Introduction
In recent years, with the rapid evolution of tumor
treatment technologies, immunotherapy, especially immune checkpoint
blockade, such as occurring via used of PD-1/PD-L1 inhibitors, has
emerged as a novel and promising therapeutic modality for thoracic
tumors (1). This approach offers
new hope to countless patients with cancer. One study found that
low-dose targeted radionuclide therapy (TRT) combined with immune
checkpoint inhibitors (ICIs) could render immunologically cold
tumors responsive to ICIs. This combination improves tumor
treatment outcomes, reduces metastatic burden, increases the
complete response rate and prolongs survival time (2). The PACIFIC (3) study indicated that immunoconsolidation
therapy after concurrent chemoradiotherapy increases the 5-year
progression-free survival (PFS) and 5-year overall survival (OS)
rates in patients with locally advanced non-small cell lung cancer
(NSCLC). Therefore, radiotherapy, integrated with immunotherapy
such as ICI therapy, has become a standard treatment strategy for
patients with NSCLC. (1). However,
another study has revealed some concerning issues. The results of
the study indicated that both radiotherapy and immunotherapy may
disrupt immune homeostasis, thereby triggering systemic immune
responses and adverse events, such as pneumonitis, myocarditis and
anemia (4). These issues have
received significant attention, and corresponding treatment
strategies are currently available. Clinicians need to conduct a
comprehensive assessment of the adverse reactions caused by
immunotherapy (1). After
comprehensively considering factors such as the patient's
condition, physical status and potential risks, they must carefully
weigh the pros and cons and, as appropriate, use immunosuppressive
agents for treatment. This is done to minimize the impact of
adverse reactions on patients, and to ensure the safety and
effectiveness of the treatment.
Given the widespread clinical use of radiotherapy
combined with immunotherapy for thoracic tumors and the uncertainty
regarding the impact of treatment timing on pneumonia, the present
retrospective study examined the data of patients with thoracic
tumors who received this combined treatment. The study focused on
analyzing the influence of different scheduling of chest
radiotherapy and immunotherapy on the incidence of pneumonia.
Additionally, it identified relevant risk factors to provide data
and theoretical support for optimizing clinical treatment protocols
and enhancing patient quality of life.
Patients and methods
Patient selection
The present retrospective analysis included 35
patients with thoracic tumors (29 cases of lung cancer and 6 cases
of esophageal cancer), aged 30–78 years old, consisting of 32 males
and 2 females, who underwent radiotherapy combined with
immunotherapy between January 2021 and December 2023 at Capital
Medical University, affiliated with Beijing Luhe Hospital (Beijing,
China). The median follow-up time was 21 months (range, 6–52
months). The total radiotherapy dose received by the lungs was
converted to the 2 Gy per fraction equivalent dose according to the
biologically effective dose formula, with a 50–62 Gy dose range.
The patients were divided into 2 cohorts: Group A, or the
sequential group, which comprised 17 patients who received
immunotherapy either 2 weeks before radiotherapy or from 2 weeks to
6 months before and after radiotherapy, and Group B, or the
concurrent group, which included 18 patients who received
immunotherapy within 2 weeks before or after radiotherapy. Grouping
was based firstly on clinical experience and secondly on a
retrospective study referenced in the literature (5). In that study, mouse experiments showed
that lung damage may occur around day 10 after the start of
combined radiotherapy and immunotherapy. None of the patients had
chronic obstructive pulmonary disease or interstitial lung disease.
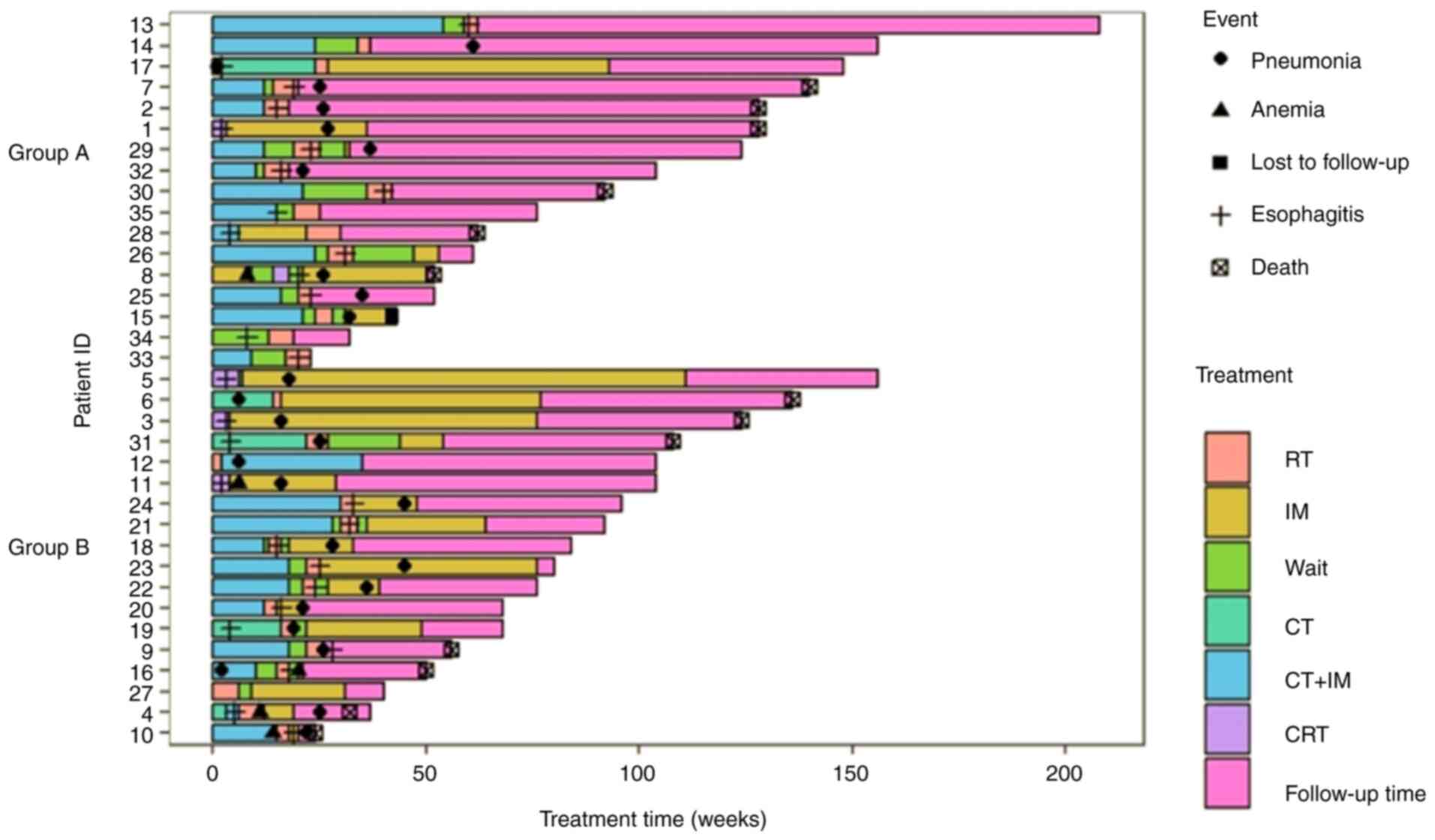
Fig. 1 presents the details of the
treatment course and endpoints.
Adverse events were graded based on the Common
Terminology Criteria for Adverse Events 5.0 (6). The incidence and severity of
pneumonia, esophagitis, myocarditis and anemia between the two
groups were compared. Furthermore, the risk factors for lung injury
and the time of chest radiotherapy in combination with
immunotherapy were assessed. Moreover, the median and 3-year
survival rates of patients with lung cancer (stage III and IV)
under combined treatment were also evaluated.
Statistical analysis
This study employed SPSS 20.0 (IBM Corp.) and SAS9.4
(SAS Institute, Inc.) for all the statistical analyses. Dose
information was assessed for clinical baseline characteristics
using unpaired Student's t-test when data conformed with a normal
distribution (assessed by Shapiro-Wilk test) and by Wilcoxon
rank-sum test when data did not conform with a normal distribution.
Categorical variables were analyzed using the χ2 test.
If the expected count in any cell was <5, Fisher's exact test
was applied as an alternative, and these results were marked with
an asterisk. For categorical variables with multiple categories,
Fisher's exact test was directly used. In the overall patient
cohort, univariate and multivariate logistic regression analyses
were performed to elucidate factors influencing pneumonia
incidence. In univariate analysis, the variables with P≤0.10 were
selected for the multivariate logistic regression analysis, where
lung volume receiving ≥20 Gy (V20) was the influencing
factor for pneumonia, which was also included in the analysis. For
multivariate analyses, the forward stepwise method was employed.
All the statistical assessments were two-tailed, with P<0.05
used to indicate a statistically significant difference.
Furthermore, swim plots indicating treatment processes and each
patient's disease status were presented using R software (7). Furthermore, Kaplan-Meier curves were
generated with log-rank test used to assess the survival outcomes
in the patients with advanced lung cancer undergoing co-treatment
with radiotherapy and immunotherapy.
Results
General and therapeutic
characteristics of the patients
The median follow-up time for the 35 patients (stage
III and IV) was 21 months (range, 6–52 months). No statistical
difference was observed in terms of tumor location, stage (8), age, tumor type, Eastern Cooperative
Oncology Group (ECOG) score (9),
TNM stage, coronary heart disease and sex between the two cohorts
(Table I). However, in the
selection of immunotherapeutic drugs, the sequential group used
more PD-1 drugs than the concurrent group (P=0.018; Table I).
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Characteristic | Group A (n=18) | Group B (n=17) | P-value |
|---|
| Sex, n (%) |
|
| 0.603a |
| Male | 17 (94.4) | 15 (88.2) |
|
|
Female | 1 (5.6) | 2 (11.8) |
|
| Median age,
years | 63 | 61.8 | 0.105 |
| Tumor type, n
(%) |
|
| 0.141a |
| Small
cell carcinoma of the lungs | 4 (22.2) | 7 (41.2) |
|
| Squamous
lung cancer | 5 (27.8) | 6 (35.3) |
|
|
Adenocarcinoma of the
lungs | 2 (11.1) | 3 (17.6) |
|
|
Esophageal cancer | 7 (38.9) | 1 (5.9) |
|
| ECOG PS, n (%) |
|
| 0.603a |
| 1 | 17 (94.4) | 15 (88.2) |
|
| 2 | 1 (5.6) | 2 (11.8) |
|
| Tumor location, n
(%) |
|
| 0.267a |
| Lung
(upper left) | 5 (27.8) | 3 (17.6) |
|
| Lung
(lower left) | 1 (5.6) | 1 (5.9) |
|
| Lung
(upper right) | 3 (16.7) | 6 (35.3) |
|
| Lung
(middle right) | 1 (5.6) | 1 (5.9) |
|
| Lung
(lower right) | 1 (5.6) | 4 (23.5) |
|
|
Esophagus | 7 (38.9) | 2 (11.8) |
|
| TNM staging, n
(%) |
|
|
|
| T |
|
| 0.256a |
|
T2 | 3 (16.7) | 6 (35.3) |
|
|
T3 | 0 (0.0) | 2 (11.8) |
|
|
T4 | 9 (50.0) | 5 (29.4) |
|
|
Tx | 6 (33.3) | 4 (23.5) |
|
| N |
|
| 0.480a |
|
N0 | 2 (11.1) | 0 (0.0) |
|
|
N1 | 1 (5.6) | 0 (0.0) |
|
|
N2 | 6 (33.3) | 8 (47.1) |
|
|
N3 | 3 (16.7) | 5 (29.4) |
|
|
Nx | 6 (33.3) | 4 (23.5) |
|
| M |
|
| 0.738 |
|
M1 | 9 (50.0) | 10 (58.8) |
|
|
M2 | 9 (50.0) | 7 (41.2) |
|
| Tumor staging, n
(%) |
|
| 0.401a |
| II | 1 (5.6) | 0 (0.0) |
|
|
III | 7 (38.9) | 10 (58.8) |
|
| IV | 10 (55.6) | 7 (41.2) |
|
| Coronary heart
disease, n (%) |
|
| 0.603 |
| No | 15 (83.3) | 16 (94.1) |
|
|
Yes | 3 (16.7) | 1 (5.9) |
|
| Immunization, n
(%) |
|
| 0.018 |
|
PD-1 | 14 (77.8) | 6 (35.3) |
|
|
PD-L1 | 4 (22.2) | 11 (64.7) |
|
In the sequential group, 66.7 and 33.3% of patients
received intensity-modulated radiation therapy (IMRT) and
volumetric arc therapy (VMAT), respectively. In the synchronized
group, 58.8 and 41.2% of patients received VMAT and IMRT,
respectively (P=0.181). The V20 for the bilateral lungs
was 15±7.3 and 15.2±8.0% in the sequential and synchronized
cohorts, respectively (P=0.947). Furthermore, the mean lung dose
(Dmean) value for the bilateral lungs was 9±3.2 and
11±3.7 Gy in the sequential and synchronized groups, respectively
(P=0.99). Overall, no statistically essential differences between
groups were observed for radiotherapy technique or dose (Table II).
 | Table II.Radiotherapy techniques. |
Table II.
Radiotherapy techniques.
| Parameter | Group A (n=18) | Group B (n=17) | P-value |
|---|
| Radiotherapy
techniques, n (%) |
|
| 0.181 |
|
VMAT | 6 (33.3) | 10 (58.8) |
|
|
IMRT | 12 (66.7) | 7 (41.2) |
|
| Lung dose |
|
|
|
|
Dmean, Gy | 9.0±3.2 | 11.0±3.7 | 0.990 |
|
V5, % | 34.0±11.8 | 36.4±13.1 | 0.604 |
|
V10, % | 23.4±9.6 | 25.0±10.0 | 0.629 |
|
V20, % | 15.0±7.3 | 15.2±8.0 | 0.947 |
|
V30, % | 8.2±5.2 | 9.6±6.1 | 0.487 |
Treatment-related adverse
reactions
The results indicated no grade 4 or higher adverse
reactions in both groups. The incidence of grade 0, 1, 2 and 3
pneumonia in the sequential group was 38.9, 38.9, 22.2 and 0.0%,
respectively, while in the synchronous group it was 11.8, 52.9,
11.8 and 23.5%, respectively (P=0.061). Moreover, the incidence of
grade 0, 1, 2, and 3 esophagitis in the sequential group was 6.3,
37.5, 37.5 and 18.8%, respectively, while it was 13.3, 60.0, 13.3
and 13.3% in the synchronous group (P=0.441), respectively.
Similarly, the grade 0, 1 and 2 incidence of anemia was 93.8, 0.0
and 6.3%, respectively, in the sequential group, and 73.3, 26.7 and
0.0%, respectively, in the synchronous group (P=0.069). There were
no statistically essential differences observed for adverse
reactions between the cohorts (Table
III).
 | Table III.Adverse reactions. |
Table III.
Adverse reactions.
| Adverse
reaction | Group A (n=18) | Group B (n=17) | P-value |
|---|
| Pneumonia, n
(%) |
|
| 0.061a |
| 0 | 7 (38.9) | 2 (11.8) |
|
| 1 | 7 (38.9) | 9 (52.9) |
|
| 2 | 4 (22.2) | 2 (11.8) |
|
| 3 | 0 (0.0) | 4 (23.5) |
|
| Esophagitis, n
(%) |
|
| 0.226a |
| 0 | 2 (11.1) | 2 (11.8) |
|
| 1 | 6 (33.3) | 11 (64.7) |
|
| 2 | 7 (38.9) | 2 (11.8) |
|
| 3 | 3 (16.7) | 2 (11.8) |
|
| Anemia, n (%) |
|
| 0.128a |
| 0 | 16 (88.8) | 12 (70.6) |
|
| 1 | 1 (5.6) | 5 (29.4) |
|
| 2 | 1 (5.6) | 0 (0.0) |
|
Symptoms, incidence and duration of
different levels of pneumonia
The analysis of pneumonia-related symptoms in the
sequential and synchronized groups (Table IV) revealed that chest tightness
(16.7 and 47.1%; P=0.075), cough (50.0 and 52.9%;
P>0.999) and fever (5.6 and 17.6%; P=0.338) symptoms were
not statistically different between groups. The pneumonia incidence
in the sequential and synchronized groups was 61.1 and 88.2%,
respectively (P=0.073; Table V).
The subgroup analysis of the 26 patients who developed pneumonia
revealed that the mean time to pneumonia development in the
sequential and synchronized groups was 3.18±1.83 and 3.27±1.98
months, respectively (P=0.912). The incidence of grade 2 and higher
pneumonia in the sequential and synchronized groups was 22.2 and
35.3%, respectively (P=0.471), whereas the incidence of grade 3 and
higher pneumonia was 0.0 and 23.5%, respectively (P=0.045). The
incidence of grade 3 and higher pneumonia exhibited the only
statistically significant difference between the groups (Table V).
 | Table IV.Symptoms of pneumonia. |
Table IV.
Symptoms of pneumonia.
| Symptom | Group A (n=18) | Group B (n=17) | P-value |
|---|
| Chest distress, n
(%) |
|
| 0.075 |
| No | 15 (83.3) | 9 (52.9) |
|
|
Yes | 3 (16.7) | 8 (47.1) |
|
| Cough, n (%) |
|
| >0.999 |
| No | 9 (50.0) | 8 (47.1) |
|
|
Yes | 9 (50.0) | 9 (52.9) |
|
| Fever, n (%) |
|
| 0.338a |
| No | 17 (94.4) | 14 (82.4) |
|
|
Yes | 1 (5.6) | 3 (17.6) |
|
 | Table V.Grading analysis of pneumonia. |
Table V.
Grading analysis of pneumonia.
| Parameter | Group A (n=18) | Group B (n=17) | P-value |
|---|
| Pneumonia, n
(%) |
|
| 0.073a |
| No | 7 (38.9) | 2 (11.8) |
|
|
Yes | 11 (61.1) | 15 (88.2) |
|
| Time to pneumonia,
months | 3.18±1.83 | 3.27±1.98 | 0.912 |
| Pneumonia grade, n
(%)a |
|
| 0.471b |
| ≥2 | 4 (22.2) | 6 (35.3) |
|
|
<2 | 14 (77.8) | 11 (64.7) |
|
| Pneumonia grade, n
(%)a |
|
| 0.045a |
| ≥3 | 0 (0.0) | 4 (23.5) |
|
|
<3 | 18 (100.0) | 13 (76.5) |
|
Influencing factors of pneumonia
The univariate analysis revealed that lung
Dmean and V30 were the factors influencing
pneumonia incidence rate. Following multivariate analysis, only
V30 retained statistical significance as an independent
prognostic factor (Table VI).
 | Table VI.Univariate analysis and multivariate
analysis. |
Table VI.
Univariate analysis and multivariate
analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
|
Dmean | 0.027 | 1.412 | 1.041–1.040 | 0.731 | 1.125 | 0.575–2.198 |
| V5 | 0.131 | 1.055 | 0.984–1.132 |
|
|
|
| V10 | 0.057 | 1.110 | 1.132–1.236 | 0.145 | 1.581 | 0.854–2.928 |
| V20 | 0.223 | 1.073 | 0.958–1.201 | 0.043 | 0.313 | 0.102–0.962 |
| V30 | 0.031 | 1.308 | 1.024–1.670 | 0.027 | 3.069 | 1.138–8.277 |
| Radiotherapy timing
(sequential/synchronization) | 0.081 | 4.773 | 0.826–27.562 | 0.341 | 0.278 | 0.020–3.868 |
| Radiotherapy
technology (VMAT/IMRT) | 0.391 | 2.010 | 0.410–9.757 |
|
|
|
| Immunization | 0.160 | 3.501 | 0.609–20.13 |
|
|
|
| Age, years | 0.304 | 0.953 | 0.87–27.562 |
|
|
|
| ECOG | 0.753 | 1.502 | 0.119–18.836 |
|
|
|
Overall survival rates in stage III/IV
lung cancer
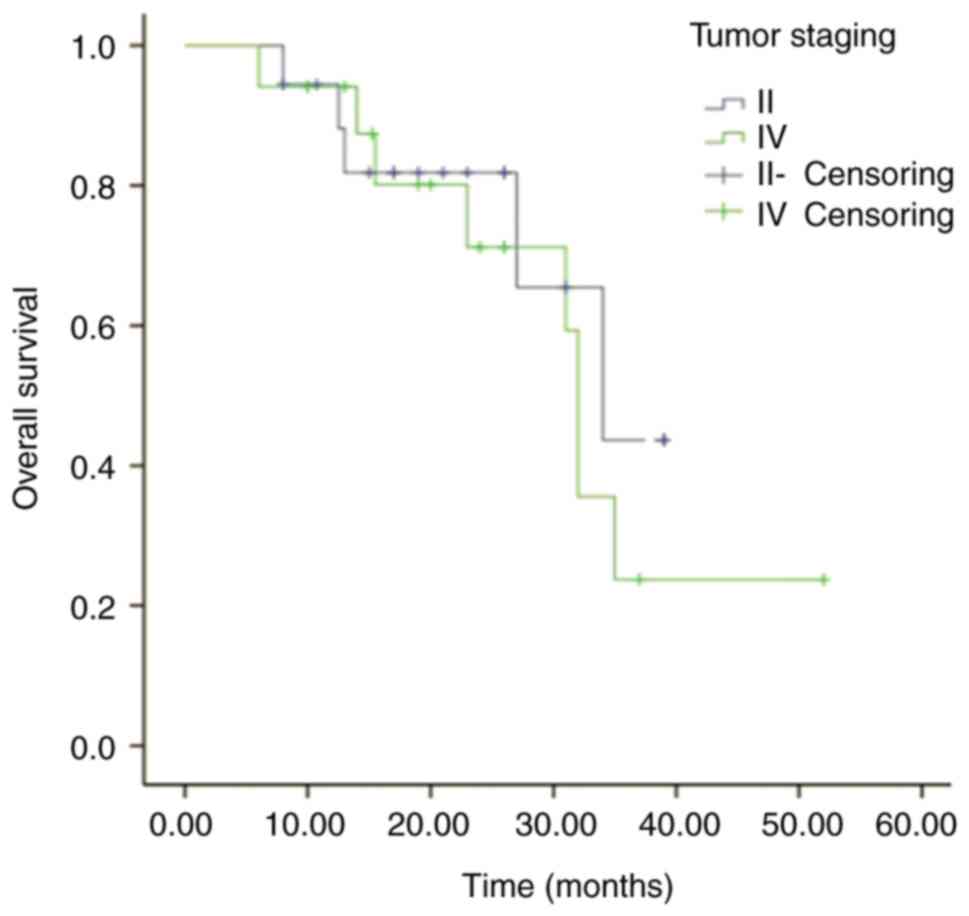
The survival analysis was performed on 29 patients
with lung cancer, with a median follow-up time of 21 months (range,
6–52 months). The 3-year OS rates were 44.8 and 22.5% for stage III
and IV patients, respectively (Fig.
2). There was no significant difference in the survival curves
between the two groups (Log-rank test, P>0.05).
Discussion
Co-treatment with radiotherapy and immunotherapy has
become a new modality for cancer treatment. The KEYNOTE-00l
(10) study indicated that patients
with stage IV NSCLC who underwent radiotherapy before immunotherapy
had longer 5-year OS and 5-year progression-free survival (PFS)
rates than those who did not undergo radiotherapy. Furthermore, the
PACIFIC study revealed that patients with unresectable stage III
lung cancer who underwent simultaneous radiotherapy and
chemotherapy, and then continued with sequential divalizumab
consolidation therapy, had 5- and 3-year survival rates of 42.9 and
56.7%, respectively (3). The
present study showed 3-year OS rates of 44.8 and 22.5% after
radiotherapy combined with immunotherapy for stage III and IV lung
cancer, respectively. This may be due to the fact that SCLC, which
has a poorer prognosis, was also included in the present research.
Overall, it was validated that immunotherapy combined with
radiotherapy has a survival benefit over any mono-treatment
(3,10). However, clinical applications
require careful monitoring, as both radiotherapy and immunotherapy
can promote the development of pneumonia.
Radiotherapy predominantly kills tumor cells by
ionizing radiation-induced DNA damage (11). Radiotherapy also affects the immune
system in various ways, such as tumor immune microenvironment
remodeling, cytokine and chemokine release, immune cell
infiltration and increasing tumor cell sensitivity to immunogenic
cell death (12). Furthermore, it
can be employed to generate ‘immunovaccines’ to promote antitumor T
cell immune responses (13).
Radiotherapy is also employed for immunomodulation and
reconstructing the immune microenvironment of the tumor.
Immunopharmaceuticals block pathways that inhibit the immune
response to maintain and restore the cancer cell recognition and
immune system response (14).
Radiotherapy-induced antitumor immune response and its synergy with
immunotherapy has evolved as a potential therapeutic modality
(15).
A meta-analysis by Nishino et al (16) indicated that patients who underwent
combination therapy were significantly more prone to pneumonia than
those who received monotherapy for all grades of pneumonia [odds
ratio (OR), 2.04] and grade 3 or above (OR, 2.86). Furthermore, a
recent phase I study revealed that 26% of the 23 patients who
underwent co-treatment with paborizumab and chemoradiotherapy had
grade 2 or higher pneumonia (17).
Another study retrospectively analyzed 196 patients who underwent
chest radiotherapy with immunotherapy and revealed that the
incidence of grade 2 or higher pneumonia was 25.5%, while the
incidence of grade 3 or higher pneumonia was 4.1% (18). Moreover, another study revealed that
in patients with thoracic tumors, the incidence of
treatment-related pneumonia was ~30% after immunotherapy combined
with radiotherapy (19). The data
of the present study revealed that the incidence of grade 2 or
higher pneumonia, which requires drug treatment, for the entire
cohort was 28.5% (22.2 and 35.3% in the sequential and synchronous
groups, respectively), which was higher than the incidence in the
sequential group. However, there was no statistically significant
difference between the two cohorts (P=0.471). These results were
consistent with previous studies. Moreover, the incidence of grade
3 or higher pneumonia was 11.4% for the whole cohort (0.0% in the
sequential group and 23.5% in the synchronous group), and the
difference was statistically significant between groups (P=0.045).
This shows that the application of immune drugs within 2 weeks
before and after radiotherapy may aggravate the incidence of grade
3 or higher pneumonia.
The timing of the pneumonia onset is also important.
The mean pneumonia onset time after immunotherapy has been reported
as 2.5 months (range, 9 days to 19 months) (20). Delaunay et al (21) diagnosed immunopneumonia in 64 out of
1,826 patients, and the median time to checkpoint inhibitor
pneumonitis onset was 2.3 months. The majority of patients (42.2%)
developed pneumonia within 2 months of receiving immunotherapy. The
pneumonia onset time was 2–4 months in 26.6% of the patients, 4–6
months in 17.2% of the patients and >6 months in 14.1% of the
patients (21). In another study,
the median time between the end of chest radiotherapy and the onset
of pneumonia was 88.5 days (22).
In an aforementioned retrospective study (19), the median pneumonia onset time after
immunotherapy followed by combined radiotherapy was ~65 days
(19). The results of the present
study showed that the mean pneumonia onset time was 2.45 and 2.88
months in the sequential and synchronized groups, respectively,
which is consistent with the aforementioned studies. Therefore, the
clinical review and follow-up of patients is recommended after 2–4
months of treatment.
Saito et al (23) retrospectively analyzed the data from
275 patients with locally advanced NSCLC who received concurrent
radiotherapy followed by consolidation therapy with duvarizumab.
The mean V20 of the enrolled patients was 19.4% (range,
1.4–37.9%) and the Dmean was 10.9 Gy (range, 1.5–31.3
Gy). Furthermore, logistic regression analysis indicated that
V20 ≥25% was an independent risk factor for grade ≥2
pneumonia (23). In another study,
the receiver operating characteristic curve showed that in the
V20 ≥21% group, the prevalence of grade ≥2 pneumonia was
33.3%, which was significantly higher than the 4.8% in the
V20 <21% group (14).
Moreover, the incidence of pneumonia was significantly associated
with a history of chronic lung disease (P=0.050), Dmean
(P=0.038), V5 (P=0.012) and V20 (P=0.030)
(22). Emphysema, lung
V20, V30, Dmean and V5
are risk factors for symptomatic pneumonia (18,24).
In line with the results of previous studies, in the present study,
the lung Dmean and V30 were observed as the
influential factors of pneumonia.
Radiotherapy combined with immunotherapy has
survival benefits for patients with advanced NSCLC (14); however, the treatment can increase
the incidence of pneumonia. The appropriate combination modality,
suitable radiotherapy dose and precise timing of the combination
are therefore particularly important. The PACIFIC study (3) investigated the effect of consolidation
therapy with immunomedicine 1–42 days after standard radiotherapy
treatment for stage III unresectable lung cancer. The immunized and
placebo groups indicated grade 3 or higher radiation pneumonitis
rates of 3.4 and 2.6%, respectively (3), but no further time stratification was
performed. Furthermore, another study performed multifactorial
analysis and suggested that an interval of <3 months between
chest radiotherapy and immunotherapy was independently associated
with grade 2 or higher pneumonia (P=0.004). However, in clinical
practice, a number of patients cannot wait for 1 or 3 months before
starting systemic therapy due to the disease severity. Therefore,
immunotherapy should be started within 1 month before or after
radiotherapy; however, the precise time remains elusive. A recent
in vivo study (5) found that
when radiotherapy is combined with immunotherapy, lung injury may
progress faster from days 15 to 20 after the start of treatment. In
the study (25), 40 patients were
studied retrospectively, and the radiotherapy and immunotherapy
intervals of <3 weeks and 3 weeks to 6 months were also
compared. There was no difference in the incidence of pneumonia,
which might due to the small sample size. Therefore, a large sample
is required to validate these analyses.
In summary, the present study revealed that
radiotherapy combined with immunotherapy has a survival benefit for
patients with advanced thoracic tumors; however, it also increases
the risk of pneumonia, with an increase in the incidence of grade 3
or higher pneumonia within a 2-week interval between radiotherapy
and immunotherapy. Furthermore, the identified factors that
influenced the incidence of pneumonia included lung
Dmean, V30 and the interval between radiation
therapy combined with immunotherapy. Moreover, pneumonia typically
develops 2–4 months after radiotherapy; therefore, the clinical
review and follow-up of patients 2–4 months after treatment is
recommended.
The number of cases included in this study is
relatively small, which may introduce some bias. Additionally, the
chest tumors in this study included both lung cancer and esophageal
cancer, which differ in terms of radiation field and dose,
potentially impacting the occurrence of pneumonia. Data collecrion
will continue and further studies will investigate the specifics of
each individual cancer type.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY contributed to the preparation, creation and
description of the work for publication, in particular writing a
first draft (including substantial translations). JY and YYG
contributed to presentation of research ideas and the development
and formation of overall research objectives. QTL and XL
contributed to the application of statistical, mathematical,
computer and other analyses to assess and integrate the research
data. XJS, XXN and XBZ contributed to implementing the research and
data/evidence collection. YYG performed the review and revision
(both pre- and post-publication phases). SYZ and BS helped with
data collection. PH and SW helped with the analysis and design of
the article. JY and YYG confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study obtained ethical approval from the
Ethics Office of Beijing Luhe Hospital, Capital Medical University
(approval no. 2024-LHKY-070-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
National Comprehensive Cancer Network
(NCCN), . Non-Small Cell Lung Cancer (NCCN Guidelines®).
NCCN; Plymouth Meeting, PA: 2024
|
|
2
|
Patel RB, Hernandez R, Carlson P,
Grudzinski J, Bates AM, Jagodinsky JC, Erbe A, Marsh IR, Arthur I,
Aluicio-Sarduy E, et al: Low-dose targeted radionuclide therapy
renders immunologically cold tumors responsive to immune checkpoint
blockade. Sci Transl Med. 13:eabb36312021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: PACIFIC investigators. Overall survival with durvalumab after
chemoradiotherapy in stage III NSCLC. N Engl J Med. 379:2342–2350.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stucci S, Palmirotta R, Passarelli A,
Silvestris E, Argentiero A, Lanotte L, Acquafredda S, Todisco A and
Silvestris F: Immune-related adverse events during anticancer
immunotherapy: Pathogenesis and management. Oncol Lett.
14:5671–5680. 2017.PubMed/NCBI
|
|
5
|
Wang CL, Ho AS and Cheng CC, Sie ZL, Peng
CL, Chang J and Cheng CC: Radiotherapy enhances
CXCR3highCD8+ T cell activation through
inducing IFNγ-mediated CXCL10 and ICAM-1 expression in lung cancer
cells. Cancer Immunol Immunother. 72:1865–1880. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events Version
5.0 (CTCAE v5.0).
|
|
7
|
Chang W: R Graphics Cookbook. O'Reilly
Media; 2013
|
|
8
|
Goldstraw P, Crowley J, Chansky K,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for the revision of the TNM stage groupings in
the forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 10:1260–1271. 2015.
|
|
9
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaverdian N, Lisberg AE, Bomazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
8:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross GM: Induction of cell death by
radiotherapy. Endocr Relat Cancer. 6:41–44. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vinod SK and Hau E: Radiotherapy treatment
for lung cancer: Current status and future directions. Respimlogy.
25 (Suppl 2):S61–S71. 2020. View Article : Google Scholar
|
|
13
|
Uribe-Herranz M, Rafail S, Beghi S,
Gil-de-Gómez L, Verginadis I, Bittinger K, Pustylnikov S, Pierini
S, Perales-Linares R, Blair IA, et al: Gut microbiota modulate
dendritic cell antigen presentation and radiotherapy-induced
antitumor immune response. J Clin Invest. 130:466–479. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Bai H, Wang C, Seery S, Wang Z,
Duan J, Li S, Xue P, Wang G, Sun Y, et al: Efficacy and safety of
first-line lmmunotherapy combinations for advanced NSCLC: A
systematic review and network meta-analysis. J Thorac Oncol.
16:1099–1117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hen'era FG, Irving M, Kandalaft LE and
Coukos G: Rational combinations of immunotherapy with radiotherapy
inovarian cancer. Lancet Oncol. 20:e417–e433. 2019. View Article : Google Scholar
|
|
16
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi F: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
systematic review and meta-analysis. JAMA Oncol. 2:1607–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jabbour SK, Berman AT, Decker RH, Lin Y,
Feigenberg SJ, Gettinger SN, Aggarwal C, Langer CJ, Simone CB,
Bradley JD, et al: Prospective phase I multi-institutional trial of
PD-1 blockade with pembrolizumab during concurrent chemoradiation
for locally advanced, unresectable non-small cell lung cancer. J
Clin Oncol. 37:85112019. View Article : Google Scholar
|
|
18
|
Lu X, Wang J, Zhang T, Zhou Z, Deng L,
Wang X, Wang W, Liu W, Tang W, Wang Z, et al: Comprehensive
pneumonitis profile of thoracic radiotherapy followed by immune
checkpoint inhibitor and risk factors for radiation recall
pneumonitis in lung cancer. Front Immunol. 13:9187872022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang HY, Gong HY and Song QB: Influencing
factors of pneumonitis in the period of thoracic radiotherapy
combined with immunotherapy. J Int Oncol. 50:102–106. 2023.(In
Chinese).
|
|
20
|
Chen K and Sun B: Incidence and risk of
PD-1/PD-L1 inhibitor-associated pneumonia in advance cancer
patients: A meta-analysis. Zhongguo Fei Ai Za Zhi. 23:927–940.
2020.(In Chinese). PubMed/NCBI
|
|
21
|
Delaunay M, Cadranel J, Lusque A, Meyer N,
Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N,
Guisier F, et al: Immune checkpoint inhibitors associated with
interstitial lung disease in cancer patients. Eur Respir J.
50:17000502017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu XT, Wang JY, Zhang T, Zhou Z, Deng L,
Wang X, Wang W, Liu W, Tang W, Wang Z, et al: Comprehensive
Pneumonitis Profile of Thoracic Radiotherapy Followed by Immune
Checkpoint Inhibitor and Risk Factors for Radiation Recall
Pneumonitis in Lung Cancer. Front Immunol. 13:9187872022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saito G, Oya Y, Taniguchi Y, Kawachi H,
Daichi F, Matsumoto H, Iwasawa S, Suzuki H, Niitsu T, Miyauchi E,
et al: Real-world survey of pneumonitis/radiation pneumonitis among
patients with locally advanced non-small cell lung cancer treated
with chemoradiotherapy after durvalumab approval: A multicenter
retrospective cohort study (HOPE-005/CRIMSON). J Clin Oncol.
38:90392020. View Article : Google Scholar
|
|
24
|
Chen Y, Liu X, Huang Z, Zhao K, Wang Y,
Ren F, Yu J and Meng X: Safety of thoracic radiotherapy after
PD-(L)1 inhibitor treatment in patients with lung cancer. Cancer
Med. 10:8518–8529. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin SH, Lin Y, Yao L, Kalihor N, Carter
BW, Altan M, Blumenschein G, Byers LA, Fossella F, Gibbons DL, et
al: Phase II Trial of Concurrent Atezolizumab With Chemoradiation
for Unresectable NSCLC. J Thorac Oncol. 15:248–257. 2020.
View Article : Google Scholar : PubMed/NCBI
|