Introduction
Lung cancer is one of the most common malignant
tumors, with the highest morbidity and mortality rates worldwide.
According to the American Cancer Society, ~350 individuals succumb
to lung cancer each day (1). In
addition, lung cancer is the most common cause of cancer-associated
mortality and morbidity and mortality rates are continually
increasing (2). Notably, lung
cancer is divided into two major histological types; namely,
non-small cell lung cancer (NSCLC) and small cell lung cancer
(SCLC) (3), with NSCLC accounting
for ~85% of all lung cancer cases (4). NSCLC is characterized by early
metastasis, high rates of recurrence and a lack of symptoms in the
early stage of disease that leads to late-stage diagnosis in the
majority of patients. Although treatment options, including
surgery, radiotherapy, chemotherapy, molecular targeted therapy and
anti-angiogenic drugs, have improved the survival rates of patients
(5), tumor heterogeneity, drug
resistance and immune side effects remain challenging, leading to
poor clinical outcomes. Thus, further explorations into the
mechanisms underlying lung cancer progression may aid in the
development of novel, effective therapeutic targets.
Circular RNAs (circRNAs) are a class of endogenous
non-coding RNA (6). CircRNAs differ
from classical linear RNAs, forming covalently-closed and stable
loops through precursor-specific shearing. Notably, these are
resistant to nucleic acid exonuclease-mediated digestion (7), leading to higher levels of stability
and abundance compared with linear RNAs (8). Previous studies have suggested that
circRNAs are products of incorrectly sheared precursor mRNAs.
However, improvements in high-throughput technologies have led to
the discovery of numerous types of circRNAs that exert regulatory
effects on gene expression in eukaryotic organisms and these serve
a role in the development of a variety of diseases (9). For instance, research indicates that
hsa_circ_0002005 exhibits overexpression in OS tissues and cells,
with its downregulation leading to a decrease in cellular
proliferation, migration, invasion and metastatic capabilities
(10). Moreover, circRNAs may act
as molecular signals that interact with other RNAs or proteins
(11), regulating the expression of
downstream genes through several mechanisms. CircRNAs may also
participate in a variety of oncogenic signaling pathways, which, in
turn, are involved in the regulation of gene transcription and
protein translation (12). Notably,
circRNAs may act as microRNA (miRNA) sponges that compete with
miRNAs for binding sites, thus regulating miRNA activity to affect
the expression of their target proteins (13). For example, circRNA_000166 inhibits
the proliferation and apoptosis of breast cancer cells through the
miR-326/ETS transcription factor ELK1 (ELK1) and miR-330-5p/ELK1
pathways (14). In lung cancer,
circRNAs regulate apoptosis-associated genes and signaling pathways
through various mechanisms, thereby affecting the apoptosis of lung
cancer cells. For example, circ_0000620 exerts its effects on lung
adenocarcinoma (LUAD) cell apoptosis through the miR-216b-5p/KRAS
signaling pathway (15). In
addition, circRNA circHIPK3 inhibits the apoptosis of NSCLC cells
by regulating the expression of apoptosis-associated protein
forkhead box protein M1 (16).
Results of a previous study indicated that circRNAs
are closely associated with proliferation, metastasis and drug
resistance in tumor cells (17).
Overexpression of circRNA_102171 promoted papillary thyroid cancer
progression via activation of the catenin β interacting protein
1-dependent Wnt/β-catenin pathway (18). Moreover, results of a previous study
revealed that tumor-associated macrophage-induced circMRCKα encodes
a peptide that facilitates glycolysis, ultimately accelerating
hepatocellular carcinoma progression (19). Therefore, circRNAs may serve a key
role in the regulation of cancer progression (20). The aberrant expression of circRNA is
associated with the pathophysiology of numerous diseases; thus,
current research is focused on the use of circRNAs as potential
diagnostic biomarkers and therapeutic targets (21). Research has shown that circKIAA0182
promotes cisplatin resistance and tumor progression in NSCLC both
in vitro and in vivo (22).
Nutrients, energy and biosynthetic activity are
required for cell metabolism and function (23). Glucose, lipid and amino acid
metabolism are the key metabolic pathways in eukaryotic organisms
and these processes are balanced and interconnected to provide
nutrients and energy for cell growth and development. However,
tumor cells exhibit a loss of function of energy metabolism and
undergo reprogramming of glucose, lipid and amino acid metabolism.
Through metabolic reprogramming, tumor cells obtain high levels of
energy and nutrients that are required for proliferation and
growth. Results of a previous study demonstrated that
N-acetyltransferase 10/N4-acetylcytidine/forkhead box protein P1
axis may promote the malignant progression and immunosuppression of
cervical cancer through reprogramming glycolytic metabolism
(24). Thus, metabolic
reprogramming may serve a key role in tumor development.
Due to the heterogeneity of tumors, the metabolic
requirements of tumor cells in different states change according to
the developmental process of NSCLC (25). The metabolic reprogramming of tumor
cells creates high levels of energy and nutrients, subsequently
impacting tumor growth and cell proliferation (26). Results of a previous study highlight
that circRNAs are critical contributors to both cancer progression
and metabolic reprogramming (27).
The present review aimed to discuss the mechanisms underlying the
circRNA-mediated metabolic reprogramming of lung cancer cells and
aimed to provide a novel theoretical basis for the clinical
treatment of NSCLC.
Characterization and function of
circRNAs
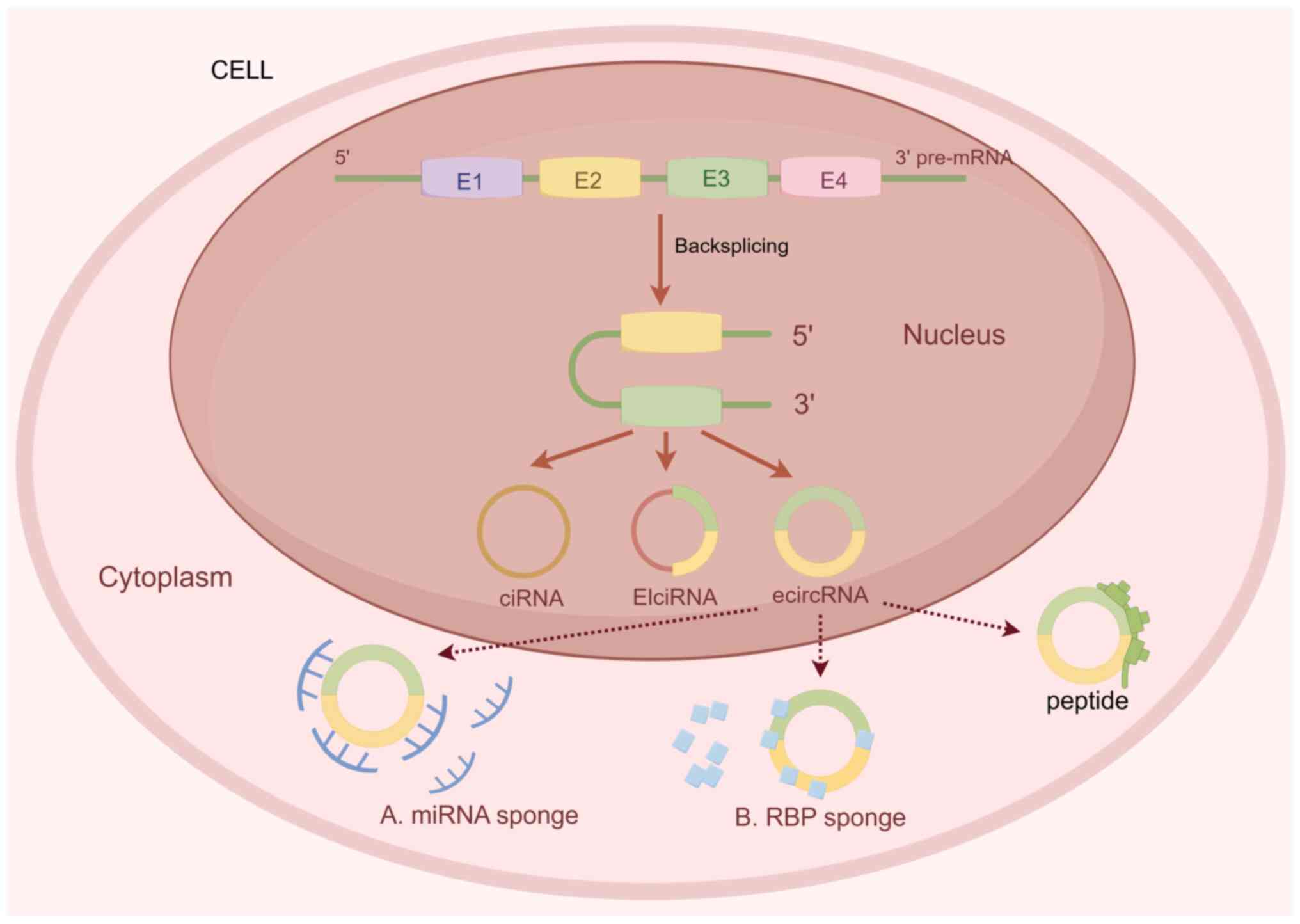
CircRNAs are covalently-closed non-coding RNAs
formed through the reverse shearing of precursor mRNAs. Notably,
circRNAs are not affected by the action of nucleic acid
exonucleases and are stably expressed in the human body with high
tissue specificity. Exonic circRNA is the most common subtype of
circRNAs, accounting for ~80% of all identified circRNAs (28). CircRNAs were discovered in Murine
respirovirus using electron microscopy (29) and were initially considered
by-products of incorrect shearing (30). However, developments in
bioinformatics and high-throughput sequencing technologies have
furthered the understanding of circRNAs and results of a previous
study demonstrated that these are widely expressed in eukaryotic
organisms (31). CircRNAs are
species-conserved, tissue-specific, disease-specific and serve key
regulatory roles as epigenetic regulators in a variety of diseases
(32).
CircRNAs also act as miRNA molecular sponges that
bind miRNAs through competing endogenous (ce)RNA mechanisms to
regulate the expression of downstream target genes. CeRNA is not a
specific type of RNA, but rather a regulatory mechanism. In this
mechanism, different types of RNAs (such as mRNA, lncRNA, circRNA,
etc.) can interact through common microRNA (miRNA) response
elements (MREs) and competitively bind to the same miRNA, thereby
regulating each other's expression levels. In addition, circRNAs
act as protein decoys or scaffolds that bind to single proteins or
chelate with multiple proteins for the formation of circRNA-protein
complexes. Thus, circRNAs may directly or indirectly affect the
expression of target proteins. Liang et al (33) demonstrate that circDCUN1D4 acts as a
scaffold for the formation of the protein ternary complex of
circDCUN1D4-human antigen R-thioredoxin interacting protein (TXNIP)
RNA, leading to the inhibition of NSCLC metastasis and improved
TXNIP mRNA stability (33).
Although circRNAs are categorized as a class of non-coding RNA
without protein translational capacity, results of a previous study
demonstrated that a small proportion of circRNAs may mediate
translation in a non-cap-dependent manner. This process involves
internal ribosomal entry sites and N6-methyladenosine (m6A)
epigenetic modifications (34).
Yang et al (35)
demonstrated that circFBXW7 inhibited the occurrence of glioma
through protein encoding. Specifically, circFBXW7 was able to
encode a novel protein, FBXW7-185aa. This protein inhibits the
development of malignant gliomas by antagonizing USP28-induced
c-Myc stability and reducing the half-life of c-Myc. This finding
suggests that circFBXW7 and its encoded proteins have important
regulatory roles in glioma tumorigenesis and may provide new
targets for the treatment of glioma. Collectively, these results
demonstrated that circRNAs may impact the progression of numerous
diseases, including cancer (Fig.
1). For instance, circRNA_0039480, which is abundant in plasma
exosomes, exhibits heightened expression levels in individuals with
gestational diabetes mellitus and holds potential as a biomarker
for early diagnosis (36). Notably,
circRNAs may exhibit potential as diagnostic and prognostic
biomarkers in numerous cancer types, due to high levels of
stability and specificity in expression (37). Research has demonstrated that the
expression levels of circACVR2A and circCCNB1 can serve as a
distinguishing marker to differentiate non-small cell lung cancer
(NSCLC) between adenocarcinoma and squamous cell carcinoma subtypes
(38). Furthermore, CiRS-7/CDR1as
has been extensively utilized as a prognostic indicator in various
cancers, including colon (39),
lung (40) and breast cancer
(41). This circular RNA has shown
potential in predicting patient outcomes and guiding treatment
strategies in these malignancies.
Effects of circRNAs on metabolic
reprogramming in tumors
Glucose, lipid and amino acid metabolism are altered
in tumor cells for the maintenance of uncontrolled cell
proliferation and survival under conditions of low nutrients, low
PH and a lack of oxygen. Alterations in metabolic pathways are
known as metabolic reprogramming. Results of previous studies
demonstrated that the metabolic reprogramming of tumors may impact
the natural differentiation of tumor stem cells, leading to
tumorigenesis and tumor development (42,43).
Through metabolic reprogramming, tumor cells acquire high levels of
ATP, lipids, proteins and nucleotides, thus promoting tumor cell
growth, proliferation and metastasis.
Cancer cells are characterized by abnormalities in
glucose metabolism (44) and most
of the energy in tumor cells is preferentially synthesized through
the glycolytic pathway, even under aerobic conditions. This process
is known as the Warburg effect (45). Tumor cells utilize the metabolic
pattern of aerobic glycolysis, resulting in high glucose
consumption and lactic acid accumulation. The development of a
hypoxic and acidic tumor microenvironment facilitates angiogenesis,
invasion, metastasis and therapeutic resistance of tumor cells
(46,47). Studies have revealed that tumor
cells fulfill their energy requirements for rapid proliferation by
enhancing glycolysis, a phenomenon known as the Warburg effect.
This process not only results in the production of lactic acid but
also alters the tumor microenvironment, thereby inhibiting the
immune response and facilitating drug resistance (48). During the growth and development of
tumors, immunosurveillance serves a key role in eliminating and
controlling malignant cells. However, tumor cells evade
immunosurveillance through the increased proliferation and function
of effector T-cells in the tumor microenvironment, using lactic
acid produced by aerobic glycolysis (49).
Alterations in glucose metabolism also affect tumor
cell growth and proliferation. Notably, glycolysis differs to
oxidative phosphorylation, in that it does not utilize the
tricarboxylic acid cycle pathway, providing ATP to cells in a more
efficient manner. Thus, tumor cells obtain energy through
glycolysis, leading to higher levels of growth and proliferation
compared with healthy cells (50).
Moreover, lipid metabolic reprogramming in malignant tumors cells
involves fatty acid uptake and biosynthesis, fatty acid β-oxidation
and lipid desaturation. In addition, amino acid metabolic
reprogramming is required for tumor cell growth and development due
to increased levels of amino acids and nutrients. Thus, tumor cells
may mediate the activation of amino acid metabolism-associated
pathways, leading to amino acid metabolism disorders and the
promotion of tumor development (51).
Metabolic reprogramming also impacts glucose
transporter proteins (GLUTs), metabolism-associated enzymes,
transcription factors and signaling pathways and this serves an
important role in sustaining tumorigenesis and progression. Results
of a previous study reveal that circITCH inhibits melanoma cancer
cell proliferation through the downregulation of glucose uptake via
GLUT1 (52). Moreover, circRNF20
upregulated hexokinase 2 expression through the
miR-487a/hypoxia-inducible factor (HIF)-1α axis, leading to the
promotion of glycolysis and breast cancer development (53). Results of a previous study reveal
that circMAT2B promotes glycolysis and the proliferation and
invasion of hepatocellular carcinoma cells, through upregulation of
pyruvate kinase muscle isozyme M2 (PKM2) by sponging miR-338-3p
(54). Moreover, circ_0006677 may
inhibit NSCLC progression and glycolysis during NSCLC cell growth
and development, by acting as a miR-578 sponge for the regulation
of suppressor of cytokine signaling 2 expression (55). Collectively, these results reveal
that circRNAs serve an important role in regulating the metabolic
reprogramming of tumors, leading to increased tumor cell growth and
proliferation.
CircRNAs are conserved due to a specific loop
structure that is resistant to nucleic acid exonucleases and are
associated with numerous diseases. The metabolic environment of
tumors impacts tumor cell survival and previous studies have
demonstrated the key regulatory role of circRNAs in the tumor
metabolism network. CircRNAs may impact tumorigenesis and disease
progression through alterations in tumor metabolism, acting on
specific target molecules or proteins. Thus, circRNAs have
potential as therapeutic targets in the treatment of cancer. In
addition, determining metabolic differences between malignant and
healthy cells may lead to the development of targeted therapies for
use in clinical practice (Fig.
2).
Effects of circRNAs on glucose
metabolism in NSCLC
Even under aerobic conditions, tumor cells favor
aerobic glycolysis without the tricarboxylic acid cycle, leading to
the evasion of mitochondria-induced oxidative stress damage
(56,57). Glucose metabolic reprogramming is a
key metabolic adaptation in NSCLC that promotes the growth and
proliferation of NSCLC cells, under hypoxic and non-hypoxic
conditions.
CircRNAs act as miRNA sponges and bind to proteins
to affect mRNA and protein expression. Notably, circRNAs manipulate
glucose metabolism-associated enzymes or kinases to regulate the
metabolic reprogramming of glucose. Glucose molecules are ingested
into the cell via GLUT and a series of glucose metabolic reactions
are carried out through the regulation of associated metabolic
enzymes. For instance, in tumor cells, the expression of PKM2 is
elevated, facilitating glycolysis and biosynthesis (58). Results of previous studies
demonstrated that circRNAs affect the growth and proliferation of
tumor cells through regulating glucose transporter proteins and key
enzymes associated with glucose metabolism. Among the identified 14
GLUT isoforms, GLUT1, GLUT3 and GLUT4 are upregulated in malignant
tumor cells (59), suggesting that
they may facilitate glucose transport to provide more energy for
tumor cell growth (60). In
addition, protein expression levels of GLUT1 are decreased in NSCLC
cells following circACACA knockdown, leading to the inhibition of
tumor growth and proliferation (61). Results of a previous study
demonstrate that circENO1 knockdown attenuates glycolysis in LUAD
cells through enolase 1 (62).
Xiong et al (63) report
that circMYLK acts as a molecular sponge for miR-195-5p and
circMYLK knockdown suppresses the expression levels of GLUT3. NSCLC
cells do not favor aerobic glycolysis and inhibit tumor cell
growth, proliferation and lactic acid production (63). For instance, it has been discovered
that the aconitine alkaloid inhibits aerobic glycolysis and
decreases lactate production in NSCLC cells through modulation of
the PI3K/Akt-mTOR signaling pathway (64). Aerobic glycolysis promotes the
expression of glucose transporter proteins to enhance glucose
uptake and glycogen synthesis (65), serving a key role in the regulation
of glucose metabolism. Following aerobic glycolysis in NSCLC, a
tumor microenvironment with high levels of lactate and hypoxia is
established. Notably, activation of glucose metabolism-associated
pathways and increased HIF-1 expression may promote the regulation
of glycolytic metabolism through PKM2.
In addition, circRNAs regulate multiple signaling
pathways, including PI3K/Akt, c-myc and Wnt/β-catenin pathways,
which enable tumor cells to obtain the energy required for rapid
growth. Results of a previous study demonstrate that circHIPK3
absorbs miR-381-3p to regulate the Akt/mTOR signaling pathway,
leading to increased glycolysis and the promotion of tumor cell
growth and proliferation (66).
CircRNA-mediated glucose metabolic reprogramming
results in a hypoxic tumor environment with a low pH and low levels
of nutrients (67). In addition,
high levels of lactic acid may aid tumor cell survival and impair
the growth and development of healthy cells. Targeting key
metabolism-associated pathways and enzymes of the glycolytic
pathway may control the growth and proliferation of NSCLC cells,
thus exhibiting potential in the treatment of cancer. Collectively,
these results demonstrate that circRNAs may be potential targets
for the treatment of lung cancer.
Effects of circRNAs on lipid
metabolism in NSCLC
In healthy cells, the source of fatty acids is
almost entirely dependent on exogenous intake; however, tumor cells
require a large amount of fatty acids to synthesize cell membranes
for rapid growth. Reprogramming of lipid metabolism not only
provides raw materials for cell membrane synthesis in tumor cells
but also provides energy for cell proliferation through fatty acid
oxidation. Notably, products produced through lipid metabolism may
also serve as important components of signal transmission.
Reprogramming of lipid metabolism in tumor cells promotes
tumorigenesis and development via interaction with the tumor
microenvironment. Lipid uptake and storage are increased in tumor
cells to maintain rapid proliferation. Tumor growth and
proliferation are promoted through enhanced lipid synthesis and
reduced catabolism (68). Results
of a previous study reveal that acute fat reduction may lead to
increased tumor malignancy in patients with triple negative breast
cancer (69).
Under non-cancerous conditions, lipids are mainly
produced in hepatocytes and adipocytes. However, tumor cells may
activate lipogenesis in response to high metabolic demands or a
lack of serum-derived lipids in the tumor microenvironment
(70). Disordered lipid metabolism
results in the accumulation of lipid oxides, which, in turn,
induces the activation of oncogenic signaling pathways, e.g., the
PI3K/AKT/mTOR pathway, the MAPK/ERK pathway and the HIF-1α pathway
(71). These pathways promote the
malignant phenotype of tumors and impact neighboring healthy cells.
For instance, JAG1 exerts an influence on vascular endothelial
cells within the tumor microenvironment by stimulating angiogenesis
(72). Cheng et al (73) reveal that alterations in healthy
lipid composition affects the antitumor immunity of T-cells.
Reprogramming of lipid metabolism in tumor cells is
mediated through the production of ATP, the biosynthesis of
macromolecules and maintenance of an appropriate redox state that
supports carcinogenesis, progression, distal metastasis and
chemoresistance. For instance, circIPO7 enhances cisplatin
resistance in NPC cells by interacting with YBX1, thereby promoting
its phosphorylation and nuclear localization, which in turn boosts
the cells' DNA damage repair capability (74). Furthermore, circITGB6 stabilizes
FGF9 mRNA via IGF2BP2, which facilitates the polarization of M2
macrophages and modulates the tumor immune microenvironment,
ultimately promoting ovarian cancer resistance to cisplatin
(75). During the reprogramming of
lipid metabolism, circRNAs alter the metabolic environment of
tumors via regulation of lipid metabolism-associated transcription
factors and key enzymes of lipid metabolism pathways. Thus,
dysregulation of circRNAs may lead to lipid metabolism disorders,
tumor progression and increased drug resistance (76).
Results of a previous study reveal that circFARSA
may affect fatty acid biosynthesis in NSCLC cells through binding
to miR-330-5p and miR-326. When circFARSA binds to these miRNAs, it
alleviates their inhibitory effect on fatty acid synthesis, leading
to an enhancement in fatty acid synthesis (77). Furthermore, circRNA_101093 regulated
ferroptosis in LUAD through lipid peroxidation. This mechanism
holds significant importance in the progression of lung
adenocarcinoma, chemotherapy resistance and the regulation of the
tumor microenvironment. It offers novel targets and insights for
clinical treatment strategies (78). Collectively, these results may
provide a novel theoretical basis for targeting lipid metabolism in
cancer. However, further investigations are required to determine
the specific mechanistic role of circRNAs in regulating lipid
metabolism in NSCLC.
Effects of circRNAs on amino acid
metabolism in NSCLC
Amino acids are involved in protein, purine and
pyrimidine synthesis, bioenergy generation and the maintenance of
redox balance. During the rapid growth and proliferation of tumor
cells, there is an increased demand for energy, amino acids and
nutrients. Tumor cells regulate key pathways of amino acid
metabolism and amino acid transport, for increased amino acid
synthesis that meets the nutritional demands of rapid cell growth.
Moreover, tumor cells may suppress tumor immunity through the
depletion of amino acids and nutrient competition in the
microenvironment. Thus, the reprogramming of amino acid metabolism
may serve an important role in the development and progression of
tumors (79).
Glutamate, as the most abundant non-essential amino
acid in humans, provides a source of carbon or nitrogen for the
synthesis of nucleotides and lipids. In addition, glutamate
provides the biological macromolecules and energy required for cell
growth. Glutaminase catalyzes glutamate for conversion to
glutamine, which participates in the synthesis of glutathione.
Notably, this serves an important role in cellular antioxidant
stress.
Tumor cells have an increased metabolic demand for
glutamine during growth and proliferation and glutamine relies on
glutamine carriers on tumor cell membranes for increased entry into
cells. Results of a previous study reveal that circ_0000518 may
promote NSCLC cell proliferation, invasion and glutamine metabolism
through competitive binding with miR-330-3p. Circ_0000518 inhibited
the activity of miR-330-3p through competitive binding to it
(80). Furthermore,
circOXCT1-mediated upregulation of solute carrier family 1 member 5
(SLC1A5) expression promotes glutamine metabolism and malignant
progression in NSCLC (81). Results
of a previous study demonstrate that IMD-0354 specifically blocks
SLC1A5 and reduces glutamine levels (82), thereby inhibiting tumor
proliferation. Thus, targeting tumor metabolism may exhibit
potential in clinical practice.
In addition, serine/glycine metabolic disorders
serve a key role in tumor progression (83). Serine is involved in nucleotide
metabolism and the tricarboxylic acid cycle and is converted to
glycine to provide carbon units for one-carbon metabolism. However,
research focused on circRNA-mediated synthesis and the catabolic
metabolism of serine/glycine in NSCLC is currently limited.
Numerous circRNAs have been identified as key
regulators of metabolism in NSCLC; however, further investigations
are required to determine the specific role of these non-coding
RNAs. Determining the metabolic differences between patients with
NSCLC and healthy individuals may exhibit potential in the
development of novel therapeutic targets for the treatment of
NSCLC.
Further investigations
CircRNAs serve key regulatory roles in numerous
physiological processes in eukaryotic organisms. Research to date
has focused on the role of circRNAs in gene expression and the
abnormal expression of circRNAs which may be a target for the
treatment of several human diseases (84). In addition, research has focused on
the effect of circRNAs on tumor cells at the molecular level;
however, studies focused on the effect of extracellular factors are
limited. Metabolic reprogramming is considered a key hallmark of
cancer and circRNA-mediated reprogramming of glucose, lipid and
amino acid metabolism provides energy and nutrients for NSCLC cell
growth, leading to tumor progression. Thus, oncogenic signals of
metabolic reprogramming may exhibit potential as therapeutic
targets for the treatment of NSCLC. For instance, studies have
shown that inhibiting the PI3K/Akt/mTOR pathway decreases the
glycolytic activity of NSCLC cells, subsequently impeding tumor
growth (85). Furthermore, it is
noteworthy that distinct cancer types exhibit unique metabolic
reprogramming characteristics (86). For instance, circMBOAT2 promotes the
cytoplasmic export of fatty acid synthase mRNA by stabilizing
polypyrimidine tract-binding protein 1, thereby facilitating lipid
metabolic reprogramming in intrahepatic cholangiocarcinoma
(87). Additionally, studies have
shown that GC-MSC-derived circ_0024107 promotes gastric cancer cell
lymphatic metastasis via fatty acid oxidation metabolic
reprogramming mediated by the miR-5572/6855-5p/carnitine
palmitoyltransferase 1A axis (88).
Furthermore, the 127-amino acid peptide encoded by circSpdyA has
been reported to enhance lipid metabolic reprogramming in breast
cancer, contributing to immune suppression in the tumor
microenvironment (89). These
differences in metabolic reprogramming are probably attributable to
the divergent metabolic demands of various cancers, as well as the
tumor microenvironment's inherent heterogeneity. Immune cells
display considerable heterogeneity within the tumor
microenvironment (TME), with tumor-associated macrophages (TAMs)
capable of exhibiting either a pro-tumor M2 phenotype or an
anti-tumor M1 phenotype (90).
Moreover, the variability in the activity of metabolic enzymes
across different cancers further contributes to these metabolic
discrepancies. Understanding such metabolic differences between
tumors is of paramount importance for the development of targeted
therapeutic strategies. Further investigations into the expression
patterns and functional effects of circRNAs in different tumor
types are required.
CircRNAs exhibit distinct gene expression patterns
across different tumor stages and cancer types (91) and expression is highly specific in
both temporal and tissue contexts (92). Thus, circRNA expression profiles may
be associated with the differing biological behavior of tumor cells
at different stages of lung cancer. Notably differentially
expressed circRNAs exhibit potential as biomarkers for early
diagnosis, prognostic evaluation and monitoring of therapeutic
responses. For instance, circ0001785 has demonstrated superior
diagnostic accuracy compared to traditional markers like CEA and
CA15-3 in detecting breast cancer, and it holds significant
prognostic potential in predicting histological grade, TNM stage
and the occurrence of distant metastasis during breast cancer
progression (93). Furthermore,
previous studies have reported both shared and unique circRNA
expression patterns between LUAD and lung squamous cell carcinoma
(38,94). For example, results of a previous
study reveal that circ_0001821 is markedly upregulated in LUAD;
however, circ_0077837 is notably downregulated in lung squamous
cell carcinoma (95). Thus, the
expression patterns of circRNAs may be specific to different lung
cancer subtypes and disease stages. CircRNAs may exhibit potential
as biomarkers that aid in tumor staging, subtype differentiation
and the development of subtype-specific cancer therapies.
CircRNAs exhibit complex functions and diverse types
and their functional mechanisms remain to be fully elucidated.
CircRNAs exhibit potential in the treatment of cancer due to
diversity in their biological functions. Notably, circRNAs are
endogenous molecules that exert therapeutic effects through
specialized targets. CircRNAs exhibit high levels of stability in
expression; thus, they have potential as therapeutic targets or for
drug delivery. A previous study discusses the development of
circRNA vaccines for coronavirus, with increased use observed in
clinical practice (96). In
addition, the antitumor effects of circRNA vaccines in a mouse
model of advanced malignant melanoma demonstrates the potential of
these non-coding RNAs in the treatment of tumors (97). Notably, circRNAs are generated
through intronic shearing and therefore exert fewer effects on
immunosuppression compared with other RNAs. Therefore, circRNA
vaccines are safer and more stable (98). Further investigations into the
mechanisms underlying circRNAs in tumor metabolism may aid in the
development of novel treatment options and highlight the role of
metabolic reprogramming in the treatment of NSCLC.
CircRNA-mediated metabolic reprogramming may be
closely associated with the efficacy of immune checkpoint
inhibitors (ICIs). Metabolic reprogramming exerts notable effects
on immune cell function within the tumor microenvironment (67) and circRNAs may affect the
effectiveness of ICIs through the direct or indirect regulation of
tumor metabolism and immune modulation pathways (99). Specifically, circRNAs can alter the
tumor microenvironment by driving metabolic reprogramming
processes, such as enhanced glycolysis, lipid metabolism and
glutamine metabolism, resulting in an immunosuppressive
environment. These metabolic changes deplete key nutrients (such as
glucose and glutamine) and lead to the accumulation of metabolic
byproducts (such as lactate), which suppress the function of
effector immune cells, such as T cells and natural killer (NK)
cells, thereby promoting tumor immune evasion. For example,
circSOBP inhibits the progression of glioblastoma by disrupting
glycolysis and promoting the quantity and activity of CD8 T and NK
cells (100). Additionally,
research has demonstrated that circRHBDD1 enhances aerobic
glycolysis in hepatocellular carcinoma and limits the efficacy of
anti-programmed cell death protein 1 (PD-1) therapy (101).
The combined use of circRNA-targeted interventions
and ICI therapy may exhibit potential in improving antitumor immune
responses. For example, the use of specific antisense
oligonucleotides that target and silence circPIAS1, in combination
with PD-1 inhibitors, demonstrated notable tumor inhibition effects
in a model of melanoma. CircPIAS1 inhibits the phosphorylation of
STAT1 and activates the SLC7A11/GPX4 signaling pathway through the
encoding of circPIAS1-108AA, ultimately suppressing immunogenic
ferroptosis and impeding the effectiveness of PD-1 inhibitors
(102). Prior to the
implementation of immunotherapy, analysis of circRNA expression
profiles in patients may provide valuable insights into their
metabolic state and immune microenvironment, leading to the
optimization of personalized ICI treatment strategies.
CircRNAs exhibit potential in vaccine development,
gene regulation and adoptive cell therapy (103). However, the development of
circRNA-targeted therapies for the treatment of lung cancer remains
challenging. The partial sequence overlap between circRNAs and
their linear RNA counterparts leads to challenges in the specific
targeting of circRNAs, without inadvertently affecting linear RNAs
(104). The use of small molecules
or RNA interference tools with a high specificity are therefore
required to target the unique splice junctions of circRNAs. In
addition, effective drug delivery is fundamental for improving
treatment outcomes (105).
Delivering circRNAs or their corresponding inhibitors to specific
cancer sites, while minimizing off-target effects on healthy
tissues, remains challenging. Thus, the development of novel
targeted delivery systems, such as lipid nanoparticles and
exosome-based carriers, are required (106). Furthermore, exogenous circRNAs or
their delivery systems may activate immune responses, leading to
immune-related side effects (107). Thus, chemical modifications of
circRNAs and their delivery vehicles may exhibit potential in
reducing immunogenicity. A previous study reported that m6A
modifications may aid in controlling circRNA-mediated immune
activation (108).
Following additional technological innovation and
interdisciplinary collaboration, circRNAs could be a novel
therapeutic strategy for the treatment of lung cancer. Notably,
lung cancer may metastasize to soft tissues (109). CircRNAs serve a pivotal role in
regulating the metabolic reprogramming of lung cancer cells,
providing adaptive support for cancer cells within soft tissue
metastatic lesions. This regulation enables cancer cells to survive
and proliferate in hypoxic or nutrient-deprived environments
(33). However, further
investigation is required to determine the impact of
circRNA-mediated metabolic reprogramming on lung cancer soft tissue
metastasis.
The metabolic regulatory network is complex and
circRNAs are involved in the regulation of tumor metabolic pathways
that involve multiple target pathways. Moreover, circRNA expression
may be affected by the negative feedback regulation of tumor
metabolites. For instance, in research on gastric cancer (GC), it
has been discovered that the RNA-binding protein QKI promotes the
onset and progression of the disease by regulating the splicing of
genes associated with epithelial-mesenchymal transition (EMT),
leading to the formation of circRNAs. Notably, QKI expression is
elevated during EMT, and it establishes a negative feedback loop
with miR-200. This loop helps maintain a homeostatic balance in
response to signals induced by EMT (110). Thus, the expression profiles of
circRNAs in tumor metabolic pathways should be determined using
metabolomics and spatial transcriptomics, to identify circRNAs that
are co-expressed in tumor metabolism pathways in NSCLC. This may
aid in the development of novel therapeutic drugs that exhibit a
high level of specificity for tumor metabolism.
Conclusions
The present review provides insights into the
association between circRNAs and glucose, lipid and amino acid
metabolism in lung cancer, leading to a novel theoretical basis for
the clinical treatment of lung cancer. CircRNAs exhibit potential
as treatment targets for tumors due to their unique structure and
wide-ranging effects. Exploring the regulatory mechanisms
underlying circRNAs in the metabolic reprogramming of lung cancer
cells may further the current understanding of the tumor metabolic
regulatory network. CircRNA-mediated interference of the metabolic
reprogramming of lung cancer cells may reverse the malignant
transformation of NSCLC cells, highlighting the potential of
circRNAs in lung cancer treatment. However, the challenge of
accurately identifying and detecting circRNAs arises due to their
low cellular abundance and the coexistence of linear mRNAs with
identical sequences. As a result, there is a pressing need for the
development of more precise detection methodologies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science and
Technology Fund of Guizhou Province [grant nos. Qiankehe Basic-ZK
(2022) General 644; Qiankehe Support (2021) General 082].
Availability of data and materials
Not applicable.
Authors' contributions
PZ, ZZ and XZ conceived and designed the review. PZ,
ZZ and YS wrote the manuscript. GX, CC and XK critically revised
and polished the manuscript. Data authentication is not applicable.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen R, Manochakian R, James L, Azzouqa
AG, Shi H, Zhang Y, Zhao Y, Zhou K and Lou Y: Emerging therapeutic
agents for advanced non-small cell lung cancer. J Hematol Oncol.
13:582020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng C, Wang P, Yang Y, Du X, Xia H, Liu
J, Lu L, Wu H and Liu Q: Smoking-induced M2-TAMs, via circEML4 in
EVs, promote the progression of NSCLC through ALKBH5-regulated m6A
modification of SOCS2 in NSCLC cells. Adv Sci (Weinh).
10:e23009532023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu T, Wang X, Jia Y and Cui H:
Autophagy and glycometabolic reprograming in the malignant
progression of lung cancer: A review. Technol Cancer Res Treat.
22:153303382311905452023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng XY, Zhu SX, Pu KJ, Huang HJ, Chen YQ
and Wang WT: New insight into circRNAs: Characterization,
strategies, and biomedical applications. Exp Hematol Oncol.
12:912023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alkhathami AG, Sahib AS, Al Fayi MS,
Fadhil AA, Jawad MA, Shafik SA, Sultan SJ, Almulla AF and Shen M:
Glycolysis in human cancers: Emphasis circRNA/glycolysis axis and
nanoparticles in glycolysis regulation in cancer therapy. Environ
Res. 234:1160072023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J: Circular RNAs: Novel players in
cancer mechanisms and therapeutic strategies. Int J Mol Sci.
25:101212024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du Z: CircNRIP1: An emerging star in
multiple cancers. Pathol Res Pract. 241:1542812023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Hu Z, Ru X, He M, Hu Z, Qin X,
Xiao S, Liu D, Huang H and Wei Q: Hsa_circ_0002005 aggravates
osteosarcoma by increasing cell proliferation, migration, and
invasion. Gene. 942:1492212025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Cai ZR, Huang RZ, Wang DS, Ju HQ and
Chen DL: Circular RNA circPHLPP2 promotes tumor growth and
anti-PD-1 resistance through binding ILF3 to regulate IL36γ
transcription in colorectal cancer. Mol Cancer. 23:2722024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Gu J, Huang J, Wen K, Zhang G,
Chen Z and Wang Z: Characterization of circRNAs in established
osimertinib-resistant non-small cell lung cancer cell lines. Int J
Mol Med. 52:1022023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamali MJ, Salehi M, Mostafavi M,
Morovatshoar R, Akbari M, Latifi N, Barzegari O, Ghadimi F and
Daraei A: Hijacking and rewiring of host CircRNA/miRNA/mRNA
competitive endogenous RNA (ceRNA) regulatory networks by
oncoviruses during development of viral cancers. Rev Med Virol.
34:e25302024. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang MH, Liu ZH, Zhang HX, Liu HC and Ma
LH: Hsa_circRNA_000166 accelerates breast cancer progression via
the regulation of the miR-326/ELK1 and miR-330-5p/ELK1 axes. Ann
Med. 56:24245152024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wang Y, Cheng J, Qiu L, Wang R,
Zhang Y and Wang H: METTL3 -mediated m6A modification of
circ_0000620 regulates cisplatin sensitivity and apoptosis in lung
adenocarcinoma via the MiR-216b-5p/KRAS axis. Cell Signal.
123:1113492024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu H, Han X, Ren J, Ren K, Li Z and Sun Z:
Circular RNA HIPK3 induces cell proliferation and inhibits
apoptosis in non-small cell lung cancer through sponging miR-149.
Cancer Biol Ther. 21:113–121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gadaleta E, Thorn GJ, Ross-Adams H, Jones
LJ and Chelala C: Field cancerization in breast cancer. J Pathol.
257:561–574. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi W, Huang J, Nie C, Liu B, He G, Han J,
Pang R, Ding Z, Xu J and Zhang J: CircRNA circRNA_102171 promotes
papillary thyroid cancer progression through modulating
CTNNBIP1-dependent activation of β-catenin pathway. J Exp Clin
Cancer Res. 37:2752018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Su S, Wang P, Li J, Chen C, Xin H,
Gong Y, Wang H, Ye X, Mao L, et al: Tumor-associated
macrophage-induced circMRCKα encodes a peptide to promote
glycolysis and progression in hepatocellular carcinoma. Cancer
Lett. 591:2168722024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Zhao X, Xu Y, Yan Y, Han L, Wei M
and He M: Potential therapeutic strategy for cancer:
Multi-dimensional cross-talk between circRNAs and parental genes.
Cancer Lett. 588:2167942024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Yin S, Yang K, Zhang B, Wu X, Zhang
M and Gao D: CircRNA regulation of t cells in cancer: Unraveling
potential targets. Int J Mol Sci. 25:63832024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang M, Sun J, Jiang Q, Zhao X, Huang H,
Lei M, Jiang S, Yuan F and Liu Z: CircKIAA0182-YBX1 axis: A key
driver of lung cancer progression and chemoresistance. Cancer Lett.
612:2174942025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin J, Zhao Q, Wei Z, Chen K, Su Y, Hu X
and Peng X: Glycolysis-cholesterol metabolic axis in
immuno-oncology microenvironment: Emerging role in immune cells and
immunosuppressive signaling. Cell Biosci. 13:1892023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Hao Y, Liu Y, Zhong S, You Y, Ao
K, Chong T, Luo X, Yin M, Ye M, et al: NAT10/ac4C/FOXP1 promotes
malignant progression and facilitates immunosuppression by
reprogramming glycolytic metabolism in cervical cancer. Adv Sci
(Weinh). 10:e23027052023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Sun S, Qi Y, Dai Y, Hao Y, Xin M,
Xu R, Chen H, Wu X, Liu Q, et al: Characterization of tumour
microenvironment reprogramming reveals invasion in epithelial
ovarian carcinoma. J Ovarian Res. 16:2002023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Peng M, Tan S, Oyang L, Lin J, Xia
L, Wang J, Wu N, Jiang X, Peng Q, et al: The roles and molecular
mechanisms of non-coding RNA in cancer metabolic reprogramming.
Cancer Cell Int. 24:372024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu CY, Faisal A, Jumaa SS, Gilmanova NS,
Ubaid M, Athab AH, Mirzaei R and Karampoor S: Exploring the impact
of circRNAs on cancer glycolysis: Insights into tumor progression
and therapeutic strategies. Noncoding RNA Res. 9:970–994. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng Y, Jiang J and Wu C: Function and
clinical significance of circRNAs in solid tumors. J Hematol Oncol.
11:982018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang C, Zeng X, Shan R, Wen W, Li J, Tan
J, Li L and Wan R: The emerging picture of the roles of
CircRNA-CDR1as in cancer. Front Cell Dev Biol. 8:5904782020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi
G, Zhichen W, Zirui W and Shengwang W: The roles of miRNA, lncRNA
and circRNA in the development of osteoporosis. Biol Res.
53:402020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Conn VM and Conn SJ: Past, present,
and future strategies for detecting and quantifying circular RNA
variants. FEBS J. Feb 11–2025.(Epub ahead of print). View Article : Google Scholar
|
|
32
|
Yang X, Xia J, Peng C and Cai W:
Expression of plasma exosomal circLPAR1 in patients with gastric
cancer and its clinical application value. Am J Cancer Res.
13:4269–4276. 2023.PubMed/NCBI
|
|
33
|
Liang Y, Wang H, Chen B, Mao Q, Xia W,
Zhang T, Song X, Zhang Z, Xu L, Dong G and Jiang F: circDCUN1D4
suppresses tumor metastasis and glycolysis in lung adenocarcinoma
by stabilizing TXNIP expression. Mol Ther Nucleic Acids.
23:355–368. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang B, Zhang J, Sun X, Yang C, Cheng G,
Xu M, Li S and Wang L: Circulating exosomal hsa_circRNA_0039480 is
highly expressed in gestational diabetes mellitus and may be served
as a biomarker for early diagnosis of GDM. J Transl Med. 20:52022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Safi A, Saberiyan M, Sanaei MJ, Adelian S,
Davarani Asl F, Zeinaly M, Shamsi M and Ahmadi R: The role of
noncoding RNAs in metabolic reprogramming of cancer cells. Cell Mol
Biol Lett. 28:372023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu
Y, Liu X, Cheng W, Wei YQ, Peng Y and Li W: RNA-Seq profiling of
circular RNA in human lung adenocarcinoma and squamous cell
carcinoma. Mol Cancer. 18:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huijbers A, Tollenaar RAEM, v Pelt GW,
Zeestraten ECM, Dutton S, McConkey CC, Domingo E, Smit VTHBM,
Midgley R, Warren BF, et al: The proportion of tumor-stroma as a
strong prognosticator for stage II and III colon cancer patients:
Validation in the VICTOR trial. Ann Oncol. 24:179–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang T, Xu J, Shen H, Dong W, Ni Y and Du
J: Tumor-stroma ratio is an independent predictor for survival in
NSCLC. Int J Clin Exp Pathol. 8:11348–11355. 2015.PubMed/NCBI
|
|
41
|
Gujam FJA, Edwards J, Mohammed ZMA, Going
JJ and McMillan DC: The relationship between the tumour stroma
percentage, clinicopathological characteristics and outcome in
patients with operable ductal breast cancer. Br J Cancer.
111:157–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Riester M, Xu Q, Moreira A, Zheng J,
Michor F and Downey RJ: The Warburg effect: Persistence of
stem-cell metabolism in cancers as a failure of differentiation.
Ann Oncol. 29:264–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cai ZR, Hu Y, Liao K, Li H, Chen DL and Ju
HQ: Circular RNAs: Emerging regulators of glucose metabolism in
cancer. Cancer Lett. 552:2159782023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T
and Shu Y: CircRNAs in cancer metabolism: A review. J Hematol
Oncol. 12:902019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
San-Millan I, Sparagna GC, Chapman HL,
Warkins VL, Chatfield KC, Shuff SR, Martinez JL and Brooks GA:
Chronic lactate exposure decreases mitochondrial function by
inhibition of fatty acid uptake and cardiolipin alterations in
neonatal rat cardiomyocytes. Front Nutr. 9:8094852022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brooks GA: Lactate as a fulcrum of
metabolism. Redox Biol. 35:1014542020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qin R, Fan X, Huang Y, Chen S, Ding R, Yao
Y, Wu R, Duan Y, Li X, Khan HU, et al: Role of glucose metabolic
reprogramming in colorectal cancer progression and drug resistance.
Transl Oncol. 50:1021562024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Watson MJ, Vignali PDA, Mullett SJ,
Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV,
Rittenhouse NL, DePeaux K, Whetstone RD, et al: Metabolic support
of tumour-infiltrating regulatory T cells by lactic acid. Nature.
591:645–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Zhou H, Liu Y, Zhao X, Wang S and
Lin Z: miR-485-5p/NQO1 axis drives colorectal cancer progression by
regulating apoptosis and aerobic glycolysis. Cancer Cell Int.
25:412025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Ren B, Ren J, Gu M, You L and Zhao
Y: The significant role of amino acid metabolic reprogramming in
cancer. Cell Commun Signal. 22:3802024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin Q, Jiang H and Lin D: Circular RNA
ITCH downregulates GLUT1 and suppresses glucose uptake in melanoma
to inhibit cancer cell proliferation. J Dermatolog Treat.
32:231–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao L, Wang M, Dong Y, Xu B, Chen J, Ding
Y, Qiu S, Li L, Karamfilova Zaharieva E, Zhou X and Xu Y: Circular
RNA circRNF20 promotes breast cancer tumorigenesis and Warburg
effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 11:1452020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Q, Pan X, Zhu D, Deng Z, Jiang R and
Wang X: Circular RNA MAT2B promotes glycolysis and malignancy of
hepatocellular carcinoma through the miR-338-3p/PKM2 axis under
hypoxic stress. Hepatology. 70:1298–1316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang B, Zhao F, Yao L, Zong Z and Xiao L:
CircRNA circ_0006677 inhibits the progression and glycolysis in
non-small-cell lung cancer by sponging miR-578 and Regulating SOCS2
expression. Front Pharmacol. 12:6570532021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
DeBerardinis RJ and Thompson CB: Cellular
metabolism and disease: What do metabolic outliers teach us? Cell.
148:1132–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pascale RM, Calvisi DF, Simile MM, Feo CF
and Feo F: The Warburg effect 97 years after its discovery. Cancers
(Basel). 12:28192020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L
and Jiang F: PKM2 and cancer: The function of PKM2 beyond
glycolysis. Oncol Lett. 11:1980–1986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fan C, Tang Y, Wang J, Xiong F, Guo C,
Wang Y, Zhang S, Gong Z, Wei F, Yang L, et al: Role of long
non-coding RNAs in glucose metabolism in cancer. Mol Cancer.
16:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yadav D, Yadav A, Bhattacharya S, Dagar A,
Kumar V and Rani R: GLUT and HK: Two primary and essential key
players in tumor glycolysis. Semin Cancer Biol. 100:17–27. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu W, Xi W, Li H, Yang M and Yao X:
Circular RNA circ-ACACA regulates proliferation, migration and
glycolysis in non-small-cell lung carcinoma via miR-1183 and
PI3K/PKB pathway. Int J Mol Med. 45:1814–1824. 2020.PubMed/NCBI
|
|
62
|
Zhou J, Zhang S, Chen Z, He Z, Xu Y and Li
Z: CircRNA-ENO1 promoted glycolysis and tumor progression in lung
adenocarcinoma through upregulating its host gene ENO1. Cell Death
Dis. 10:8852019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiong S, Li D, Wang D, Huang L, Liang G,
Wu Z, Long J, Yang D, Teng Y, Lei S and Li Y: Circular RNA MYLK
promotes glycolysis and proliferation of non-small cell lung cancer
cells by sponging miR-195-5p and increasing glucose transporter
member 3 expression. Cancer Manag Res. 12:5469–5478. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang W, Cai S, Qin L, Feng Y, Ding M, Luo
Z, Shan J and Di L: Alkaloids of aconiti lateralis radix praeparata
inhibit growth of non-small cell lung cancer by regulating
PI3K/Akt-mTOR signaling and glycolysis. Commun Biol. 7:11182024.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Starska K, Forma E, Jóźwiak P, Bryś M,
Lewy-Trenda I, Brzezińska-Błaszczyk E and Krześlak A: Gene and
protein expression of glucose transporter 1 and glucose transporter
3 in human laryngeal cancer-the relationship with regulatory
hypoxia-inducible factor-1α expression, tumor invasiveness, and
patient prognosis. Tumour Biol. 36:2309–2321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gu F, Zhang J, Yan L and Li D:
CircHIPK3/miR-381-3p axis modulates proliferation, migration, and
glycolysis of lung cancer cells by regulating the AKT/mTOR
signaling pathway. Open Life Sci. 15:683–695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lan T, Gao F, Cai Y, Lv Y, Zhu J, Liu H,
Xie S, Wan H, He H, Xie K, et al: The protein circPETH-147aa
regulates metabolic reprogramming in hepatocellular carcinoma cells
to remodel immunosuppressive microenvironment. Nat Commun.
16:3332025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bian X, Liu R, Meng Y, Xing D, Xu D and Lu
Z: Lipid metabolism and cancer. J Exp Med. 218:e202016062021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gómez de Cedrón M and Ramírez de Molina A:
Microtargeting cancer metabolism: Opening new therapeutic windows
based on lipid metabolism. J Lipid Res. 57:193–206. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Khan F, Elsori D, Verma M, Pandey S,
Obaidur Rab S, Siddiqui S, Alabdallah NM, Saeed M and Pandey P:
Unraveling the intricate relationship between lipid metabolism and
oncogenic signaling pathways. Front Cell Dev Biol. 12:13990652024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu J, Shi Y, Wu M, Xu M, Zhang F, He Z
and Tang M: JAG1 promotes migration, invasion, and adhesion of
triple-negative breast cancer cells by promoting angiogenesis. Nan
Fang Yi Ke Da Xue Xue Bao. 42:1100–1108. 2022.(In Chinese).
PubMed/NCBI
|
|
73
|
Cheng X, Wang W, Zhang Z, Zhang H, Zhu P,
He R, Wu M, Zhou T, Jiang Y, Jiang L, et al: Distinctly altered
lipid components in hepatocellular carcinoma relate to impaired T
cell-dependent antitumor immunity. Hepatol Int. 18:582–594. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hong X, Li Q, Li J, Chen K, He Q, Zhao Y,
Liang Y, Zhao Y, Qiao H, Liu N, et al: CircIPO7 promotes
nasopharyngeal carcinoma metastasis and cisplatin chemoresistance
by facilitating YBX1 nuclear localization. Clin Cancer Res.
28:4521–4535. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li H, Luo F, Jiang X, Zhang W, Xiang T,
Pan Q, Cai L, Zhao J, Weng D, Li Y, et al: CircITGB6 promotes
ovarian cancer cisplatin resistance by resetting tumor-associated
macrophage polarization toward the M2 phenotype. J Immunother
Cancer. 10:e0040292022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu YL, Li HF, Chen HH and Lin H: Emergent
roles of circular RNAs in metabolism and metabolic disorders. Int J
Mol Sci. 23:10322022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hang D, Zhou J, Qin N, Zhou W, Ma H, Jin
G, Hu Z, Dai J and Shen H: A novel plasma circular RNA circFARSA is
a potential biomarker for non-small cell lung cancer. Cancer Med.
7:2783–2791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X,
Shi Y, Guo S, Xue X, Wang Y, et al: Essential roles of exosome and
circRNA_101093 on ferroptosis desensitization in lung
adenocarcinoma. Cancer Commun (Lond). 42:287–313. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zheng Y, Yao Y, Ge T, Ge S, Jia R, Song X
and Zhuang A: Amino acid metabolism reprogramming: Shedding new
light on T cell anti-tumor immunity. J Exp Clin Cancer Res.
42:2912023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lv H, Shi Z, Sui A, Zhang Y, Peng L, Wang
M and Zhang F: hsa_circ_0000518 facilitates non-small-cell lung
cancer progression via moderating miR-330-3p and positively
regulating SLC1A5. J Immunol Res. 2022:49969802022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Luo H, Peng J and Yuan Y: CircRNA OXCT1
promotes the malignant progression and glutamine metabolism of
non-small cell lung cancer by absorbing miR-516b-5p and
upregulating SLC1A5. Cell Cycle. 22:1182–1195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Feng Y, Pathria G, Heynen-Genel S, Jackson
M, James B, Yin J, Scott DA and Ronai ZA: Identification and
characterization of IMD-0354 as a glutamine carrier protein
inhibitor in melanoma. Mol Cancer Ther. 20:816–832. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qiu W, Zhang S, Yu W, Liu J and Wu H:
Non-coding RNAs in hepatocellular carcinoma metastasis: Remarkable
indicators and potential oncogenic mechanism. Comput Biol Med.
180:1088672024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Luo J, Ng W, Liu Y, Wang L, Gong C, Zhou
Y, Fang C, Zhu S and Yao C: Rocaglamide promotes infiltration and
differentiation of T cells and coordinates with PD-1 inhibitor to
overcome checkpoint resistance in multiple tumor models. Cancer
Immunol Immunother. 73:1372024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Faubert B, Solmonson A and DeBerardinis
RJ: Metabolic reprogramming and cancer progression. Science.
368:eaaw54732020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yu X, Tong H, Chen J, Tang C, Wang S, Si
Y, Wang S and Tang Z: CircRNA MBOAT2 promotes intrahepatic
cholangiocarcinoma progression and lipid metabolism reprogramming
by stabilizing PTBP1 to facilitate FASN mRNA cytoplasmic export.
Cell Death Dis. 14:202023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang L, Wu C, Xu J, Gong Z, Cao X, Huang
J, Dong H, Zhu W, Huang F, Zhou C and Wang M: GC-MSC-derived
circ_0024107 promotes gastric cancer cell lymphatic metastasis via
fatty acid oxidation metabolic reprogramming mediated by the
miR-5572/6855-5p/CPT1A axis. Oncol Rep. 50:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao X, Sun Z, Liu X, Luo J, Liang X, Wang
H, Zhou J, Yang C, Wang T and Li J: 127aa encoded by circSpdyA
promotes FA synthesis and NK cell repression in breast cancers.
Cell Death Differ. Oct 14–2024.(Epub ahead of print). View Article : Google Scholar
|
|
90
|
Sabit H, Arneth B, Pawlik TM, Abdel-Ghany
S, Ghazy A, Abdelazeem RM, Alqosaibi A, Al-Dhuayan IS, Almulhim J,
Alrabiah NA and Hashash A: Leveraging single-cell multi-omics to
decode tumor microenvironment diversity and therapeutic resistance.
Pharmaceuticals (Basel). 18:752025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
DeSouza NR, Nielsen KJ, Jarboe T, Carnazza
M, Quaranto D, Kopec K, Suriano R, Islam HK, Tiwari RK and
Geliebter J: Dysregulated expression patterns of circular RNAs in
cancer: Uncovering molecular mechanisms and biomarker potential.
Biomolecules. 14:3842024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xie C, Hao X, Yuan H, Wang C, Sharif R and
Yu H: Crosstalk between circRNA and tumor microenvironment of
hepatocellular carcinoma: Mechanism, function and applications.
Onco Targets Ther. 17:7–26. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yin WB, Yan MG, Fang X, Guo JJ, Xiong W
and Zhang RP: Circulating circular RNA hsa_circ_0001785 acts as a
diagnostic biomarker for breast cancer detection. Clin Chim Acta.
487:363–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang C, Yu Q, Song T, Wang Z, Song L, Yang
Y, Shao J, Li J, Ni Y, Chao N, et al: The heterogeneous immune
landscape between lung adenocarcinoma and squamous carcinoma
revealed by single-cell RNA sequencing. Signal Transduct Target
Ther. 7:2892022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nicot C: RNA-Seq reveal the circular RNAs
landscape of lung cancer. Mol Cancer. 18:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Qu L, Yi Z, Shen Y, Lin L, Chen F, Xu Y,
Wu Z, Tang H, Zhang X, Tian F, et al: Circular RNA vaccines against
SARS-CoV-2 and emerging variants. Cell. 185:1728–1744.e16. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li H, Peng K, Yang K, Ma W, Qi S, Yu X, He
J, Lin X and Yu G: Circular RNA cancer vaccines drive immunity in
hard-to-treat malignancies. Theranostics. 12:6422–6436. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang W, Xu C, Yang Z, Zhou J, Peng W,
Zhang X, Li H, Qu S and Tao K: Circular RNAs in tumor immunity and
immunotherapy. Mol Cancer. 23:1712024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhou J, Xu H, Li X, Liu H, Sun Z, Li J,
Tang Y, Gao H, Zhao K, Ding C and Gao X: Targeting tumorous
Circ-E-Cadherinencoded C-E-Cad inhibits the recruitment and
function of breast cancer-associated myeloid-derived suppressor
cells. Pharmacol Res. 204:1072042024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mu M, Niu W, Chu F, Dong Q, Hu S and Niu
C: CircSOBP suppresses the progression of glioma by disrupting
glycolysis and promoting the MDA5-mediated immune response.
iScience. 26:1078972023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cai J, Chen Z, Zhang Y, Wang J, Zhang Z,
Wu J, Mao J and Zuo X: CircRHBDD1 augments metabolic rewiring and
restricts immunotherapy efficacy via m6A modification in
hepatocellular carcinoma. Mol Ther Oncolytics. 24:755–771. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zang X, He XY, Xiao CM, Lin Q, Wang MY,
Liu CY, Kong LY, Chen Z and Xia YZ: Circular RNA-encoded oncogenic
PIAS1 variant blocks immunogenic ferroptosis by modulating the
balance between SUMOylation and phosphorylation of STAT1. Mol
Cancer. 23:2072024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cai J, Qiu Z, Chi-Shing Cho W, Liu Z, Chen
S, Li H, Chen K, Li Y, Zuo C and Qiu M: Synthetic circRNA
therapeutics: Innovations, strategies, and future horizons. MedComm
(2020). 5:e7202024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dance A: Circular logic: Understanding
RNA's strangest form yet. Nature. 635:511–513. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Aquino-Jarquin G: CircRNA knockdown based
on antisense strategies. Drug Discov Today. 29:1040662024.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhao X, Zhong Y, Wang X, Shen J and An W:
Advances in circular RNA and its applications. Int J Med Sci.
19:975–985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen YG, Kim MV, Chen X, Batista PJ,
Aoyama S, Wilusz JE, Iwasaki A and Chang HY: Sensing self and
foreign circular RNAs by intron identity. Mol Cell. 67:228–238.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen YG, Chen R, Ahmad S, Verma R, Kasturi
SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, et al:
N6-methyladenosine modification controls circular RNA immunity. Mol
Cell. 76:96–109.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hashimoto K, Nishimura S and Akagi M: Lung
adenocarcinoma presenting as a soft tissue metastasis to the
shoulder: A case report. Medicina (Kaunas). 57:1812021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cui YS, Zheng X, Wu YN, Yao YH, Wang J,
Liu ZQ and Sun GG: The RNA binding protein QKI can promote gastric
cancer by regulating cleavage of EMT-related gene transcripts to
form circRNAs. Chin Pharmacol Bull. 40:1462–1473. 2024.(In
Chinese).
|