radiochemotherapy according to Stupp
et al (11) was initiated,
followed by six cycles of adjuvant temozolomide, administered at
200 mg/m2 daily for the first 5 days of a 4-week cycle,
and tumor-treating fields.
In January 2024, at the age of 58 years, the patient
experienced a new extra-axial lesion at the planum sphenoidale with
homogeneous contrast-enhancement, radiographically consistent with
meningioma but suspicious for a distant glioblastoma manifestation
due to its de novo appearance. The case was discussed, and a
[68Ga]-DOTA-octreotide (DOTATOC) PET/MRI was
recommended. The imaging was performed 2 weeks later (dosage, 85
MBq; uptake time, 30 min after injection). MRI confirmed the
extra-axial lesion and additionally showed local tumor recurrence
in the right temporal lobe. [68Ga]-DOTATOC PET
demonstrated pronounced tracer accumulation in the pituitary gland
and doubled maximum standardized uptake (SUVmax) values
in the extra-axial lesion compared to the glioblastoma recurrence
[SUVmax extra-axial lesion of the planum sphenoidale
(lesion_1), 2.91; SUVmax local glioblastoma recurrence
(lesion_2), 1.35; SUVmax pituitary gland, 8.23]. The
[68Ga]-DOTATOC PET/MRI investigation is illustrated in
Fig. 1A-C. Due to the uncertainty
of the diagnosis of the extra-axial lesion, the patient underwent
tumor removal, involving both the right temporal lesion and the
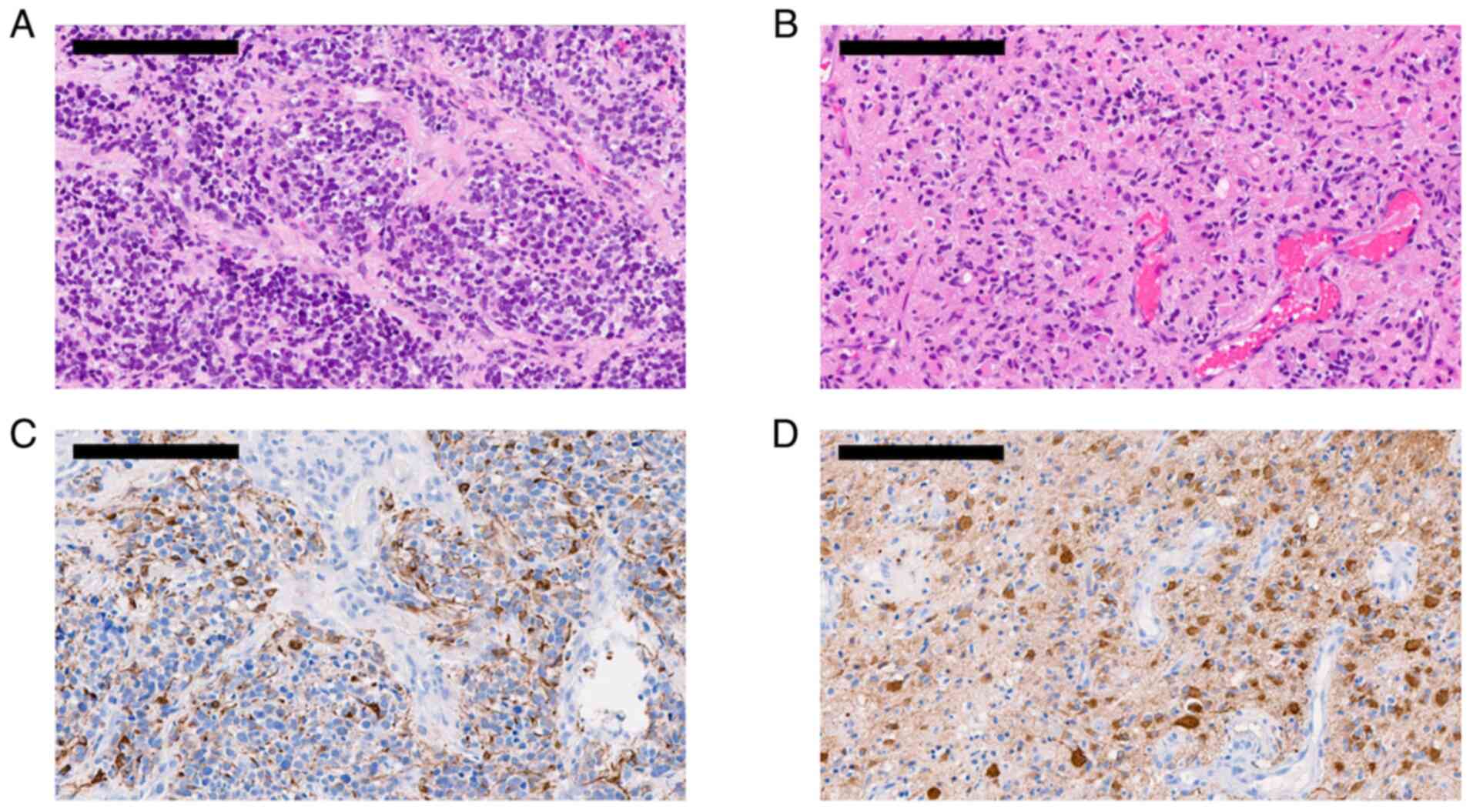
extra-axial lesion. The neuropathological assessment revealed a
glioblastoma diagnosis in both lesions. The right temporal lesion
demonstrated a diffusely infiltrative glioma, composed of
astrocytic, differentiated tumor cells with marked pleomorphism,
while the extra-axial lesion revealed a highly cellular glioma with
a small cell-like appearance. Representative neuropathological
slices are presented in Fig. 2.
Retrospectively, SSTR2 expression was assessed, and consistent with
[68Ga]-DOTATOC PET imaging, the right temporal lesion
showed weak positivity for SSTR2, while the extra-axial lesion
demonstrated increased SSTR2 expression. Representative staining is
presented in Fig. 1D. Post-surgery,
systemic treatment with lomustine, administered at 110
mg/m2 on the first day of a 6-week cycle, combined with
tumor-treating fields was initiated. Following the second
recurrence, the treatment strategy was adjusted to systemic therapy
with trofosfamide and etoposide, administered at 100 and 25
mg/m2, respectively, following a ‘1 week on - 1 week
off’ schedule within a 4-week cycle, while continuing the use of
tumor-treating fields. At the most recent follow-up in May 2024,
the patient remains alive and under clinical observation, with a
survival period of 2 years and 8 months since the initial
diagnosis.
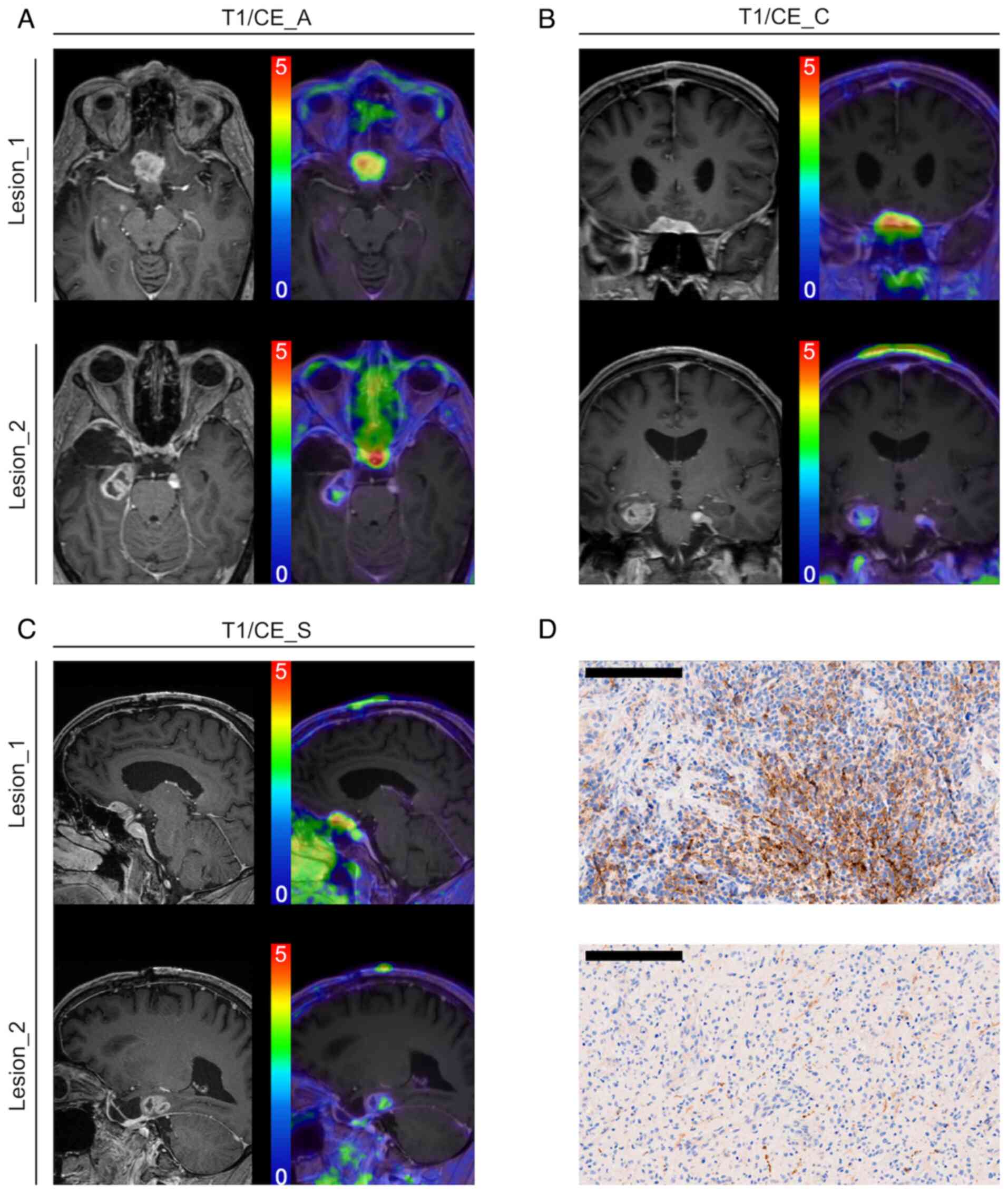 | Figure 1.Preoperative imaging results of both
lesions. (A-C) Preoperative MRI (left) and
[68Ga]-DOTA-octreotide-PET/MRI (right) images focusing
on the extra-axial tumor manifestation at the planum sphenoidale
(lesion_1; above) and the right temporal glioblastoma recurrence
(lesion_2; below). MRI images demonstrate contrast-enhanced
T1-sequences. All lesions are presented in (A) axial, (B) coronal
and (C) sagittal images. The following SUVmax values were obtained:
Lesion_1, 2.91; and lesion_2, 1.35. (D) Representative sections
were stained for SSTR2, with high expression of SSTR2 found in
lesion_1 (top) and weak positivity for SSTR2 found in lesion_2
(bottom) (scale bar, 200 µm). CE, contrast-enhanced; SSTR2,
somatostatin receptor 2; SUV, standardized uptake value; MRI,
magnetic resonance imaging; T1/CE_A, axial, contrast-enhanced T1
MRI; T1/CE_C, coronal, contrast-enhanced T1 MRI; T1/CE_S, sagittal,
contrast-enhanced T1 MRI. |
Literature review
To provide a comprehensive review of all reports on
[68Ga]-DOTA-conjugated SSTR-targeting peptide PET and
glioma, a broad search strategy was implemented. To avoid database
bias, research was conducted using PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), Scopus
(https://www.elsevier.com/en-gb/products/scopus),
Embase (https://embase.com) and Web of Science
(https://webofscience.com), utilizing the search
string: [(‘68-Ga-DOTA*’ OR ‘68Ga-DOTA*’ OR ‘Gallium-68 DOTA*’) AND
(‘glioma*’ OR ‘glioblastoma*’ OR ‘astrocytoma*’ OR ‘ependymoma*’].
Following the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (12), a computerized literature search was
performed on May 25, 2024. All duplicates were excluded. The titles
and abstracts of the remaining publications were reviewed, and
suitable articles were extracted. All articles were independently
screened by two investigators for their suitability. The references
of eligible publications were reviewed, and additional citations
were extracted. Research studies were considered eligible for
inclusion if they addressed the use of
[68Ga]-DOTA-conjugated SSTR-targeting peptide PET in
glioma, including preclinical and clinical research. Review
articles or conference abstracts were not included. The resulting
publication list was used to prepare this review, and all included
studies were subsequently analyzed in detail. The final publication
list was reviewed by two investigators, and relevant data points
were extracted.
The systematic literature search and subsequent
analysis identified six publications investigating the use of
[68Ga]-DOTA-conjugated SSTR-targeting peptide PET in
glioma patients or preclinical glioma models (8,13–17).
Five publications were clinically oriented and examined patients
with either primary or recurrent tumor entities. Results are
presented in Table SI. One
publication referred to the preclinical examination of PET imaging
on chemically induced glioma in animal models. Details are provided
in Table SII. In total, 44
patients with glioma underwent PET imaging. Extensive heterogeneity
was observed among the studied entities, as the inclusion extended
beyond high-grade tumors, with individual tumor grading
occasionally unspecified. Additionally, the study protocols often
differed regarding the administered tracer dose and uptake time,
with some details occasionally missing. Absolute tumor-to-brain
ratio or SUV values were rarely reported; however, a common finding
was tracer uptake in malignant gliomas of WHO grades III and IV,
corresponding to contrast enhancement in MRI according to the 2016
WHO classification (10).
Discussion
The use of [68Ga]-DOTA PET imaging as a
surrogate marker for SSTR-expressing tumors is well established in
oncology, and is particularly valuable in neuro-oncology for
assessing meningiomas. Compared to MRI, [68Ga]-DOTA PET
offers superior sensitivity and specificity for diagnosing
meningiomas based on imaging alone (18). Traditionally, intracranial tumors
require histopathological confirmation before initiating specific
treatment. However, in cases of meningioma, [68Ga]-DOTA
PET is gaining recognition as a sufficiently specific imaging
modality, enabling radiotherapy to be initiated for non-surgical
cases without the need for prior tissue biopsy.
Recently, case reports and series have raised
concerns about the specificity of SSTR expression for meningiomas,
noting uptake in intracerebral tumors such as high-grade gliomas,
including glioblastoma (8,19,20).
Both gliomas and meningiomas can demonstrate increased uptake of
[68Ga]-DOTA compounds, particularly in cases of elevated
SSTR expression or high vascularization (7,16).
This overlap in tracer uptake can make differentiation challenging,
especially in cases with atypical presentations or unusual tumor
locations. While gliomas and meningiomas are generally
distinguishable, certain features may blur the distinction.
Gliomas, particularly high-grade ones, often exhibit heterogeneous
uptake due to necrosis and irregular blood-brain barrier
permeability (8). They may also
occasionally express SSTRs, though typically at lower levels than
meningiomas (13). By contrast,
meningiomas are predominantly extra-axial tumors with robust,
homogeneous [68Ga]-DOTA uptake, reflecting their high
SSTR density. However, tumor location, such as the parasagittal
region or skull base, can cause gliomas to mimic meningiomas on
imaging (21). Additionally,
atypical or recurrent meningiomas may display more aggressive
features, further complicating the diagnostic process (22).
The current study presents a case of glioblastoma
with two distinct SSTR-expressing intracranial lesions showing
tracer enhancement on [68Ga]-DOTATOC PET. While MRI and
PET data initially suggested recurrent glioblastoma and a distant
meningioma, histological analysis confirmed both lesions as
glioblastoma, further supporting concerns about the specificity of
SSTR PET in meningioma diagnosis.
Currently, evidence-based data on
[68Ga]-DOTA PET imaging for gliomas is limited. The
present systematic literature review confirmed minimal data on the
role of [68Ga]-DOTA PET in glioma imaging, although
existing studies report tracer uptake in gliomas. The only
preclinical study, which investigated [68Ga]-DOTA uptake
in chemically induced gliomas in rodents, showed minimal tracer
enhancement (14).
Comparative studies on SSTR expression in
meningiomas vs. gliomas are sparse but indicate that while
[68Ga]-DOTA uptake is detectable in gliomas, the uptake
levels are lower than those observed in meningiomas (13). Studies by Kiviniemi et al
(8), Li et al (16) and Lapa et al (15) emphasized the role of blood-brain
barrier integrity in tracer uptake, with reduced uptake linked to
an intact blood-brain barrier. Consequently, high-grade gliomas
with disrupted barriers may be more appropriate for
[68Ga]-DOTA PET imaging than lower-grade gliomas, which
often lack contrast enhancement on MRI.
In conclusion, the present complex case, coupled
with the limited available literature, suggests that
[68Ga]-DOTA PET imaging is not exclusively specific to
meningiomas and may demonstrate uptake in gliomas, particularly
high-grade types. All reviewed studies report some degree of glioma
tracer enhancement in [68Ga]-DOTA PET, although
meningiomas generally demonstrate higher uptake. Establishing
standardized cut-off values to differentiate between tumor types
remains a critical need, as no validated SSTR-PET uptake scale
specific to meningiomas currently exists (23). Models that examine the diagnostic
performance of a combined application of PET and MRI would be of
interest, incorporating meningioma-specific PET parameters (e.g.,
high SSTR2 expression with a yet-to-be-defined tumor-to-brain
ratio) and MRI parameters (e.g., homogeneous contrast enhancement
and the dural tail sign in dura-attached tumors). Consequently,
clinicians must currently integrate clinical presentation, MRI
findings and PET imaging results, maintaining caution in presuming
meningioma specificity for [68Ga]-DOTA PET imaging.
Accurate determination of the patient's prognosis is crucial, as
the therapeutic approaches for meningiomas and gliomas differ
significantly in treatment intensity. This becomes even more
critical when some tumors are diagnosed as meningiomas based solely
on PET imaging and are subsequently treated with radiation without
prior histological confirmation. Further clinical studies are
essential to improve PET imaging specificity for differentiating
brain tumor types and to define specific cut-off values for
clinical decision-making.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank The West German
Biobank, University Hospital Essen, University Duisburg-Essen
(Essen, Germany) who assisted in transporting and storing
biosamples and handling patient data (project identifier ID:
19-WBE-074).
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LR and FG conceived the study, contributed to data
acquisition, participated in the case analysis and interpretation,
conducted the systematic literature review, and drafted the
manuscript. CB conducted imaging analyses and interpretation,
provided technical expertise on PET, and assisted with manuscript
preparation. TB supported the acquisition and interpretation of
tissue data, contributed to case management discussions, and
reviewed the manuscript for clinical relevance. SK, CDo, and CDe
provided input on study design, contributed to data interpretation,
and offered expertise on imaging and tissue analyses. US and KH
provided clinical oversight and the necessary infrastructure. PD
supervised the study, contributed to conceptual design, critically
revised the manuscript, and provided guidance on study direction
and methodology. All authors have read and approved the final
manuscript. LR and FG confirm the authenticity of all the raw
data.
Ethical approval and consent to
participate
Tissue sampling was performed in accordance with the
guidelines of the Ethics Committee of the Medical Faculty at the
University of Duisburg-Essen (Essen, Germany; ethical approval ID
19-8706-BO).
Patient consent for publication
The patient provided written informed consent for
data use and publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: primary brain and other central nervous system tumors
diagnosed in the United States in 2016–2020. Neuro Oncol. 25 (12
Suppl 2):iv1–iv99. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiemels J, Wrensch M and Claus EB:
Epidemiology and etiology of meningioma. J Neurooncol. 99:307–314.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sales AHA, Beck J, Schnell O, Fung C,
Meyer B and Gempt J: Surgical treatment of glioblastoma:
State-of-the-art and future trends. J Clin Med. 11:53542022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen WC, Lucas CG, Magill ST, Rogers CL
and Raleigh DR: Radiotherapy and radiosurgery for meningiomas.
Neurooncol Adv. 5 (Suppl 1):i67–i83. 2023.PubMed/NCBI
|
|
5
|
Tirosh A and Kebebew E: The utility of
(68)Ga-DOTATATE positron-emission tomography/computed tomography in
the diagnosis, management, follow-up and prognosis of
neuroendocrine tumors. Future Oncol. 14:111–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu W, Zhou Y, Wang Y, Liu L, Lou J, Deng
Y, Zhao P and Shao A: Clinical significance of somatostatin
receptor (SSTR) 2 in meningioma. Front Oncol. 10:16332020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rachinger W, Stoecklein VM, Terpolilli NA,
Haug AR, Ertl L, Pöschl J, Schüller U, Schichor C, Thon N and Tonn
JC: Increased 68Ga-DOTATATE uptake in PET imaging discriminates
meningioma and tumor-free tissue. J Nucl Med. 56:347–353. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiviniemi A, Gardberg M, Frantzen J,
Pesola M, Vuorinen V, Parkkola R, Tolvanen T, Suilamo S, Johansson
J, Luoto P, et al: Somatostatin receptor subtype 2 in high-grade
gliomas: PET/CT with (68)Ga-DOTA-peptides, correlation to
prognostic markers, and implications for targeted radiotherapy.
EJNMMI Res. 5:252015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rison RA, Kidd MR and Koch CA: The CARE
(CAse REport) guidelines and the standardization of case reports. J
Med Case Rep. 7:2612013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wesseling P and Capper D: WHO 2016
Classification of gliomas. Neuropathol Appl Neurobiol. 44:139–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collamati F, Pepe A, Bellini F, Bocci V,
Chiodi G, Cremonesi M, De Lucia E, Ferrari ME, Frallicciardi PM,
Grana CM, et al: Toward radioguided surgery with β-decays: Uptake
of a somatostatin analogue, DOTATOC, in meningioma and high-grade
glioma. J Nucl Med. 56:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiviniemi A, Gardberg M, Autio A, Li XG,
Heuser VD, Liljenbäck H, Käkelä M, Sipilä H, Kurkipuro J,
Ylä-Herttuala S, et al: Feasibility of experimental BT4C glioma
models for somatostatin receptor 2-targeted therapies. Acta Oncol.
53:1125–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lapa C, Linsenmann T, Luckerath K, Samnick
S, Herrmann K, Stoffer C, Ernestus RI, Buck AK, Löhr M and Monoranu
CM: Tumor-associated macrophages in glioblastoma multiforme-a
suitable target for somatostatin receptor-based imaging and
therapy? PLoS One. 10:e01222692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Tian Y and He Y: Late
pseudoprogression: A potential pitfall in 68Ga-DOTATATE PET/CT for
glioma. Clin Nucl Med. 48:e207–e208. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savelli G and Muni A: Somatostatin
receptors in an anaplastic oligodendroglioma relapse evidenced by
68Ga DOTANOC PET/CT. Clin Nucl Med. 40:e363–e365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afshar-Oromieh A, Giesel FL, Linhart HG,
Haberkorn U, Haufe S, Combs SE, Podlesek D, Eisenhut M and
Kratochwil C: Detection of cranial meningiomas: Comparison of
68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J
Nucl Med Mol Imaging. 39:1409–1415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He JH, Wang J, Yang YZ, Chen QX, Liu LL,
Sun L, Hu WM and Zeng J: SSTR2 is a prognostic factor and a
promising therapeutic target in glioma. Am J Transl Res.
13:11223–11234. 2021.PubMed/NCBI
|
|
20
|
Kiviniemi A, Gardberg M, Kivinen K, Posti
JP, Vuorinen V, Sipilä J, Rahi M, Sankinen M and Minn H:
Somatostatin receptor 2A in gliomas: Association with
oligodendrogliomas and favourable outcome. Oncotarget.
8:49123–49132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel M, Nguyen HS, Doan N, Gelsomino M,
Shabani S and Mueller W: Glioblastoma mimicking meningioma: Report
of 2 cases. World Neurosurg. 95:624e9–624e13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson TA, Huang L, Ramanathan D,
Lopez-Gonzalez M, Pillai P, De Los Reyes K, Kumal M and Boling W:
Review of atypical and anaplastic meningiomas: Classification,
molecular biology, and management. Front Oncol. 10:5655822020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Roytman M, Madera G, Magge RS,
Liechty B, Ramakrishna R, Pannullo SC, Schwartz TH, Karakatsanis
NA, Osborne JR, et al: Evaluating diagnostic accuracy and
determining optimal diagnostic thresholds of different approaches
to [68Ga]-DOTATATE PET/MRI analysis in patients with
meningioma. Sci Rep. 12:92562022. View Article : Google Scholar : PubMed/NCBI
|