Introduction
Upper tract urothelial carcinoma (UTUC) accounts for
~40% of all UC cases, with a higher prevalence among women than men
in Taiwan (1,2). The incidence of UTUC has been rising
in previous years, predominantly impacting the elderly population
(3,4). As UTUC is aggressive and ~60% of UTUCs
are invasive at presentation, improving the oncological outcome is
imperative, especially in the current comorbid patient population
(5). Malnutritional status is also
a poor prognostic factor of survival outcome in patients with UTUC
receiving radical surgery (6).
Radical nephroureterectomy (RNU) with bladder cuff
excision is a curative treatment in UTUC. Although early-stage
disease achieves durable long-term control, the 5-year overall
survival rate for patients with non-organ-confined UTUC is <50%
and it drops to <35% for those with nodal metastases. Given the
limited effectiveness of surgery alone in treating locally advanced
UTUC, there is a crucial need to develop multimodal treatment
strategies to improve the survival rates for patients with poor
prognostic UTUC (7).
The optimal timing for perioperative systemic
chemotherapy is still controversial. Cisplatin-based treatments are
presently administered in both neoadjuvant chemotherapy (NAC) and
adjuvant chemotherapy (AC) settings. The importance of AC for
patients with the aim of a cure has been confirmed (8). However, patient eligibility must be
evaluated, especially concerning renal function in relation to
definitive surgical treatment. The eligibility for cisplatin-based
chemotherapy decreases after RNU, as some patients are unable to
benefit from the advantage of AC. A previous systematic review and
meta-analysis reported that patients with nonmetastatic UTUC who
received NAC before RNU experienced improved OS and CSS compared
with those who underwent RNU alone (9). NAC has the potential to offer these
patients the benefits of chemotherapy. However, NAC presents
several disadvantages. Firstly, it may postpone definitive RNU,
potentially allowing disease progression, especially in patients
who are chemoresistant. Secondly, chemotherapy-related toxicities
in NAC recipients can further delay surgery. Lastly, there is a
risk of overtreatment in patients who do not have pathologically
confirmed muscle-invasive disease (9).
A phase 3 randomized trial from the UK presented
compelling evidence supporting the use of AC. These results showed
a 55% improvement in disease-free survival at a median follow-up
period of 30.3 months. This study showed the use of AC may be
superior when compared with RNU alone. The primary disadvantage of
AC in the treatment of patients with UTUC is the decline in renal
function following RNU (8) In
addition, in the clinical trial, a subgroup analysis revealed that
patients treated with cisplatin experienced notable benefits,
whereas those treated with carboplatin did not (8,9).
The decision to administer NAC is difficult due to
challenges in obtaining an accurate diagnosis. Currently,
conventional imaging and endoscopic biopsy often underestimate the
cancer stage (10). Therefore,
developing nomograms that predict an advanced pathological stage at
RNU could enhance the ability to identify patients who are most
likely to benefit from preoperative chemotherapy. The aim of the
present study was to create a model to predict renal function after
RNU for improved planning of perioperative chemotherapy.
Materials and methods
Study cohort and clinical
characteristics
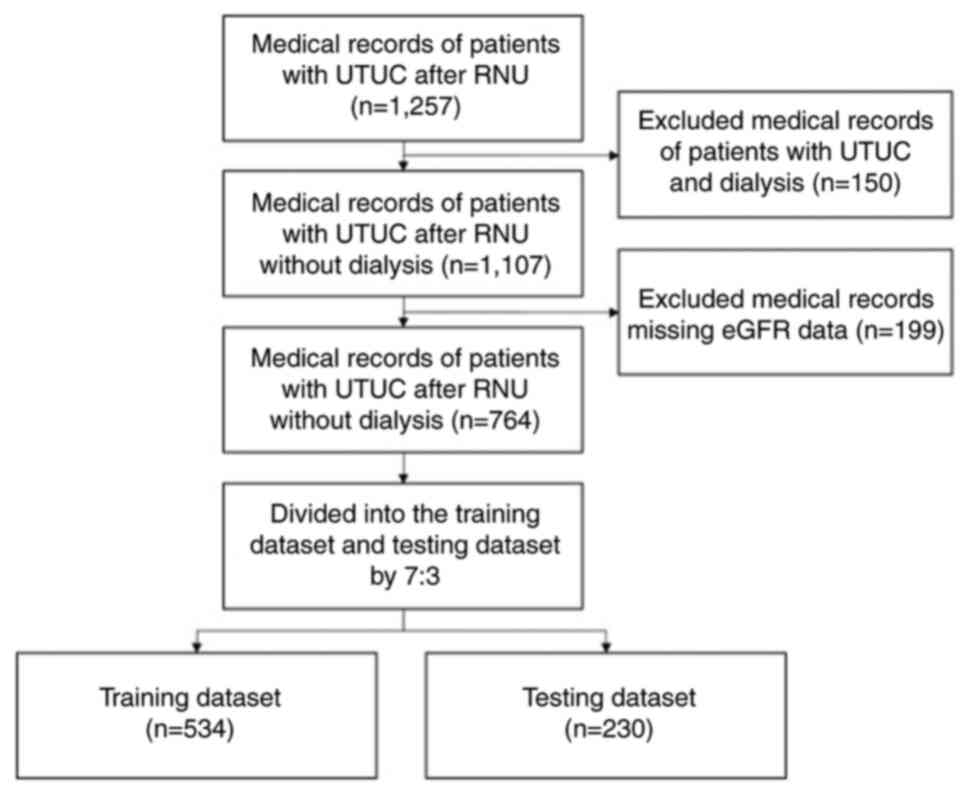
A total of 764 medical records of patients with
non-metastatic UTUC who received RNU from January 2008 to December
2022 at Kaohsiung Medical University Hospital (Kaohsiung, Taiwan)
and Kaohsiung Municipal Ta-Tung Hospital (Kaohsiung, Taiwan) were
retrospectively included in the present study. The present study
was approved by Kaohsiung Medical University Hospital ethics
committee [approval no. KMUHIRB-E(I)-20180214]. The inclusion
criteria used were as follows: i) Patients with UTUC; and ii)
patients who underwent RNU. The exclusion criteria used were as
follows: i) Patients undergoing dialysis; and ii) missing eGFR data
from medical records. The included data was divided into the
training (n=534) and testing (n=230) datasets. The flowchart of the
inclusion process is shown in Fig.
1. The training dataset was used to preliminarily train the
prediction model, and the model was subsequently tested using the
testing dataset. Several demographic and clinicopathological
parameters were collected to predict postoperative renal function,
including preoperative estimated glomerular filtration rate (eGFR),
tumor size, body mass index (BMI), Charlson Comorbidity Index
(CCI), pathological tumor stage ≥2 (pT2), pathological lymph node
stage ≥Nx/N0 (pN), lymphovascular invasion (LVI), diabetes mellitus
(DM), sex, hypertension (HTN), smoking, radiotherapy (RT), surgical
margin, grade, carcinoma in situ (CIS), perineural invasion
(PNI) and time. The Chronic Kidney Disease (CKD)-Epidemiology
Collaboration equation was used to calculate the GFR, defined as:
GFR=141 × min(Scr/κ, 1)α × max(Scr/κ,
1)−1.209 × 0.993age × 1.018 (if female) ×
1.159 (if black), where Scr is serum creatinine (mg/dl), κ is 0.7
for women and 0.9 for men, α is-0.329 for female patients and
−0.411 for male patients, min indicates the minimum of Scr/κ or 1
and max indicates the maximum of Scr/κ or 1 (11). The definition of CKD according to
current The Kidney Disease: Improving Global Outcomes international
guidelines (2012) is GFR <60 ml/min per 1.73 m2
(12,13).
Statistical analysis
The unpaired t-test (normalized data) and
Mann-Whitney U test (non-normalized data) were used to assess the
association between the dependent variable and continuous
variables, respectively. Pearson's chi-square test or Fisher's
exact test were used to assess the association between dependent
and categorical variables. P<0.05 was used to indicate a
statistically significant difference.
Linear regression is a foundational algorithm in
machine learning that predicts continuous outcomes by modeling the
relationship between independent and dependent variables. Logistic
regression estimates probabilities using the logistic function and
is employed for binary classification tasks. Regularization
techniques like Lasso regression and Ridge regression address
overfitting by penalizing large coefficients. The Elastic Net
combines both Lasso and Ridge penalties for greater flexibility.
Huber Regression is robust to outliers, blending squared and
absolute loss functions. Support Vector Machines are versatile for
classification and regression, leveraging hyperplanes for
separation. K-Nearest Neighbors is a simple, non-parametric method
relying on proximity for predictions. Naïve Bayes assumes feature
independence and is efficient for text classification. Decision
Trees provide interpretable models by splitting data
hierarchically, while ensemble methods like Gradient Boosting,
XGBoost, LightGBM, AdaBoost, CatBoost, Random Forest and Extra
Trees combine multiple models to enhance performance. Least-angle
regression and its Lasso variant are efficient for high-dimensional
data. Orthogonal Matching Pursuit is a greedy algorithm for sparse
representation to make locally optimal choices at each step and
find a global optimum solution. Dummy Regression serves as a
baseline model. Passive-aggressive regression is designed for
online learning scenarios (14).
All analyses were performed using Python (version
3.11; Python Software Foundation), and machine learning algorithm
was implemented with the PyCaret 3.0.4 package (Moez Ali), on a
64-bit Windows 11 (Microsoft Corporation) computer. PyCaret was
used for data preprocessing and training the model with the
available algorithms using a 10-fold cross-validation.
Hyperparameter tuning was automatically performed via random grid
search. The dataset was split into a 70% training set for model
development and a 30% test set for validation. The regression was
used to establish the prediction model for the continuous target
(eGFR) and the classification model was used for the category
target (CKD). To assess the accuracy of the machine learning
models, R2 and accuracy (the proportion of all
classifications that were correct) were utilized as performance
evaluations for predicting eGFR and CKD, respectively. The best
model with the highest accuracy would be further explored, and the
ROC curve was formulated according to the model. The SHapley
Additive exPlanations (SHAP) method was used to identify key
feature factors, presenting individual SHAP values in a violin
plot, and the ranking of the mean absolute SHAP values were shown
in a bar chart to represent the impact mode of features and the
importance ranking.
Results
Distribution of patient
characteristics
There was no significant difference between the
median age of the training [70 (64–76) years] and the testing [69
(63–76) years] datasets (Table I),
and 48.9% of the training dataset were male compared with 43.9% of
the testing dataset. In addition, there were no significant
differences in patient characteristics (BMI, preoperative eGFR, DM,
HTN, hydronephrosis, RT, CCI, time, and preoperative CKD) and tumor
characteristics (pT2, pN, grade, LVI, PNI, surgical margin, CIS,
and tumor size) between the training and test sets.
 | Table I.Characteristics and distribution of
patients after radical nephroureterectomy in the training and
testing datasets. |
Table I.
Characteristics and distribution of
patients after radical nephroureterectomy in the training and
testing datasets.
| Characteristic | Training dataset
(n=534) | Testing dataset
(n=230) | P-value |
|---|
| Age, years [median
(Q1-Q3)] | 70 (64–76) | 69 (63–76) | 0.4820 |
| BMI, kg/m2
[median (Q1-Q3)] | 24.50
(22.04–27.03) | 24.63
(22.00–27.39) | 0.8208 |
| Tumor size, cm
[median (Q1-Q3)] | 3.0 (2.2–4.5) | 3.2 (1.9–5.0) | 0.9774 |
| Charlson Comorbidity
Index, [median (Q1-Q3)] | 4.0 (3.0–5.0) | 4.5 (3.0–6.0) | 0.6381 |
| Preoperative
estimated glomerular filtration rate, ml/min/1.73 m2
[median (Q1-Q3)] | 53.84
(36.74–73.53) | 51.52
(36.76–67.75) | 0.3489 |
| Carcinoma in
situ, n (%) |
|
| 0.8810 |
| No | 449.0 (70.3) | 190.0 (29.7) |
|
|
Yes | 43.0 (69.4) | 19.0 (30.6) |
|
| Diabetes mellitus,
n (%) |
|
| 0.4503 |
| No | 370.0 (70.7) | 153.0 (29.3) |
|
|
Yes | 164.0 (68.1) | 77.0 (31.9) |
|
| Sex, n (%) |
|
| 0.2075 |
|
Female | 273.0 (67.9) | 129.0 (32.1) |
|
|
Male | 261.0 (72.1) | 101.0 (27.9) |
|
| Pathological grade,
n (%) |
|
| 0.5221 |
|
Low | 65.0 (73.0) | 24.0 (27.0) |
|
|
High | 449.0 (69.7) | 195.0 (30.3) |
|
| Hypertension, n
(%) |
|
| 0.9976 |
| No | 188.0 (69.9) | 81.0 (30.1) |
|
|
Yes | 346.0 (69.9) | 149.0 (30.1) |
|
| Hydronephrosis, n
(%) |
|
| 0.1818 |
| No | 168.0 (73.0) | 62.0 (27.0) |
|
|
Yes | 358.0 (68.2) | 167.0 (31.8) |
|
| Lymphovascular
invasion, n (%) |
|
| 0.9095 |
| No | 405.0 (70.2) | 172.0 (29.8) |
|
|
Yes | 106.0 (70.7) | 44.0 (29.3) |
|
| Surgery margin of
resection, n (%) |
|
| 0.9330 |
| No | 482.0 (69.9) | 208.0 (30.1) |
|
|
Yes | 31.0 (70.4) | 13.0 (29.6) |
|
| Perineural
invasion, n (%) |
|
| 0.9891 |
| No | 473.0 (70.3) | 200.0 (29.7) |
|
|
Yes | 38.0 (70.4) | 16.0 (29.6) |
|
| Radiotherapy, n
(%) |
|
| 0.6216 |
| No | 480.0 (70.2) | 204.0 (29.8) |
|
|
Yes | 54.0 (67.5) | 26.0 (32.5) |
|
| Smoking, n (%) |
|
| 0.5931 |
| No | 418.0 (69.4) | 184.0 (30.6) |
|
|
Yes | 116.0 (71.6) | 46.0 (28.4) |
|
| Time of
postoperative eGFR measurement, months [n (%)] |
|
| 0.6037 |
|
<3 | 235.0 (69.5) | 103.0 (30.5) |
|
|
3-12 | 194.0 (71.9) | 76.0 (28.1) |
|
|
12-24 | 105.0 (67.3) | 51.0 (32.7) |
|
| Pathological lymph
node stage, n (%) |
|
| 0.3164 |
|
N0/Nx | 118.0 (73.3) | 43.0 (26.7) |
|
|
N1/N2 | 400.0 (69.2) | 178.0 (30.8) |
|
| Pathological tumor
stage, n (%) |
|
| 0.5578 |
|
<2 | 241.0 (71.1) | 98.0 (28.9) |
|
| ≥2 | 282.0 (69.1) | 126.0 (30.9) |
|
| Postoperative CDK
status |
|
| 0.1868 |
| No | 208.0 (72.7) | 78.0 (27.3) |
|
|
Yes | 326.0 (68.2) | 152.0 (31.8) |
|
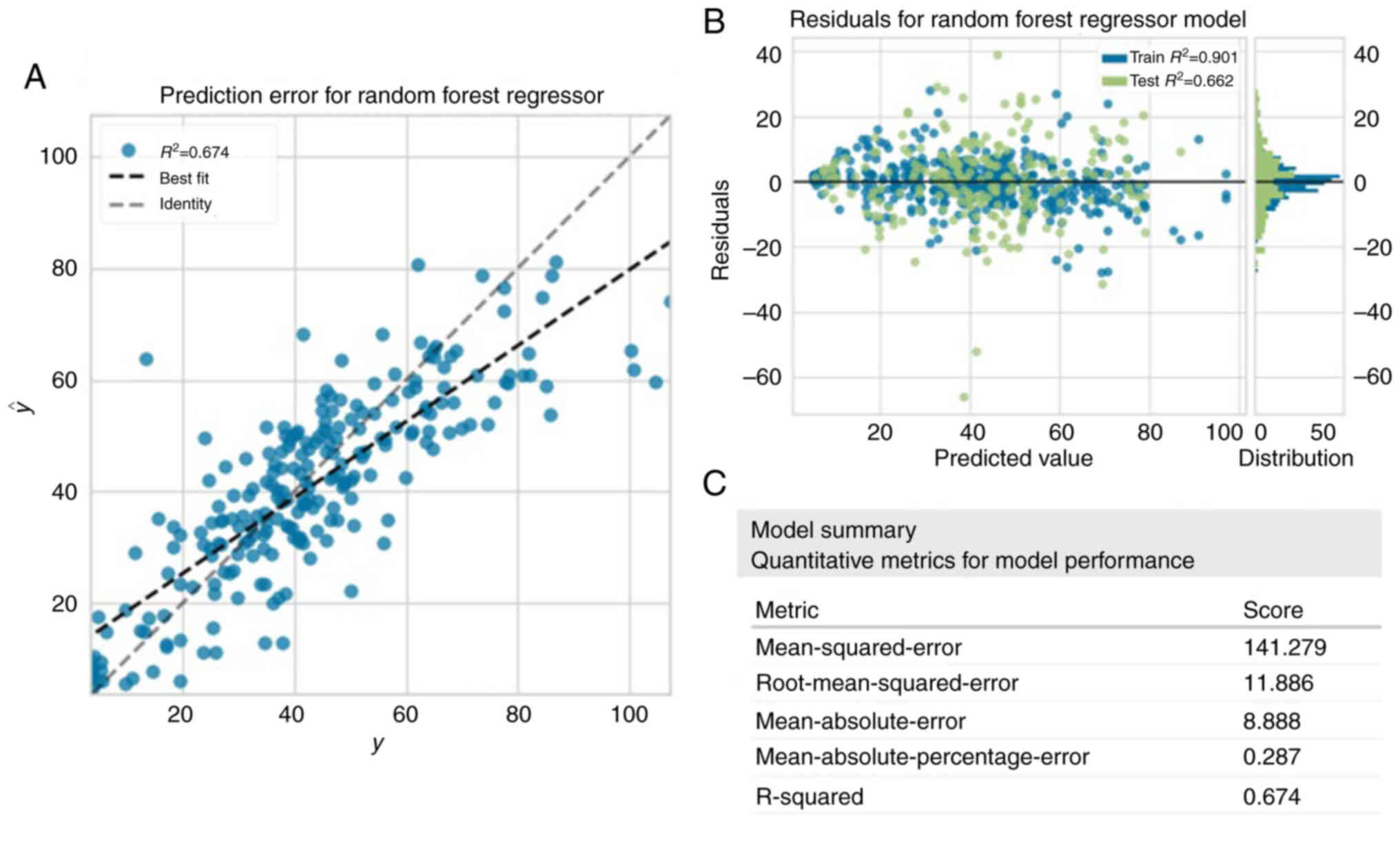
Prediction of postoperative eGFR
As shown in Table
II, the Random Forest Regressor outperformed the other
algorithms and achieved the highest average score across multiple
indicators. Fig. 2A illustrated the
correlation between the predicted and actual values of eGFR when
using Random Forest Regressor, showing that the model tended to
overestimate when the actual value was low and underestimate when
the actual value was high. Both the training and test sets achieved
an R2 >0.6, and there was a larger residual in the
test set compared with the training set (Fig. 2B). The summary of the model
performance was assessed, including the mean square error
(141.279), root mean squared error (11.886), mean absolute error
(8.888), mean absolute percentage error (0.287) and R2
(0.674) (Fig. 2C). The
R2 showed that the model explained more than one-half of
the variance.
 | Table II.Performance of 20 machine learning
algorithms for predicting estimated glomerular filtration rate in
the training dataset. |
Table II.
Performance of 20 machine learning
algorithms for predicting estimated glomerular filtration rate in
the training dataset.
| Model | MAE | MSE | RMSE | R2 | RMSLE | MAPE | TT (sec) |
|---|
| Random Forest
Regressor | 9.1430 | 172.1723 | 12.8377 | 0.6081 | 0.4496 | 0.4783 | 0.0800 |
| Light Gradient
Boosting Machine | 9.3503 | 177.7188 | 13.0598 | 0.5971 | 0.4613 | 0.4893 | 0.0860 |
| CatBoost
Regressor | 9.4182 | 184.8543 | 13.3495 | 0.5774 | 0.4592 | 0.4759 | 0.4640 |
| Extreme Gradient
Boosting | 9.5244 | 190.4045 | 13.5610 | 0.5620 | 0.4574 | 0.4498 | 0.0440 |
| Extra Trees
Regressor | 9.5420 | 195.3132 | 13.7607 | 0.5512 | 0.4751 | 0.4738 | 0.0680 |
| Gradient Boosting
Regressor | 10.3896 | 202.9164 | 14.0047 | 0.5343 | 0.4959 | 0.5524 | 0.0510 |
| Bayesian Ridge | 11.2124 | 226.7562 | 14.9715 | 0.4680 | 0.5378 | 0.6426 | 0.0340 |
| Elastic Net | 11.4288 | 229.5513 | 15.0674 | 0.4612 | 0.5385 | 0.6514 | 0.0310 |
| Huber
Regressor | 11.0380 | 230.6556 | 15.0620 | 0.4597 | 0.5358 | 0.6082 | 0.0340 |
| Ridge
Regression | 11.1474 | 230.5441 | 15.0782 | 0.4596 | 0.5420 | 0.6415 | 0.0310 |
| Linear
Regression | 11.1538 | 230.8561 | 15.0878 | 0.4589 | 0.5422 | 0.6419 | 0.0340 |
| Least Angle
Regression | 11.1538 | 230.8561 | 15.0878 | 0.4589 | 0.5422 | 0.6419 | 0.0330 |
| Lasso
Regression | 11.5126 | 231.7457 | 15.1408 | 0.4559 | 0.5398 | 0.6550 | 0.0290 |
| Lasso Least Angle
Regression | 11.5126 | 231.7457 | 15.1408 | 0.4559 | 0.5398 | 0.6550 | 0.0300 |
| Orthogonal Matching
Pursuit | 11.8809 | 242.4413 | 15.4791 | 0.4314 | 0.5439 | 0.6685 | 0.0310 |
| AdaBoost
Regressor | 12.1183 | 247.7008 | 15.5625 | 0.4287 | 0.5662 | 0.7149 | 0.0440 |
| Decision Tree
Regressor | 10.7265 | 244.8949 | 15.3545 | 0.4228 | 0.5399 | 0.4943 | 0.0310 |
| K Neighbors
Regressor | 12.1525 | 263.8043 | 16.0848 | 0.3841 | 0.5367 | 0.5995 | 0.0340 |
| Dummy
Regressor | 16.5575 | 437.9160 | 20.7940 | −0.0103 | 0.7276 | 1.1068 | 0.0340 |
| Passive Aggressive
Regressor | 16.3600 | 497.8276 | 20.7364 | −0.0947 | 0.5926 | 0.7304 | 0.0340 |
The SHAP method identified the importance of
features for the Random Forest Regressor model, and violin and bar
plots were used to evaluate the correlation between features and
predicted values, as well as their importance in prediction. In the
violin plot (Fig. 3A), the higher
feature value was colored red and the lower feature value was
colored blue. If the red points are distributed on right side of
center axis (SHAP value=0), it means that a higher feature value
corresponds to a higher SHAP value. On the contrary, the higher
feature value is associated with the lower SHAP value when the red
points are scattered on the left. In the bar plots shown in
Fig. 3B, all features were sorted
by the mean absolute SHAP values (Fig.
3B). Preoperative eGFR, tumor size, BMI, CCI and hydronephrosis
were the top five factors attributed to prediction, among which
preoperative eGFR had the greatest contribution to the model for
prediction. The features in the top five showed a positive
correlation with the SHAP value, except for CCI. Other features,
including pT stage, LVI, pN stage, DM, sex, HTN, smoking, RT,
surgical margin, grade, CIS and PNI also had medium to small
contributions to predicting post-operative eGFR. The ability to
predict time was minimal.
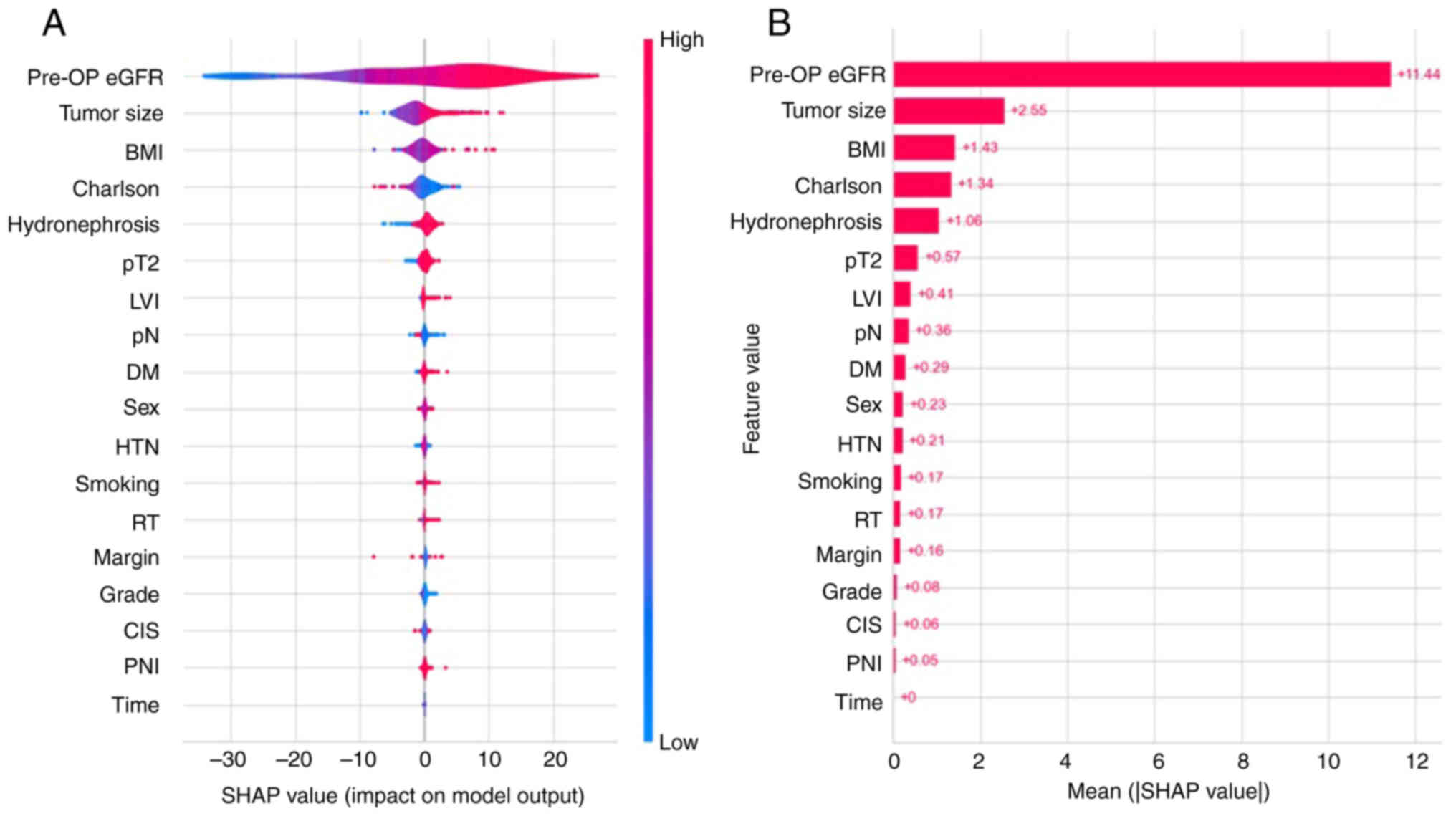 | Figure 3.SHAP method for postoperative eGFR
regression prediction model. (A) Violin plot and (B) bar chart of
the SHAP values. SHAP, SHapley Additive exPlanations; pre-OP,
preoperative; eGFR, estimated glomerular filtration rate; BMI, body
mass index; Charlson, Charlson Comorbidity Index; pT2, pathological
tumor stage ≥2; LVI, lymphovascular invasion; pN, pathological
lymph node stage ≥Nx/N02; DM, diabetes mellitus; HTN, hypertension;
RT, radiotherapy; CIS, carcinoma in situ; PNI, perineural
invasion. |
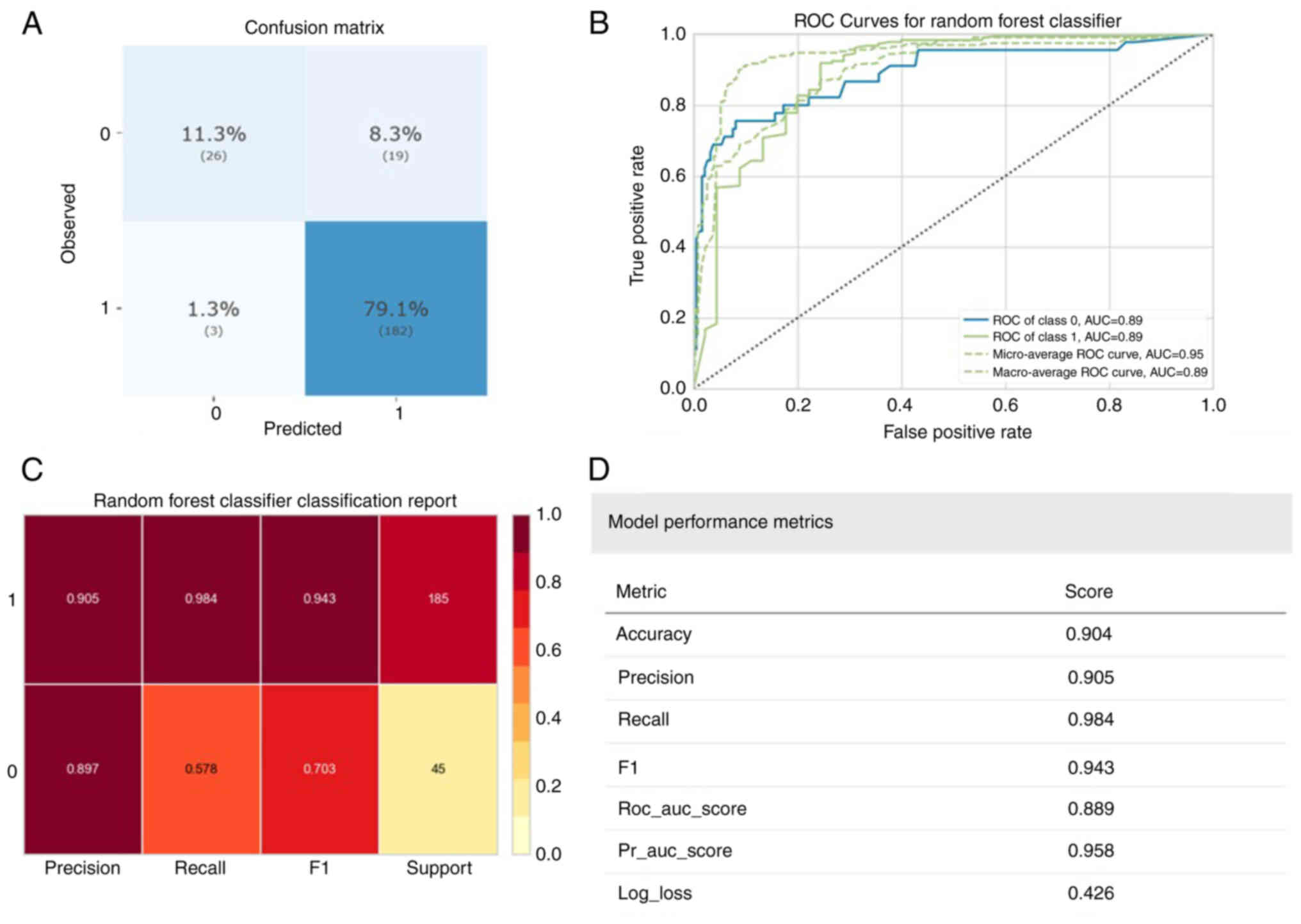
Prediction of postoperative CKD
status
Regarding CKD, the Random Forest Classifier, with
the best accuracy, also had the highest accuracy compared with
other algorithms regarding the predicted index scores (Table III). The model's performance is
illustrated in Fig. 4, with the
confusion matrix results (Fig. 4A)
indicating that 208 (90.4%) patients were accurately classified,
182 (79.1%) were patients with CKD and 26 (11.3%) were healthy
controls. Fig. 4B displays the
receiver operating characteristic (ROC) curve for the best model.
However, the model's performance in predicting healthy groups was
lower compared with patients with CKD (Fig. 4C). The overall model performance is
summarized in Fig. 4D, with
accuracy (0.904), precision (0.905), recall (0.984) and F1 score
(0.943) all >0.9. In Fig. 5, the
results from the SHAP method were consistent with the eGFR
prediction analysis, with preoperative CKD serving as the most
notable role for predicting the post-operative CKD status. The top
four factors with the highest SHAP values were preoperative CKD,
CCI, tumor size and BMI. Preoperative CKD and higher CCI had
above-average SHAP values, while higher BMI and tumor size had
below-average SHAP values. Other characteristics, including pT
stage, LVI, pN stage, DM, sex, HTM, smoking, RT, surgical margin,
grade, CIS, hydronephrosis and PNI, were also of similar importance
in predicting postoperative CKD. The SHAP values for time were
almost zero.
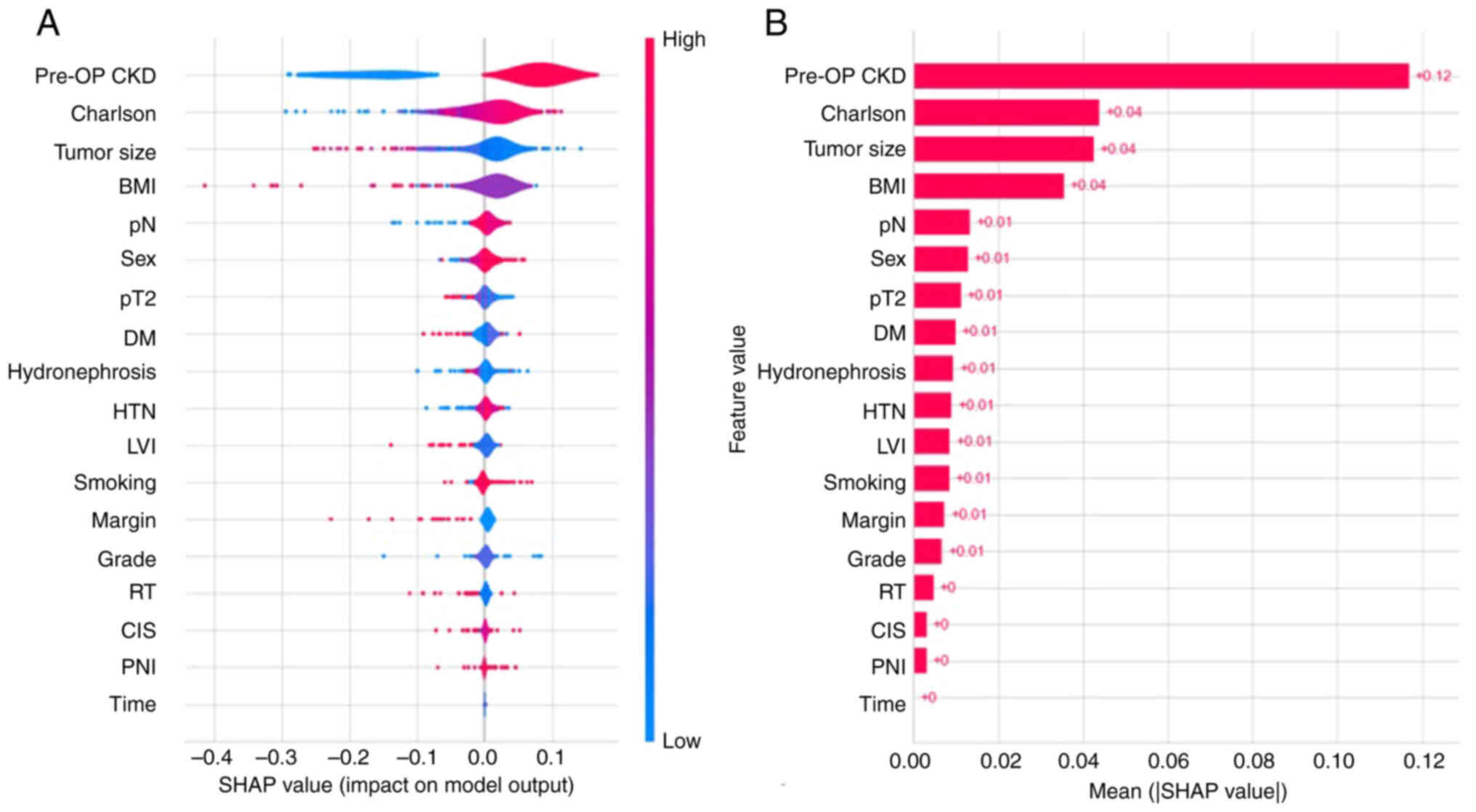 | Figure 5.SHAP method for postoperative CKD
status classification prediction model. (A) Violin plot and (B) bar
chart for the SHAP values. SHAP, SHapley Additive exPlanations;
pre-OP, preoperative; eGFR, estimated glomerular filtration rate;
BMI, body mass index; Charlson, Charlson Comorbidity Index; pT2,
pathological tumor stage ≥2; pN, pathological lymph node stage
≥Nx/N02; DM, diabetes mellitus; HTN, hypertension; LVI,
lymphovascular invasion; RT, radiotherapy; CIS, carcinoma in
situ; PNI, perineural invasion. |
 | Table III.Performance of 16 machine learning
algorithms for predicting chronic kidney disease in the training
dataset. |
Table III.
Performance of 16 machine learning
algorithms for predicting chronic kidney disease in the training
dataset.
| Model | Accuracy | AUC | Recall | Precision | F1 | k | MCC | TT (sec) |
|---|
| Random Forest
Classifier | 0.8746 | 0.8882 | 0.9559 | 0.8966 | 0.9249 | 0.5434 | 0.5585 | 0.0650 |
| CatBoost
Classifier | 0.8745 | 0.8744 | 0.9489 | 0.9022 | 0.9243 | 0.5525 | 0.5683 | 0.7260 |
| Extreme Gradient
Boosting | 0.8707 | 0.8757 | 0.9373 | 0.9070 | 0.9214 | 0.5534 | 0.5616 | 0.1450 |
| Gradient Boosting
Classifier | 0.8690 | 0.8710 | 0.9605 | 0.8871 | 0.9220 | 0.5098 | 0.5316 | 0.0490 |
| Light Gradient
Boosting Machine | 0.8689 | 0.8740 | 0.9373 | 0.9051 | 0.9202 | 0.5455 | 0.5559 | 0.1040 |
| Extra Trees
Classifier | 0.8558 | 0.8465 | 0.9187 | 0.9048 | 0.9112 | 0.5239 | 0.5291 | 0.0670 |
| Decision Tree
Classifier | 0.8502 | 0.8007 | 0.8932 | 0.9198 | 0.9055 | 0.5390 | 0.5450 | 0.0300 |
| K Neighbors
Classifier | 0.8390 | 0.7759 | 0.9490 | 0.8654 | 0.9049 | 0.3786 | 0.4056 | 0.3760 |
| Linear Discriminant
Analysis | 0.8351 | 0.8327 | 0.9374 | 0.8692 | 0.9013 | 0.3964 | 0.4173 | 0.0300 |
| Ridge
Classifier | 0.8277 | 0.0000 | 0.9722 | 0.8401 | 0.9011 | 0.2528 | 0.3107 | 0.0340 |
| Ada Boost
Classifier | 0.8238 | 0.7899 | 0.9280 | 0.8643 | 0.8947 | 0.3546 | 0.3665 | 0.0630 |
| Logistic
Regression | 0.8201 | 0.8342 | 0.9513 | 0.8456 | 0.8949 | 0.2770 | 0.3149 | 0.6260 |
| Dummy
Classifier | 0.8072 | 0.5000 | 1.0000 | 0.8072 | 0.8933 | 0.0000 | 0.0000 | 0.0340 |
| Naive Bayes | 0.8032 | 0.7821 | 0.8978 | 0.8646 | 0.8803 | 0.3211 | 0.3264 | 0.0350 |
| SVM-Linear
Kernel | 0.7920 | 0.0000 | 0.8980 | 0.8610 | 0.8722 | 0.2229 | 0.2339 | 0.0320 |
| Quadratic
Discriminant Analysis | 0.6571 | 0.5337 | 0.7374 | 0.8424 | 0.7313 | 0.0355 | 0.0451 | 0.0340 |
Discussion
For patients with advanced UTUC, optimum timing of
perioperative systemic treatment is important to decrease cancer
progression and recurrence. Although NAC can be a good strategy to
decrease the stage of the tumor, there is a lack of confidence in
the existing staging methods before surgery (15). The issue of over-staging and the
subsequent over-treatment is a concern. However, some patients who
are eligible for chemotherapy before surgery may become ineligible
after surgery. Factors predicting a decline in renal function due
to RNU may help identify patients at the highest risk of becoming
ineligible for postoperative cisplatin treatment.
In the present retrospective study, a machine
learning model that focused on preoperative prediction accuracy was
adopted and included 18 different feature values for predicting
renal function insufficiency postoperatively. The findings
indicated that the Random Forest algorithms outperformed other
models in both regression and classification tasks. In the
regression analysis, a slight difference in residual distribution
was observed between the training and testing datasets, which may
be attributed to limitations in potential features and sample size.
Regarding classification, the prediction of postoperative CKD
appears to strongly depend on the preoperative CKD status. Most
patients with preoperative CKD remain in a CKD state post-surgery.
However, for individuals with normal renal function in the healthy
group, postoperative CKD cannot be reliably predicted based on
preoperative renal function alone. Furthermore, SHAP was utilized
to gain insights into the importance and impact of clinical
features on predictions, making the machine learning model more
interpretable. Integrating clinical risk factors into a prediction
model enhances preoperative personalized risk assessment and helps
determine the best timing for perioperative cisplatin-based
chemotherapy.
Random Forest provides insights into feature
importance, aiding in feature selection and model interpretability.
It has robustness and high accuracy and can reduce overfitting. In
addition to being computationally intensive, it may struggle with
small datasets where individual trees lack sufficient diversity.
While it provides feature importance, the overall model is less
interpretable compared with simpler algorithms like linear
regression (16,17). In the present dataset, most patients
had CKD before surgery, which may have contributed to the high
accuracy of the model. The prediction of postoperative CKD depends
largely on the preoperative CKD status. The prediction model was
less effective in the preoperative CKD group in predicting the
postoperative CKD status compared with the healthy control group.
Therefore, it is necessary to establish different prediction models
based on the presence or absence of CKD before surgery.
In the present study, from the analysis of eGFR and
CKD outcomes, preoperative eGFR, tumor size, BMI and CCI were the
main predictors of postoperative renal function. It has been
previously shown that numerous clinical factors such as
preoperative eGFR <60 ml/min, increased age, coronary artery
disease and acute kidney injury on postoperative day 1 have been
identified as being associated with poorer renal function outcomes
after RNU, highlighting that preoperative renal function serves a
crucial role in preserving postoperative function (18). Kaag et al (19) reported that there is a postoperative
decline in renal function that does not improve over time observed
between the early (1–5 months) and late (>5 months)
postoperative time points. In addition, the authors discovered that
older patients with lower preoperative eGFR tend to have a poorer
renal function decline after surgery (19). Rodríguez Faba et al (20) also identified that some factors can
predict postoperative impaired renal function including
preoperative eGFR and hydronephrosis. Song et al (21) reported that preoperative renal
function and contralateral kidney volume can predict new-onset CKD
after surgery based on a multivariate logistic regression analysis,
and that a small contralateral kidney volume is associated with a
risk of postoperative CKD. The results of the present study
indicated that BMI is an independent risk factor for renal function
decline following RNU. Obesity is associated with a significantly
increased risk for CKD progression. However, malnutrition is a
prevalent issue among patients with cancer who often progress to
cachexia, which can result in a diminished response to treatment, a
poorer prognosis and a reduced quality of life. While the link
between obesity and CKD progression is intricate, its adverse
impact has been shown in several previous studies (21–23).
In the present study, hydronephrosis was
demonstrated to be a predictive factor for postoperative renal
function decline. Kanno et al (24) also discovered that the severity of
hydronephrosis has a notable impact on postoperative renal function
decline. The aforementioned study also reported that moderate to
severe hydronephrosis, but not mild hydronephrosis, was associated
with a slight decrease in renal function after surgery. The
difference between preoperative and postoperative renal function is
larger in patients without hydronephrosis or mild hydronephrosis
compared with severe hydronephrosis (24). Similarly, a study by Singla et
al (25) revealed that patients
with hydronephrosis receiving surgery experience a smaller decrease
in renal function compared with those without hydronephrosis. A
possible explanation for the findings of the present study is that
hydronephrosis was consistently associated with a thinner renal
cortex and lower eGFR (25). The
overall renal function would be compensated by the opposite kidney,
so removing the impaired kidney would not lead to a marked drop in
total eGFR. A smaller tumor size predicted impaired postoperative
overall renal function as this would be compensated by the opposite
kidney, which is in accordance with a previous study (26), suggesting that a larger tumor size
would be associated with decreased function in the affected kidney;
therefore, it is a protective factor for postoperative eGFR after
accounting for preoperative renal function. Based on this concept,
split renal function studies using nuclear renal scans may offer
preoperative contralateral kidney function information, which could
serve a key role in predicting postoperative renal function
following surgery.
Compared with previous studies (27–29),
the present model demonstrates greater flexibility, effectively
accommodating both linear and non-linear outcome distributions.
Furthermore, the performance of various models was evaluated to
ensure the final selection achieved optimal accuracy. However, the
present study does have some limitations. Firstly, although 18
features were included in the prediction model, there may be other
important features such as tumor variants, cardiovascular disease,
impact of medication and patient age, that were not added, as these
may not have been collected or the data were missing. Secondly,
this is a retrospective design study from a single institution and
the number of cases is limited. Therefore, the original data may
limit the reliability and accuracy of the model. Additionally, some
patients were excluded from the present study due to the lack of
complete preoperative data. Nevertheless, the present research
provides important predictive factors that are associated with
postoperative renal function decline. The predictors identified in
the present study may be used for treatment planning strategies
especially perioperative systemic chemotherapy.
To conclude, preoperative eGFR, tumor size, BMI, CCI
and hydronephrosis were found to be independent predictors of
postoperative eGFR in patients with UTUC. The correlation between
the predicted and observed postoperative eGFR and CKD demonstrated
a strong relationship. While further research is required to
validate these findings, the machine learning model based on these
factors could be valuable for identifying patients with impaired
renal function after RNU and those who may be suitable for NAC.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the Ministry of
Science and Technology (grant nos. MOST-111-2314-B-037-100-MY2 and
NSTC 113-2314-B-037-027), Kaohsiung Medical University Hospital
(grant nos. KMUH-111-1R55 and KMUH-112-2R57) and Regenerative
Medicine and Cell Therapy Research Center (grant no.
KMU-TC112A02).
Availability of data and materials
The data generated in the present study are included
in the figures and tables of this article.
Authors' contributions
Conception and design was performed by HYL, HCY,
WML, WJW and HLK. Acquisition of data and analysis was performed by
YCW and CCL. YCW and HYL analyzed and interpreted the data. HYL
drafted the manuscript. HYL and YCW confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Kaohsiung Medical University Hospital (Kaohsiung,
Taiwan; approval no. KMUHIRB-E(I)-20180214). A waiver for consent
to participate was granted by the IRB/Ethics Committee for this
purpose.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han J, Xian Z, Zhang Y, Liu J and Liang A:
Systematic overview of aristolochic acids: Nephrotoxicity,
carcinogenicity, and underlying mechanisms. Front Pharmacol.
10:6482019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang YH, Hsu WL, Lee YK, Chiang CJ, Yang
YW, You SL, Chen YC and Lai TS: Trends and sex-specific incidence
of upper urinary tract cancer in Taiwan: A birth cohort study.
Cancer Med. 12:15350–15357. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh HC, Chang CH, Fang JK, Chen IHA, Lin
JT, Hong JH, Huang CY, Wang SS, Chen CS, Lo CW, et al: The value of
preoperative local symptoms in prognosis of upper tract urothelial
carcinoma after radical nephroureterectomy: A retrospective,
multicenter cohort study. Front Oncol. 12:8728492022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen IA, Chang CH, Huang CP, Wu WJ, Li CC,
Chen CH, Huang CY, Lo CW, Yu CC, Tsai CY, et al: Factors predicting
oncological outcomes of radical nephroureterectomy for upper tract
urothelial carcinoma in Taiwan. Front Oncol. 11:7665762022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG;
Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract
Urothelial Carcinoma Collaboration, : Outcomes of radical
nephroureterectomy: A series from the upper tract urothelial
carcinoma collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang LW, Hung SC, Chen CS, Li JR, Chiu
KY, Wang SS, Yang CK, Lu K, Chen CC, Wang SC, et al: Geriatric
nutritional risk index as a prognostic marker for patients with
upper tract urothelial carcinoma receiving radical
nephroureterectomy. Sci Rep. 13:45542023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abouassaly R, Alibhai SMH, Shah N,
Timilshina N, Fleshner N and Finelli A: Troubling outcomes from
population-level analysis of surgery for upper tract urothelial
carcinoma. Urology. 76:895–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Birtle A, Johnson M, Chester J, Jones R,
Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto
JWF, et al: Adjuvant chemotherapy in upper tract urothelial
carcinoma (the POUT trial): A phase 3, open-label, randomised
controlled trial. Lancet. 395:1268–1277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leow JJ, Chong YL, Chang SL, Valderrama
BP, Powles T and Bellmunt J: Neoadjuvant and adjuvant chemotherapy
for upper tract urothelial carcinoma: A 2020 systematic review and
meta-analysis, and future perspectives on systemic therapy. Eur
Urol. 79:635–654. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petros FG, Qiao W, Singla N, Clinton TN,
Robyak H, Raman JD, Margulis V and Matin SF: Preoperative multiplex
nomogram for prediction of high-risk nonorgan-confined upper-tract
urothelial carcinoma. Urol Oncol. 37:292.e1–292.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Webster AC, Nagler EV, Morton RL and
Masson P: Chronic kidney disease. Lancet. 389:1238–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for
the diagnosis, evaluation, prevention, and treatment of chronic
kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int
Suppl. 76 (Suppl 113):S1–S130. 2009.
|
|
14
|
Ray S: ‘A quick review of machine learning
algorithms’. 2019 International Conference on Machine Learning, Big
Data, Cloud and Parallel Computing (COMITCon); Faridabad, India:
pp. 35–39. 2019
|
|
15
|
Favaretto RL, Shariat SF, Savage C, Godoy
G, Chade DC, Kaag M, Bochner BH, Coleman J and Dalbagni G:
Combining imaging and ureteroscopy variables in a preoperative
multivariable model for prediction of muscle-invasive and non-organ
confined disease in patients with upper tract urothelial carcinoma.
BJU Int. 109:77–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi Y: Random forest for bioinformatics.
Zhang C and Ma Y: Ensemble Machine Learning. Springer; New York,
NY: pp. 307–323. 2012
|
|
17
|
Smith PF, Ganesh S and Liu P: A comparison
of random forest regression and multiple linear regression for
prediction in neuroscience. J Neurosci Methods. 220:85–91. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tafuri A, Marchioni M, Cerrato C, Mari A,
Tellini R, Odorizzi K, Veccia A, Amparore D, Shakir A, Carbonara U,
et al: Changes in renal function after nephroureterectomy for upper
urinary tract carcinoma: Analysis of a large multicenter cohort
(radical nephroureterectomy outcomes (RaNeO) research consortium).
World J Urol. 40:2771–2779. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaag M, Trost L, Thompson RH, Favaretto R,
Elliott V, Shariat SF, Maschino A, Vertosick E, Raman JD and
Dalbagni G: Preoperative predictors of renal function decline after
radical nephroureterectomy for upper tract urothelial carcinoma.
BJU Int. 114:674–679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodríguez Faba O, Palou J, Breda A, Maroto
P, Fernández Gómez JM, Wong A and Villavicencio H: Predictive
factors for impaired renal function following nephroureterectomy in
upper urinary tract urothelial cell carcinoma. Urol Int.
92:169–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song W, Sung HH, Han DH, Jeong BC, Seo SI,
Jeon SS, Lee HM, Choi HY and Jeon HG: The effect of contralateral
kidney volume on renal function after radical nephroureterectomy:
Implications for eligibility for neoadjuvant chemotherapy for upper
tract urothelial cancer. Urol Oncol. 35:114.e1–114.e7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun HR, Kim H, Park JT, Chang TI, Yoo TH,
Kang SW, Choi KH, Sung S, Kim SW, Lee J, et al: Obesity, metabolic
abnormality, and progression of CKD. Am J Kidney Dis. 72:400–410.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madero M, Katz R, Murphy R, Newman A,
Patel K, Ix J, Peralta C, Satterfield S, Fried L, Shlipak M and
Sarnak M: Comparison between different measures of body fat with
kidney function decline and incident CKD. Clin J Am Soc Nephrol.
12:893–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanno T, Kobori G, Saito R, Ito K,
Nakagawa H, Takahashi T, Koterazawa S, Takaoka N, Somiya S, Haitani
T, et al: Hydronephrosis severity as a predictor of postoperative
renal function decline following laparoscopic radical
nephroureterectomy. Int J Clin Oncol. 29:464–472. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singla N, Hutchinson R, Haddad A,
Sagalowsky A, Lotan Y and Margulis V: Preoperative hydronephrosis
is associated with less decline in renal function after radical
nephroureterectomy for upper tract urothelial carcinoma. Can J
Urol. 23:8334–8341. 2016.PubMed/NCBI
|
|
26
|
Fang D, Zhang Q, Li X, Qian C, Xiong G,
Zhang L, Chen X, Zhang X, Yu W, He Z and Zhou L: Nomogram
predicting renal insufficiency after nephroureterectomy for upper
tract urothelial carcinoma in the Chinese population: Exclusion of
ineligible candidates for adjuvant chemotherapy. Biomed Res Int.
2014:5291862014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hashimoto T, Ohno Y, Nakashima J, Gondo T,
Nakagami Y, Namiki K, Horiguchi Y, Yoshioka K, Ohori M and
Tachibana M: Prediction of renal function after nephroureterectomy
in patients with upper tract urothelial carcinoma. Jpn J Clin
Oncol. 45:1064–1068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hensley PJ, Labbate C, Zganjar A, Howard
J, Huelster H, Durdin T, Pham J, Xiao L, Pallauf M, Lombardo K, et
al: Development and validation of a multivariable nomogram
predictive of post-nephroureterectomy renal function. Eur Urol
Oncol. 7:1313–1319. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Wu P, Lai S, Wang J, Hou H and
Zhang Y: Prognostic models for upper urinary tract urothelial
carcinoma patients after radical nephroureterectomy based on a
novel systemic immune-inflammation score with machine learning. BMC
Cancer. 23:5742023. View Article : Google Scholar : PubMed/NCBI
|