Introduction
Schwannomas, also referred to as neurilemmomas, are
the most common type of benign peripheral nerve sheath tumor
(BPNST), originating from Schwann cells and present predominantly
in the hands and upper limbs (1–4). While
PNSTs are rare, schwannomas are the most prevalent type, accounting
for nearly 5% of all soft-tissue tumors of the upper limb (5). The global incidence of schwannomas is
reported to be 3–4 cases per million individuals (6). As BPNSTs, schwannomas typically grow
slowly, with a relatively low incidence of malignant
transformation, generally ranging from 1 to 13% (7). Therefore, the prognosis for the vast
majority of patients is favorable, and the mortality rate is
relatively low. However, for patients at risk of malignant
transformation, especially those with pathogenic genes (such as
NF2, SMARCB1 and LZTR1), there may be a higher risk of
complications (8). Approximately
19% of all cases of schwannomas occur in the upper limb, with the
distal region near the elbow being the most common tumor site
(9). These tumors tend to originate
along major nerves and on the flexor surfaces of the upper and
lower limbs (10,11). Surgical intervention has been
reported as the primary treatment and is relatively effective for
schwannomas (12). However, to the
best of our knowledge, studies on multiple schwannomas in the upper
limb are scarce. The rarity of this condition and the ambiguity in
the diagnostic criteria may lead to an underestimation of the
actual incidence of multiple schwannomas. Therefore, it is
imperative to conduct standardized studies on multiple schwannomas
in the upper limb, including studies on diagnosis, surgical
management and postoperative care. In this context, the present
study discusses the case of a patient with multiple schwannomas in
the upper limb. This report also includes a brief review of the
literature on the pathogenesis, clinical features, diagnosis and
treatment of schwannomas, with the objective of providing novel
insights into their management.
Case report
A 70-year-old man presented at Guangzhou Red Cross
Hospital (Guangzhou, China) in January 2024 with a mass in the left
upper limb that had existed for >40 years. Initially, this mass
was solid and oval in shape, measuring ~1×2 cm on the medial aspect
of the left upper limb. No specific treatment was received for the
mass. Over the past 20 years, the mass had progressively enlarged,
and new masses have developed near the left elbow and the
hypothenar region. The patient reported no pain or tenderness, and
all the masses were cystic-solid upon palpation. The results of the
neurological examination were unremarkable. The masses were mobile
in the transverse plane but not along the longitudinal axis. The
overlying skin exhibited no signs of redness, swelling or
ulceration, and no increase in skin temperature was noted. No
family member had a history of schwannomas. Given these
observations, it was plausible to conclude that this case was more
likely to represent a sporadic schwannoma.
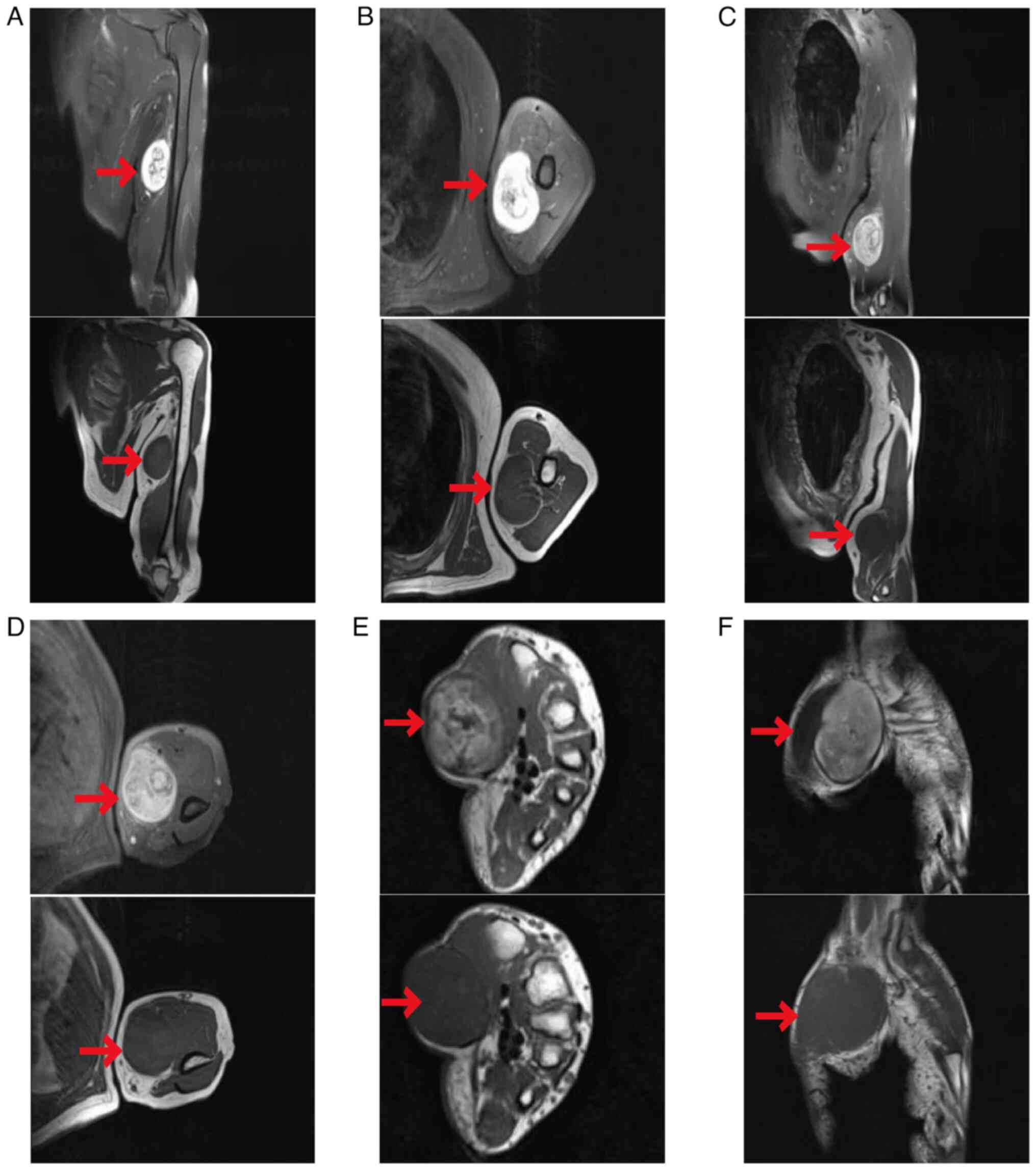
The magnetic resonance imaging (MRI) examination
conducted upon admission revealed two ovoid masses located in the
ulnar nerve path in the middle to distal part of the left upper
arm, with slight hyperintensity on T1-weighted images and
heterogeneous hyperintensity on T2-weighted images. The masses were
~4.2×5.8×6.1 and 5.0×5.6×6.5 cm in size. The T2-weighted images
revealed target signs, with the masses closely related to the
nerve. No signal suppression was noted on fat-saturated sequences,
and the enhancement of the masses was heterogeneous, with
progressive enhancement on delayed-phase imaging. The adjacent
muscles exhibited signs of compression displacement with distinct
delineations. In addition, long ovoid nodular lesions with similar
signal characteristics were observed on the radial side of the
flexor tendon sheath close to the wrist and superficial to the
thenar muscles on the volar aspect of the left forearm. These
lesions were ~1.4×2.8 and 3.0×3.7×5.1 cm in size, respectively, and
exhibited marked heterogeneous enhancement on contrast-enhanced
images (Fig. 1).
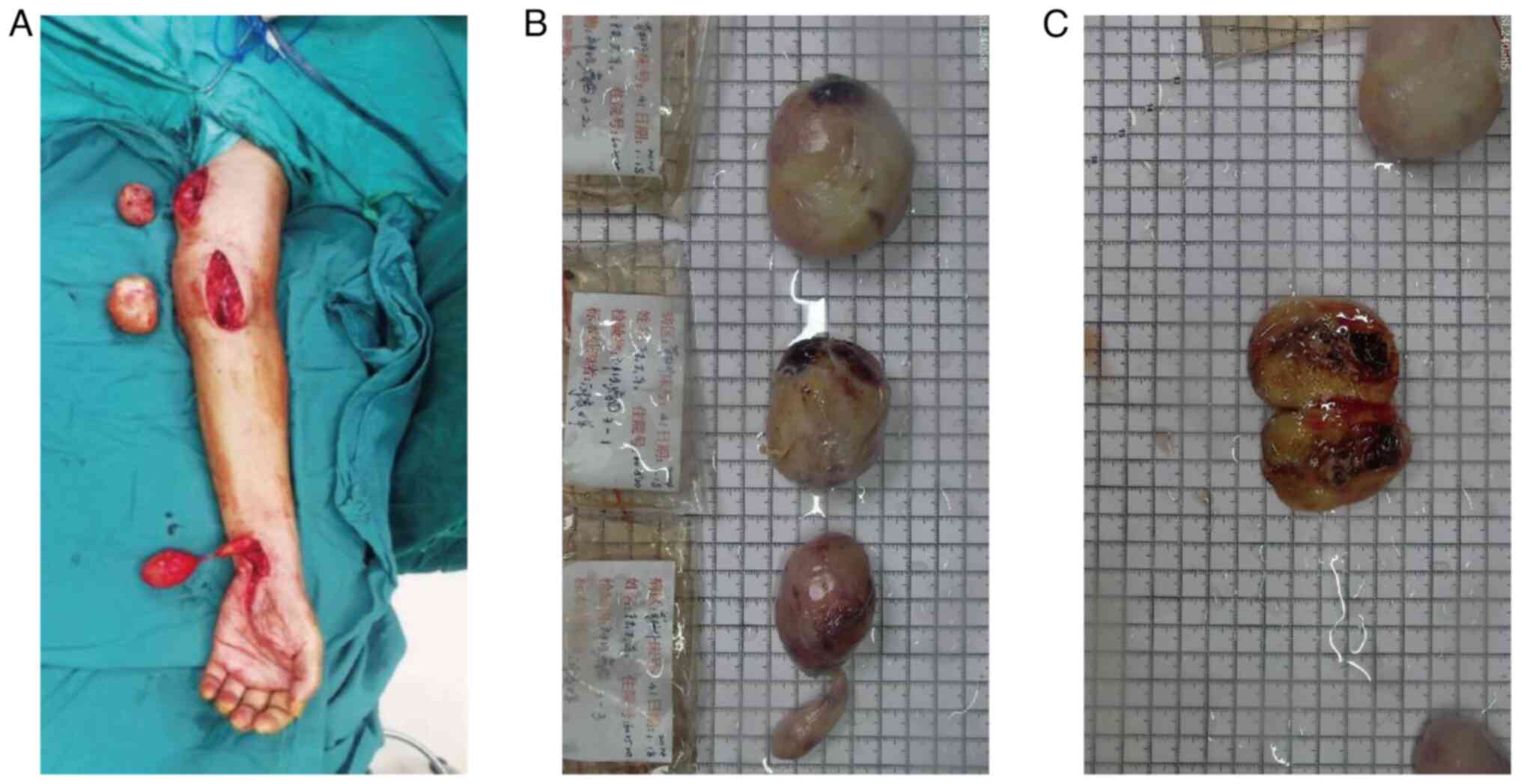
The patient underwent excision of the left upper
limb mass, median nerve exploration and pathological biopsy under
general anesthesia with endotracheal intubation. A longitudinal
incision was made along the long axis of the tumor, followed by an
incision on the skin and subcutaneous tissue. The tumor was bluntly
dissected and exposed. A capsule was observed, although it was a
focal discontinuity. The tumor adhered to the surrounding skeletal
muscle and the median nerve, which was most consistent with a
benign neurogenic tumor. Careful dissection of the surrounding
nerves, blood vessels and tendons was performed. The tumor
dimensions from proximal to distal were 6×5×3, 5×4×3.5 and 5×3.5×3
cm, respectively (Fig. 2). The
incision was closed in layers, and the tumors were sent for
pathological evaluation. The postoperative course remained
uneventful, and complete tumor resection was achieved without any
damage to the surrounding nerves. The patient exhibited no sensory
or circulatory abnormalities in the surgical region or the distal
limb, and no complications occurred. The patient was, therefore,
discharged with a healed incision.
Postoperative pathological examination revealed the
following gross findings: i) Subcutaneous mass no. 1 in the left
upper limb: A grayish-red to grayish-white mass measuring 6×5×3 cm,
with the excised surface exhibiting grayish-red to grayish-white
coloration and soft consistency. ii) Subcutaneous mass no. 2 in the
left upper limb: A grayish-red to grayish-white mass measuring
5×4×3.5 cm in size, with the excised surface exhibiting grayish-red
to grayish-yellow coloration, a moderate texture and associations
with regions of hemorrhage. iii) Subcutaneous mass no. 3 in the
left upper limb: A grayish-red mass measuring 5×3.5×3 cm, with a
tail 5 cm in length and ~1 cm in diameter, exhibiting a grayish-red
to grayish-yellow excised surface with a soft consistency and solid
texture. After tissue sampling, fixation with 10% neutral formalin
at room temperature for 8 h, dehydration, embedding with paraffin,
sectioning at a thickness of 4 µm and staining, the prepared slides
were examined under a Nikon Eclipse CI optical microscope (Nikon
Corporation). Hematoxylin and eosin (H&E) staining was
performed on the prepared slides. After dewaxing and rehydrating at
room temperature, the slides were stained with hematoxylin for 3
min, differentiated and rinsed before being stained with eosin for
9 min. Following rinsing, dehydration and clearing, the slides were
baked at 67°C for 15 min, dried for 10 min and then mounted for
observation. H&E staining revealed typical fascicular regions
(Antoni A regions) and reticular regions (Antoni B regions). The
Antoni A regions contained spindle-shaped cells arranged in
fascicular and palisading patterns, with Verocay bodies, with a few
regions densely packed, while other regions were more loosely
arranged, with visible blood vessels. The Antoni B regions
presented with hemosiderin deposition around the blood vessels,
along with chronic hemorrhage and cystic changes, suggesting a
degenerative schwannoma. Mild nuclear atypia (degenerative changes)
was observed in some cells, with no increased mitotic activity. The
stroma showed loose myxoid changes, with abundant vascular
structures exhibiting congestion and hemorrhage, accompanied by
fibrosis, hyaline degeneration and localized calcification,
indicating a degenerative schwannoma (Fig. 3A-D). To accurately describe the
tumor components, immunohistochemical (IHC) staining was performed
on several representative paraffin block sections using the
ChemMate Envision method (DakoCytomation; Agilent Technologies,
Inc.). IHC was performed using a ready-to-use ChemMate Envision kit
from DakoCytomation; Agilent Technologies, Inc., [Kit details:
Anti-p53 (DO-7) monoclonal antibody, cat. no. M7001; anti-Ki-67
(30–9) rabbit monoclonal primary antibody, cat. no. 790-4286; S100
antibody, cat. no. CSM-0101] following the manufacturer's
instructions. The immunohistochemistry analysis revealed positive
expression for S100, p53 (~2%) and Ki-67 (hotspot region ~2%).
S-100 immunohistochemical staining showed positivity in both the
cytoplasm and nuclei of tumor cells, with some regions primarily
exhibiting cytoplasmic positivity, while others demonstrated both
cytoplasmic and nuclear dual positivity, suggesting that the latter
may indicate higher tumor activity. p53 immunohistochemical
staining demonstrated focal positivity in scattered tumor cells
with variable staining intensity, showing colors ranging from light
to dark brown. Immunohistochemical staining revealed a Ki-67
proliferation index of 2%, indicating the presence of tumor cells
(Fig. 3E-H). Based on the
histological morphology of schwannomas in the microscopic
examination section of the third edition of ‘Diagnostic Pathology:
Soft Tissue Tumors’ (13), the
pathological diagnosis was established as multiple schwannomas of
the left upper limb with secondary degenerative hemorrhage. During
the 1-year postoperative follow-up period, the patient underwent
seven clinical examinations. The surgical wounds healed well
without significant complications, and physical examinations
revealed no evidence of tumor recurrence.
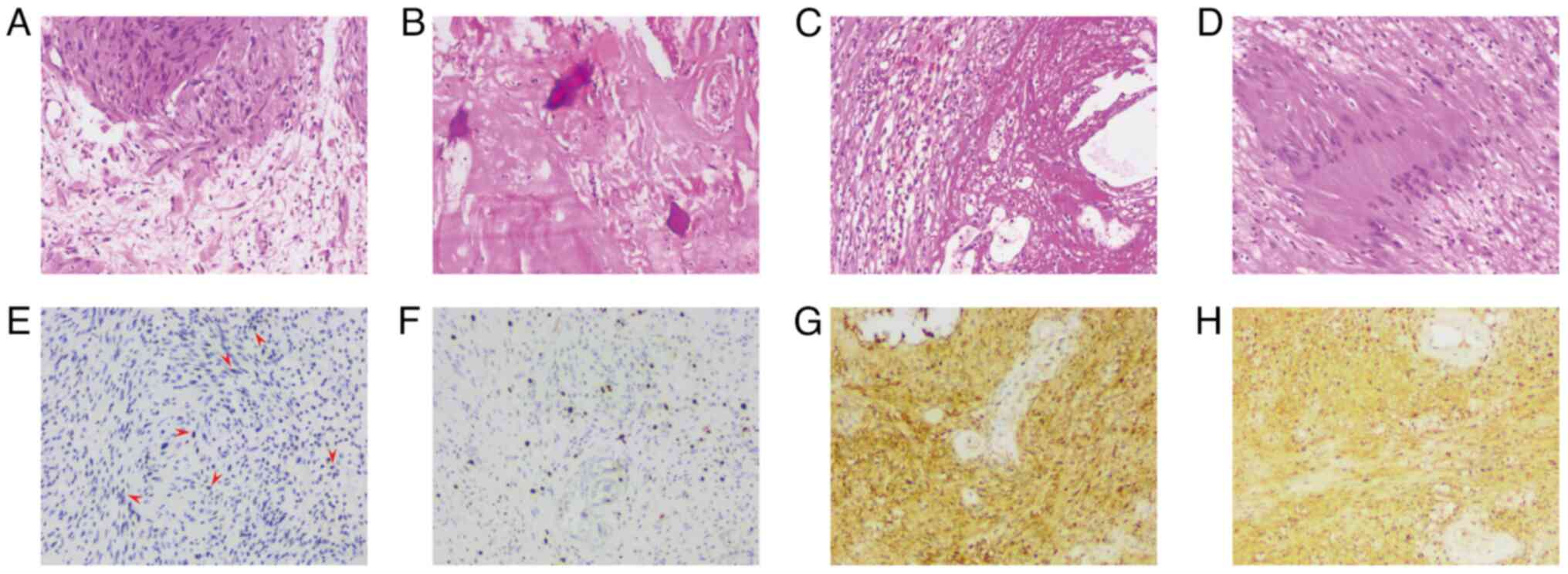 | Figure 3.Tumor H&E and immunohistochemistry
staining. (A-D) H&E staining and (E-H) immunohistochemical
staining images of schwannomas (all ×100 magnification). (A) The
diagnostic morphology of the schwannoma revealed typical fascicular
regions (Antoni A regions) and reticular regions (Antoni B
regions). The Antoni A regions contained spindle cells arranged in
fascicles or interwoven patterns, and Verocay bodies were also
observed; the lower region exhibited a loose pattern, with visible
blood vessels. (B) Noticeable hyaline degeneration of the tumor
stroma and punctate calcification were present, indicating a
degenerative schwannoma. (C) The Antoni B region presented with
hemosiderin deposition around the blood vessels, along with chronic
hemorrhage and cystic changes, suggesting a degenerative
schwannoma. (D) Antoni A regions exhibited spindle cells in
fascicular or interwoven arrangements, and Verocay bodies were
present. (E) p53 immunohistochemical staining demonstrated focal
positivity in scattered tumor cells with variable staining
intensity. Red arrows point to some of these positively stained
cells or cell clusters, which are colored in varying shades from
light to dark brown. (F) Immunohistochemical staining revealed a
Ki-67 proliferation index of 2%, indicating the presence of tumor
cells. (G and H) Immunohistochemical staining for S-100 revealed
positivity in the cytoplasm and nuclei of the tumor cells.
Cytoplasmic positivity is mainly shown in (G), while (H) exhibits
both cytoplasmic and nuclear dual positivity, suggesting that the
latter may indicate higher tumor activity. H&E, hematoxylin and
eosin. |
Discussion
Schwannomas are non-invasive tumors of the
peripheral nerve sheath. Studies have indicated that schwannomas
are the most common PNSTs in the upper limb, with an incidence rate
of 64% (12). No significant sex or
ethnicity differences have been noted in this context (14). In a 22-year epidemiological study
conducted by Sandberg et al (12), which studied a total of 68 tumors in
53 patients, the median nerve was revealed to be the most
frequently affected nerve, followed by the ulnar and digital
nerves.
Research has shown that schwannomatosis and
neurofibromatosis type 2 (NF2) are both characterized by multiple
schwannomas but represent distinct genetic entities. NF2 is caused
by mutations in the NF2 gene, whereas schwannomatosis is associated
with germline mutations in SMARCB1 or LZTR1 (15). In the epidemiological study on
schwannomas by Forde et al (16), the prevalence of LZTR1-related
schwannomatosis and SMARCB1-related schwannomatosis was ~1 in
527,000 and ~1 in 1,100,000, respectively, which is 8.4 to 18.4
times lower than the prevalence of NF2. This highlights the
significant role of NF2 in the genetic diagnosis of schwannomas
(17). In the present case, due to
the need for outsourcing high-throughput sequencing, the associated
costs and the patient's personal preferences, genetic sequencing
could not be performed to confirm the presence of any alterations
in the NF2 gene.
The clinical presentation of schwannomas varies
notably. Certain reports indicate that these tumors may be
painless, although this lack of pain may be attributed to their
slow growth, indirect compression of the nerve and absence of
sensory innervation in the tumor (18–21). A
comprehensive retrospective analysis by Iwashita, which examined
1,202 patients with 1,271 schwannomas, demonstrated a notably low
incidence of pain. The histopathological classification of the
patients revealed several distinct types: Common (n=932),
degenerative (n=238), cellular (n=19), plexiform (n=35), pigmented
(n=2), myxoma (n=16) and organoid (n=30), with pain manifestation
predominantly observed in myxoma and organoid variants (19). Additionally, symptoms such as pain,
sensory abnormalities and a positive Tinel's sign are often
associated with the location of the tumor. As the tumor grows and
compresses more sensitive nerve regions, the patient may experience
pain or other symptoms. A review of the literature on patients with
schwannomas in the hand and wrist revealed that the interval
between symptom onset and surgery ranged from a few months to 37
years (14). Tumors located in the
finger regions tend to cause symptoms earlier than those located in
the wrist and the palm. In the present case, the combination of the
patient's 40-year clinical history and subsequent histopathological
findings from H&E staining strongly indicated a degenerative
variant of schwannoma (19).
When considering differential diagnoses, it is
important to identify benign tumors that could be confused with
schwannomas. The tumors that should be distinguished from
schwannomas include neurofibromas, neuroblastomas and
ganglioneuromas. Clinically, no distinct standard exists for
differentiating schwannomas from neurofibromas. However, the
literature suggests that the primary distinction between the two
lies in the age of the patients in which they occur, as while
neurofibromas predominantly originate in individuals aged 20–30
years, schwannomas are more common in those aged 30–50 years
(12,14). Nevertheless, distinguishing
schwannomas from other tumors based solely on clinical history and
imaging findings remains considerably challenging.
Ultrasound examination may be utilized to establish
a preoperative diagnosis of soft-tissue tumors in the extremities.
According to reports, an accuracy rate of up to 77% may be achieved
when ultrasound is used as an initial diagnostic modality (22). Ultrasound, however, is unable to
differentiate between schwannomas and other tumors, as all tumors
typically present as similar hypoechoic masses with posterior
acoustic enhancement. Typically, uncharacterized soft-tissue masses
in the upper limb that are suspected to be related to PNSTs require
further evaluation through MRI examination, which reportedly has
75% diagnostic accuracy in the case of these tumors (23). Schwannomas typically present a
homogeneous central low signal and a peripheral high signal,
referred to as the target sign, although this is not exclusive to
schwannomas (22). While MRI is
more accurate for determining tumor location and establishing the
differential diagnosis of schwannomas, it does not achieve 100%
diagnostic accuracy. In addition, fine-needle aspiration or open
biopsy is unsuitable for a definitive diagnosis, as these
procedures may lead to scarring and potential fascicular injury
during subsequent excision (11).
Therefore, despite the availability of appropriate clinical and
imaging evidence, the definitive diagnosis of schwannomas continues
to rely on histopathological examination.
Currently, no pharmacological therapies are
recommended as alternatives to surgery for the treatment of
schwannomatosis (24). In
asymptomatic schwannomas that are stable in size, a period of
observation may be appropriate. In the management of benign
schwannomas, therapists prefer to perform complete excision of the
tumor along with its capsule, as recurrence is often observed in
different regions of the same nerve in the affected limb rather
than at the surgical site (25).
The optimal surgical approach involves microsurgical techniques for
intralesional dissection and meticulous tumor removal aimed at
preserving nerve structures while minimizing traction on the
surrounding nerve bundles (2,14,26,27).
Overall, a review of the relevant literature and the
findings of the present case indicate that patients with
schwannomas commonly present with soft-tissue masses, which may be
accompanied by symptoms that develop due to nerve compression.
Schwannomas originate from Schwann cells located within the nerve
sheath and are often easily separable from the nerve. The present
report highlights the fact that schwannomas can be multifocal and
affect different regions of the body. A thorough examination of the
peripheral nerves in patients with schwannomas is therefore
essential, and a complete dissection should be performed to obtain
a biopsy and thereby confirm that the tumor is not malignant. The
potential for tumor recurrence underscores the necessity for
routine follow-up examinations of affected patients.
Acknowledgements
The authors would like to thank Dr Yanmei Li
(Department of Radiology, Guangzhou Red Cross Hospital, Guangdong,
China) for providing radiography consultations.
Funding
This research was supported by the Guangzhou Science and
Technology Program of Guangzhou Red Cross Hospital (grant no.
202102010111) and the Guangzhou Municipal Key Discipline in
Medicine.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DY conceived and designed the study, and revised the
manuscript. HC collected and analyzed the patient's clinical,
imaging and pathological data, and drafted the manuscript. XY and
PW performed the surgeries, provided detailed surgical
descriptions, and contributed to the refinement of the manuscript's
conception, design and content. WY, YL, WL, YT, and JL contributed
to the writing of specific sections of the manuscript, assisted in
the development of the study's methodology, analyzed data and
prepared the pathological images for analysis. YX and HM
contributed to the overall study design, critically reviewed the
manuscript for important intellectual content, and oversaw the
processing and presentation of the figures. YY independently
analyzed the radiological images, identified key diagnostic
features and drafted the radiological findings section of the
manuscript. LL performed the histopathological examination,
conducted the immunohistochemical staining, interpreted the results
and drafted the pathology section of the manuscript. HC, XY, PW and
DY confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript, and have agreed to be
accountable for all aspects of the work, ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided specific written informed
consent for publication, which included the acquisition of clinical
data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsu CS, Hentz VR and Yao J: Tumours of the
hand. Lancet Oncol. 8:157–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai CS, Chen IC, Lan HC, Lu CT, Yen JH,
Song DY and Tang YW: Management of extremity neurilemmomas:
Clinical series and literature review. Ann Plast Surg. 71 (Suppl
1):S37–S42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhurst WD and Khachemoune A: An unknown
mass: The differential diagnosis of digit tumors. Int J Dermatol.
54:1214–1225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Payne WT and Merrell G: Benign bony and
soft tissue tumors of the hand. J Hand Surg Am. 35:1901–1910. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holdsworth BJ: Nerve tumours in the upper
limb A clinical review. J Hand Surg. 10:236–238. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrov M, Sakelarova T and Gerganov V:
Other nerve sheath tumors of brain and spinal cord. Adv Exp Med
Biol. 1405:363–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Junxiao G and Qianhui Q: Diagnosis and
surgical treatment of head and neck Schwannomas. Intern J
Otolaryngol-Head and Neck Surg. 43:216–219. 2019.(In Chinese).
|
|
8
|
Magro G, Broggi G, Angelico G, Puzzo L,
Vecchio GM, Virzì V, Salvatorelli L and Ruggieri M: Practical
approach to histological diagnosis of peripheral nerve sheath
tumors: An update. Diagnostics (Basel). 12:14632022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Das Gupta TK, Brasfield RD, Strong EW and
Hajdu SI: Benign solitary Schwannomas (neurilemomas). Cancer.
24:355–366. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang HJ, Shin SJ and Kang ES: Schwannomas
of the upper extremity. J Hand Surg Br. 25:604–607. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Russell SM: Preserve the nerve:
Microsurgical resection of peripheral nerve sheath tumors.
Neurosurgery. 61 (3 Suppl):S113–S117; discussion 117–118. 2007.
|
|
12
|
Sandberg K, Nilsson J, Soe Nielsen N and
Dahlin LB: Tumours of peripheral nerves in the upper extremity: A
22-year epidemiological study. Scand J Plast Reconstr Surg Hand
Surg. 43:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindberg M: Diagnostic Pathology: Soft
Tissue Tumors. 3rd Edition. Elsevier; Philadelphia, PA: 2019
|
|
14
|
Ozdemir O, Ozsoy MH, Kurt C, Coskunol E
and Calli I: Schwannomas of the hand and wrist: Long-term results
and review of the literature. J Orthop Surg (Hong Kong).
13:267–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kehrer-Sawatzki H, Kluwe L, Friedrich RE,
Summerer A, Schäfer E, Wahlländer U, Matthies C, Gugel I,
Farschtschi S, Hagel C, et al: Phenotypic and genotypic overlap
between mosaic NF2 and schwannomatosis in patients with multiple
non-intradermal schwannomas. Hum Genet. 137:543–552. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forde C, Smith MJ, Burghel GJ, Bowers N,
Roberts N, Lavin T, Halliday J, King AT, Rutherford S, Pathmanaban
ON, et al: NF2-related schwannomatosis and other schwannomatosis:
An updated genetic and epidemiological study. J Med Genet.
61:856–860. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BH, Chung YH, Woo TG, Kang SM, Park S,
Kim M and Park BJ: NF2-related schwannomatosis (NF2): Molecular
insights and therapeutic avenues. Int J Mol Sci. 25:65582024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khodaee M and Langston L: A painless
finger mass. J Musculoskel Neuron. 12:189–191. 2012.
|
|
19
|
Iwashita T: Neurilemmoma of the soft
tissues: An analysis of 1,271 tumors in an attempt at subtyping.
Fukuoka Igaku Zasshi. 80:355–367. 1989.(In Japanese). PubMed/NCBI
|
|
20
|
Martin AE, Martin D, Sandu AM, Neacsu A,
Rata O, Gorgan C and Gorgan MR: Nerve sheath tumor, benign
neurogenic slow-growing solitary neurilemmoma of the left ulnar
nerve: A case and review of literature. Rom Neurosur. 30:219–229.
2016.
|
|
21
|
Pinho R, Santana S, Farinha F, Cunha I,
Barcelos A and Brenha J: Shoulder giant schwannoma-a diagnosis to
be considered in painless shoulder masses. Acta Reumatol Port.
42:332–333. 2017.PubMed/NCBI
|
|
22
|
Hung YW, Tse WL, Cheng HS and Ho PC:
Surgical excision for challenging upper limb nerve sheath tumours:
A single centre retrospective review of treatment results. Hong
Kong Med J. 16:287–291. 2010.PubMed/NCBI
|
|
23
|
Nilsson J, Sandberg K, Soe Nielsen N and
Dahlin LB: Magnetic resonance imaging of peripheral nerve tumours
in the upper extremity. Scand J Plast Reconstr Surg Hand Surg.
43:153–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plotkin SR, Blakeley JO, Evans DG,
Hanemann CO, Hulsebos TJ, Hunter-Schaedle K, Kalpana GV, Korf B,
Messiaen L, Papi L, et al: Update from the 2011 International
Schwannomatosis Workshop: From genetics to diagnostic criteria. Am
J Med Genet A. 161A:405–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spinner RJ: Complication avoidance.
Neurosurg Clin N Am. 15:193–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, Peng C, Yorkashjan M, Li Y, Kong
W, Maimaitiali T and Zhao Y: Microscope-assisted treatment of rare
schwannoma of median nerve: 1 case. J Pract Hand Surg. 35:543–544.
2021.
|
|
27
|
Maimaitiali T, Kong W, Huang X, Peng C, Li
Y and Zhao Y: Microscopic technique in the treatment of proximal
thenar ulnar schwannoma in 1 case. J Pract Hand Surg. 36:569–570.
2022.(In Chinese).
|