Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth
most prevalent cancer (4.3%) and was the third leading cause of
cancer-associated deaths (7.8%) worldwide in 2022, with nearly
one-half of the global liver cancer cases occurring in China
(1). Surgery remains the primary
curative treatment for HCC; however, ~70% of patients with HCC are
diagnosed at an advanced stage, rendering them ineligible for
surgical resection (2). Unlike
other malignant tumors, treatment options for advanced HCC are
scarce, owing to its resistance to radiotherapy and conventional
chemotherapy (3). Advanced-stage
HCC treatments include local ablation therapy, interventional
therapy, radiotherapy, chemotherapy, targeted therapy and
immunotherapy. For example, the immune checkpoint inhibitors
nivolumab or pembrolizumab combined with the targeted drug
lenvatinib exhibited an improved tumor response compared with
lenvatinib in advanced-stage HCC; however, none of the treatment
effects were satisfactory in improving overall survival (4–6).
Therefore, there is an urgent need for development of novel
therapeutic modalities to improve clinical outcomes in patients
with advanced HCC.
The introduction of proteasome inhibitors (such as
bortezomib) has markedly improved the overall survival rates of
patients with multiple myeloma (7).
Bortezomib selectively and reversibly binds to the catalytic site
of the 26S proteasome, which promotes cancer cell apoptosis
(8); however, a previous study has
demonstrated that bortezomib alone is ineffective in treating most
solid tumors (9). Moreover,
attempts to combine bortezomib with docetaxel have failed to
achieve the desired clinical outcomes in advanced non-small-cell
lung cancer (10). These
limitations emphasize the necessity for further mechanistic
research to facilitate the design of targeted therapies and improve
patient outcomes.
Previous research has reported that bortezomib can
induce apoptosis in HCC cells when used in combination with tumor
necrosis factor-related apoptosis-inducing ligand or sorafenib
(8,11,12).
Furthermore, bortezomib combined with sorafenib was found to induce
apoptosis in PLC/PRF/5 cells via Akt inactivation (11). The findings suggest that proteasome
inhibitors may improve the therapeutic effects of sorafenib in HCC.
Carfilzomib, a second-generation proteasome inhibitor, irreversibly
binds to the catalytic site of the proteasome leading to prolonged
inhibition (13). In vitro
studies have demonstrated that carfilzomib exhibited superior
therapeutic efficacy compared with bortezomib in multiple myeloma
(14). In a rat model of
diethylnitrosamine-induced hepatocarcinogenesis, carfilzomib
exerted a notable preventive benefit (15). However, the efficacy of carfilzomib
alone against HCC and the precise mechanisms underlying its action
remain unclear.
The present study aimed to explore the efficacy of
carfilzomib against the progression of HCC and investigate the
underlying molecular mechanisms.
Materials and methods
Materials
Carfilzomib was obtained from MedChemExpress (cat.
no. HY-10455) and dissolved in DMSO. Antibodies were procured from
Proteintech Group, Inc., including cyclin-dependent kinase (CDK)2
(cat. no. 10122-1-AP), CDK4 (cat. no. 11026-1-AP), cyclin A2 (cat.
no. 66391-1-Ig), cyclin E1 (cat. no. 11554-1-AP), GAPDH (cat. no.
60004-1-Ig), phosphorylated (p)-JNK (Tyr185) recombinant antibody
(cat. no. 80024-1-RR), JNK monoclonal antibody (cat. no.
66210-1-Ig), p38 MAPK polyclonal antibody (cat. no. 14064-1-AP),
p-p38 MAPK (Thr180/Tyr182) polyclonal antibody (cat. no.
28796-1-AP). Additionally, anti-GADD45α antibody (cat. no. A1797)
was purchased from ABclonal Biotech Co., Ltd. A total of 12
4-week-old female BALB/c nude mice (15–16 g) were purchased from
Beijing Vital River Laboratory Animal Technology Co.
Cell culture
MHCC-97H and Huh7 cells were acquired from Wuhan
Pricella Biotechnology Co., Ltd. and cultured in Modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
1×105 U/ml penicillin and 100 mg/ml streptomycin
(Beijing Solarbio Science & Technology Co., Ltd.) in a constant
temperature incubator at 37°C and 5% CO2. Cells were
treated with different concentrations of carfilzomib and control
cells treated with the same concentration of DMSO.
Cell viability assay
The cytotoxic effects of carfilzomib on MHCC-97H and
Huh7 cells were assessed using Cell Counting Kit-8 (CCK-8; Beyotime
Institute of Biotechnology). Cells were cultured in 96-well plates
(3×104 cells/well) and subsequently treated with
different concentrations of carfilzomib or DMSO (0, 10, 20, 30, 40,
50, 60, 80 and 100 µM) for 24 h at 37°C. Following treatment, 10 µl
CCK-8 solution was added to each well and incubated for an
additional 1 h at 37°C. Absorbance was measured at 450 nm using a
Multiskan FC microplate reader (Thermo Fisher Scientific, Inc.)
(16).
Cell proliferation assay
The evaluation of MHCC-97H and Huh7 cell
proliferation was performed using an 5-ethynyl-2-deoxyuridine (EdU)
staining kit (Beyotime Institute of Biotechnology) following the
manufacturer's instructions (17).
Briefly, 5,000 cells were cultured into a 96-well plate. The
following day, the cells were treated with carfilzomib (30 µM for
MHCC-97H cells and 60 µM for Huh7 cells) at 37°C for 24 h. For the
EdU staining assay, each well was treated with 10 µM EdU working
solution and was incubated for 2 h at 37°C. The nuclei were stained
with DAPI for 10 min at room temperature and EdU-positive cells
were visualized using a fluorescence microscope (Olympus
Corporation). ImageJ software (version 1.51, National Institutes of
Health) was used for sample analysis.
Colony formation assay
MHCC-97H and Huh7 cells were seeded in 6-well plates
(1×104 cells/well) and incubated for 24 h at 37°C.
Subsequently, the cells were treated with carfilzomib (30 µM for
MHCC-97H cells and 60 µM for Huh7 cells) at 37°C and cultured for
14 days. The culture medium containing carfilzomib was replaced
every 3 days throughout this period. Colonies were washed with PBS,
fixed with 4% paraformaldehyde at room temperature for 10 min and
crystal violet (0.1%) was then used to stain the colonies at room
temperature for 20 min (18). After
washing, colony formation was observed using an inverted light
microscope. Colonies consisting of ≥50 cells were counted. ImageJ
software (version 1.51; National Institutes of Health) was used for
data analysis.
Transwell migration and invasion
assays
Cell migration and invasion were assessed using
Transwell chambers (pore size, 8 µm) in 24-well plates with (for
invasion) or without (for migration) Matrigel (BD Biosciences). A
total of 100 µl Matrigel (diluted 1:8 with serum-free DMEM medium)
was added to the upper chambers of the Transwell inserts and
incubated at 37°C for 2 h before use. MHCC-97H and Huh7 cells
(1×105 cells/well) were seeded in the upper chambers of
the Transwell inserts with serum-free DMEM medium containing
carfilzomib (30 µM for MHCC-97H cells and 60 µM for Huh7 cells).
Medium containing 20% FBS was added into the lower chamber. After
incubation for 24 h at 37°C, non-migrating cells on the top of the
filter were eliminated using a cotton swab. Subsequently, the
migrated cells on the bottom surface of the membrane were fixed and
stained with 0.1% crystal violet at room temperature for 10 min
(19). After washing, attached
cells were counted under an inverted light microscope. The number
of cells that had migrated was counted using ImageJ software
(version 1.51; National Institutes of Health).
Wound healing assays
MHCC-97H and Huh7 cells were seeded in 6-well plates
at a density of 1×106 cells/well until the confluency
reached 90%. Subsequently, the cells were scraped with the tip of a
standard 200 µl sterile pipette to create a wound and further
incubated in fresh serum-free DMEM medium containing carfilzomib
(30 µM for MHCC-97H cells and 60 µM for Huh7 cells) for 48 h at
37°C. Cells were imaged with a light microscope at 0 and 48 h and
the migratory capacity of the cells was assessed using the size of
the healed wound area. ImageJ software (version 1.51l National
Institutes of Health) was used for data analysis. Wound closure
ratio=(0 h cell migration area-48 h cell migration area)/0 h cell
migration area.
Flow cytometry detection of the cell
cycle
MHCC-97H and Huh7 cells were seeded in 6-well plates
at a density of 5×105 cells/well and treated with
carfilzomib (30 µM for MHCC-97H cells and 60 µM for Huh7 cells) at
37°C for 24 h after 12 h. After 24 h of treatment, the cells were
collected and fixed in 70% ethanol at 4°C overnight. Subsequently,
the collected cells were washed with PBS, resuspended in staining
buffer containing 50 µl PI and 450 µl RNase A (Beijing Solarbio
Science & Technology Co., Ltd.) and incubated at room
temperature for 30 min in the dark. The cells were analyzed and
calculated using BD FACSCanto™ Flow Cytometer (BD
Biosciences) and MoD FIT software (version 3.0; BD Biosciences)
(20).
Western blotting
Protein samples were extracted from MHCC-97H and
Huh7 cells using RIPA buffer supplemented with a mixture of 1% PMSF
and 1% phosphatase inhibitors (Beijing Solarbio Science &
Technology Co., Ltd.) and the concentration was measured using a
BCA Protein Assay kit (Beijing Solarbio Science & Technology
Co., Ltd.). Subsequently. 30 µg of total protein was separated by
10% SDS-PAGE and transferred onto a PVDF membrane (Merck KGaA).
After blocking with 5% non-fat milk at room temperature for 1 h,
the membrane was incubated with primary antibodies at 4°C
overnight, including anti-CDK2 (1:5,000), anti-CDK4 (1:2,000),
anti-cyclin A2 (1:5,000), anti-cyclin E1 (1:1,000), anti-p-JNK
(1:1,000), anti-JNK (1:1,000), anti-p-p38 (1:1,000), anti-p38
(1:1,000), anti-GADD45α (1:1,000) and anti-GAPDH (1:10,000),
followed by incubation with horseradish peroxidase (HRP)-conjugated
Goat Anti-Rabbit IgG (1:10,000; cat. no. SA00001-2, Proteintech
Group, Inc.) or HRP-conjugated Goat Anti-Mouse IgG (1:10,000; cat.
no. SA00001-1, Proteintech Group, Inc.) secondary antibodies at
room temperature for 1 h. The signal was developed with an enhanced
chemiluminescence reagent (New Cell & Molecular Biotechnology
Co., Ltd.), and images were captured using an Amersham ImageQuant
800 imaging system (Cytiva). The quantification of protein
abundance was performed using ImageJ software (version 1.51;
National Institutes of Health), with GAPDH as an endogenous
control.
Plasmid construction and cell
transfection
GADD45α knockdown plasmids containing pPLK GFP+Puro
plasmid backbone [short hairpin (sh)-negative control (NC) and
shGADD45α, pPLK GFP+Puro], were provided by Beijing Tsingke Biotech
Co., Ltd. The 2nd generation system was used for plasmid packaging.
A total of 1.5 µg knockdown plasmids, 1 µg pCMV–VSV-G and 0.5 µg
pCAG-dR8.9 (packaging plasmid provided by Beyotime Institute of
Biotechnology) were transfected into 293T cells in 6-well plates
using Lipofectamine 3000® transfection reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions at 37°C. Lentiviral particles were harvested at 48 h
after transfection, followed by infection of HCC cells for 24 h and
screening of the cells for 7 days with medium containing 2 µg/ml
puromycin (Beyotime Institute of Biotechnology). The target
sequences of the knockdown lentiviral plasmids were as follows:
shNC, 5′-TGTCGCGGTAAGTGCCTCATA-3′; GADD45α sh1,
5′-GCTGGAGAGCAGAAGACCGAA-3′; GADD45α sh2,
5′-GAAGACCGAAAGGATGGATAA-3′; and GADD45α sh3,
5′-CAATGGGTTCCAGTGATTAAT-3′.
In vivo studies
A total of 12 four-week-old female BALB/c nude mice
(weight, 15–16 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and housed in the animal
facility under standard conditions with a controlled temperature of
25±2°C, a humidity of 55±5%, a light/dark photoperiod of 12 h and
free access to water and food. The study was conducted in
compliance with the ‘Animal Research: Reporting of In Vivo
Experiments’ guidelines. Animal experiments were approved by the
Animal Care and Use Committee of The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China; approval no.
2023-KY-1008-002). Huh7 cells were collected in the logarithmic
growth phase and resuspended in PBS at a density of
5×107 cells/ml. Each mouse received a subcutaneous
injection of 100 µl (5×106) cell suspension into the
right flank. The mice were then randomly divided into two groups
and treated with DMSO or 4 mg/kg carfilzomib. Carfilzomib
(dissolved in DMSO and diluted in PBS) was administered
intraperitoneally twice a week. Tumor volume and mouse weight were
measured every 3–4 days. Tumor volume was calculated using the
following formula: Tumor volume=(width2 × length)/2.
After 4 weeks of treatment, mice were euthanized by cervical
dislocation. Subsequently, the subcutaneous tumors were excised and
processed for further experiments (21).
Reverse transcription-quantitative PCR
(RT-qPCR)
VeZol reagent (cat. no. R411-01; Vazyme Biotech Co.,
Ltd.) was used to extract RNA from MHCC-97H and Huh7 cells. A
NanoDrop 2000 spectrophotometer was used to measure the RNA
concentration, and cDNA was synthesized from RT of 1 µg total RNA
according to the manufacturer's protocol of the RT kit (cat. no.
R233-01; Vazyme Biotech Co., Ltd.). A qPCR machine was used to
amplify the cDNA using the SYBR Green qPCR Mix (cat. no. Q711-02;
Vazyme Biotech Co., Ltd.). The thermocycling conditions for
amplification were as follows: 95°C for 5 min, followed by 40
cycles at 95°C for 10 sec, 60°C for 30 sec and a final step at 95°C
for 15 sec, 60°C for 60 sec and 95°C for 15 sec. GAPDH was used as
an internal control. Subsequently, the 2−ΔΔCq method was
used to analyze the gene expression data (22). Primer sequences were as follows:
GADD45α forward (F), 5′-CTGGAGGAAGTGCTCAGCAAAG-3′ and reverse (R),
5′-AGAGCCACATCTCTGTCGTCGT-3′; GADD45β F,
5′-GCCAGGATCGCCTCACAGTGG-3′ and R, 5′-GGATTTGCAGGGCGATGTCATC-3′;
GADD45γ F, 5′-CGTCTACGAGTCAGCCAAAGTC-3′ and R,
5′-CGATGTCGTTCTCGCAGCAGAA-3′; and GAPDH F,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and R,
5′-ACCACCCTGTTGCTGTAGCCAA-3′.
RNA sequencing and analysis
Total RNA from Huh7 cells was extracted using VeZol
reagent (cat. no. R411-01; Vazyme Biotech Co., Ltd.). Furthermore,
a NanoDrop 2000 spectrophotometer was used to determine the purity
and quantity of RNA. The RNA 6000 Nano Kit (cat. no. 5067-1511;
Agilent Technologies, Inc.) and an Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc.) was used to determine RNA integrity.
For each sample, 1 µg of the total RNA was used for RNA-seq library
preparation using the VAHTS Universal V6 RNA-seq Library Prep Kit
(cat. no. NR604-01; Vazyme Biotech Co., Ltd.) per the
manufacturer's instructions. Then mRNA was enriched and purified
using VAHTS mRNA Capture Beads (cat. no. N401; Vazyme Biotech Co.,
Ltd.). The purified mRNA was cut into short fragments using
fragmentation buffer and reverse transcribed into cDNA using random
primers. Second-strand cDNA was synthesized using DNA polymerase I,
RNase H, dNTP and buffer oncluded in the VAHTS Universal V6 RNA-seq
Library Prep Kit. After performing end repair and adding poly (A),
the cDNA fragments were ligated using VAHTS RNA Adapters (cat. no.
N803/N804; Vazyme Biotech Co., Ltd.). The ligation products were
enriched via PCR amplification to construct the cDNA library
template (NovaSeq 6000 S4 Reagent Kit v2.5; 300 cycles; cat. no.
20028312). The quality of these cDNA libraries was evaluated with
the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). An
Illumina Novaseq 6000 (Illumina Inc.) was used for 150 bp
paired-end sequencing. The loading concentration was 4 nM, and the
concentration was quantified using the Qubit 3.0 (Thermo Fisher
Scientific, Inc.). The sequencing was performed by Shanghai OE
Biotech Co., Ltd. DESeq (2012) package (version 1.46.0) was used to
identify transcriptome differentially expressed genes (23,24).
The Hierarchical clustering analysis, volcano map and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analysis were produced
using R (https://www.r-project.org/) on the
OECloud platform (25,26). To complete these analyses, the plate
corresponding to the class volume was selected on the OECloud
platform for hierarchical clustering analysis, volcano map and KEGG
analyses. Subsequently, the sequenced gene expression data,
included in the raw data from the Gene Expression Omnibus database
(accession no. GSE284346; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE284346)
was used for further analysis.
Protein-protein interaction (PPI)
network
Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (version 11.5; http://version-11-5.string-db.org/) is an online
search tool for the analysis of PPIs and functional protein
networks, which contains confirmed and predicted direct and
indirect PPI biological data. In the present study, GADD45α, P38
and JNK were used as query conditions, and the minimum required
interaction score was set to >0.4, which is a medium level of
confidence.
Immunofluorescence staining
The xenograft tumor tissue was fixed in 10% formalin
for 48 h at room temperature, paraffin-embedded and cut into 5
µm-thick paraffin sections. Tissue sections were incubated at 60°C
for 2 h, deparaffinized through xylene, dehydrated in ethanol
gradients and subjected to thermal antigen retrieval as samples
were heated to boiling in a microwave oven for 10 min with 0.01
mol/l citrate buffer (pH 6.0). The sections were blocked in 10%
goat serum (Wuhan Servicebio Technology Co., Ltd.) for 30 min at
room temperature and incubated overnight at 4°C with proliferating
cell nuclear antigen (PCNA) primary antibodies (1:200; cat. no.
GB11010; Wuhan Servicebio Technology Co., Ltd.). Subsequently, the
sections were incubated with CoraLite594-conjugated Goat
Anti-Rabbit IgG fluorescent secondary antibodies (cat. no.
SA00013-4; 1:500; Proteintech Group, Inc.) for 1 h at room
temperature, counterstained with DAPI for 10 min at room
temperature and examined using a fluorescence microscope (27).
Hematoxylin and eosin (H&E)
staining
The xenograft tumor tissue was fixed in 10% formalin
for 48 h at room temperature, paraffin-embedded and cut into 5
µm-thick paraffin sections. Tissue sections were incubated at 60°C
for 2 h, deparaffinized using xylene for 5 min at room temperature
and dehydrated in 5 and 95% ethanol gradient 5 min at room
temperature. Then, the sections were soaked in hematoxylin solution
for 5 min, rinsed in tap water for 1 min, incubated with 1%
hydrochloric acid alcohol solution for 3 sec, rinsed in tap water
for 1 min and hematoxylin reblue solution was added for 10 sec.
Next, the sections were soaked in eosin solution for 5 min. The
sections were dehydrated using 75 and 95% gradient ethanol for 5
min at room temperature, then incubated with xylene for 5 min at
room temperature and sealed with neutral gum. Observation and
analysis were performed under an inverted light microscope. H&E
staining reagent was provided by Wuhan Servicebio Technology Co.,
Ltd. and all incubations were performed room temperature, unless
otherwise stated.
Relationship between GADD45a mRNA
expression levels and overall survival
The Kaplan-Meier (KM) plotter (http://kmplot.com/analysis/) can perform survival
analysis on >54,000 genes, including mRNA, microRNA and
proteins, across 21 tumor types, including liver cancer (28,29).
This database consolidates information from the Gene Expression
Omnibus (GEO), the Cancer Genome Atlas (TCGA) and European
Genome-phenome Archive (EGA), providing gene expression profiles
and prognostic details for a wide range of tumors (28,29).
The datasets used for liver cancer survival analysis in Kaplan
Meier plotter are available at TCGA (https://cancergenome.nih.gov/) and GEO databases
(dataset GSE9843 is available in the https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9843;
dataset GSE20017 is available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20017)
(28). In the present study, the KM
plotter was used to evaluate the prognostic value of GADD45α
in patients with HCC. GADD45α was entered into the database
and the number-at-risk was displayed under the main panel of the
OS. Based on the Kaplan-Meier plotter Online Tool, 322 patients
with HCC (follow-up of 60 months) were included in the online
database and were divided into two groups (high vs. low expression
levels) according to the median expression levels of GADD45α. All
possible cut-off values were auto selected. Statistical parameters
such as survival plot, hazard ratio and log-rank P-values were
obtained from the KM plotter Online Tool.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc.). Data are presented as the mean ± SD. An unpaired
Student's t-test was used to compare two groups and one-way ANOVA
followed by Tukey's post hoc test was used to compare multiple
groups. Kaplan-Meier analysis followed by the log-rank test was
used to evaluate the prognosis of patients. P<0.05 was
considered to indicate a statistically significant difference.
Results
Carfilzomib inhibits the viability of
HCC cells
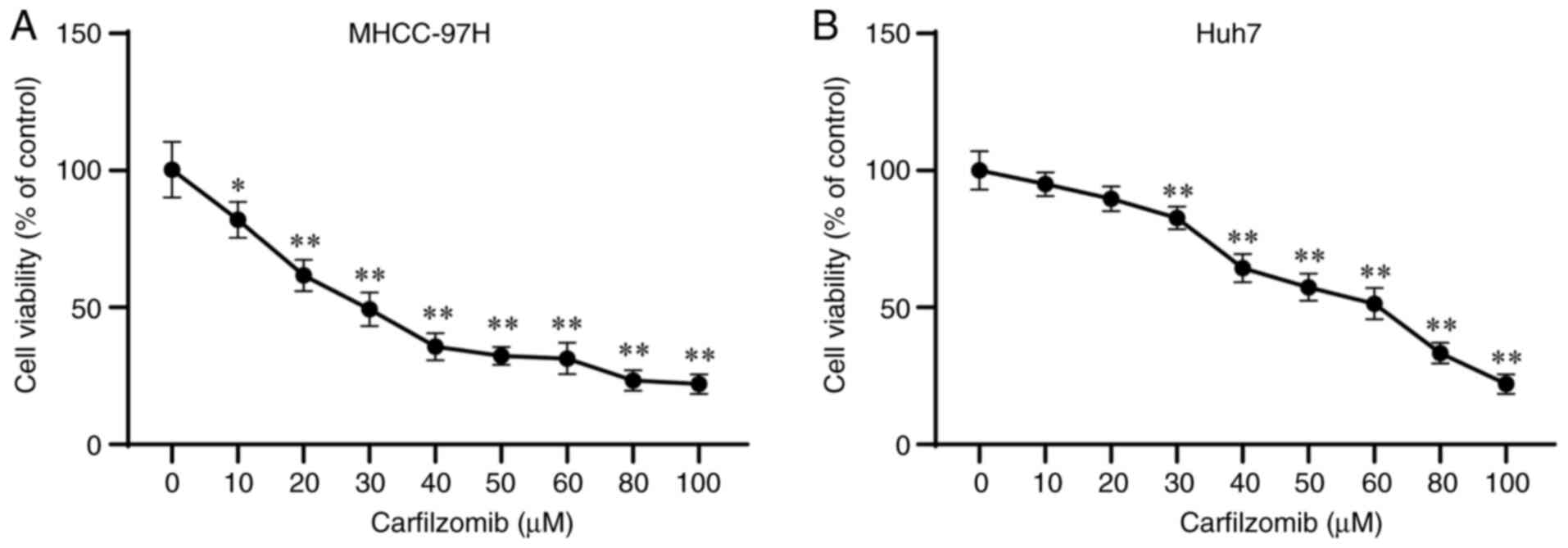
To assess the effect of carfilzomib on HCC cells,
MHCC-97H and Huh7 cells were treated with several concentrations of
carfilzomib. As shown in Fig. 1A and
B, carfilzomib decreased the viability of MHCC-97H and Huh7
cells in a dose-dependent manner. Based on the results of the CCK-8
assay, the activity of MHCC-97H and Huh7 cells decreased by ~50% at
carfilzomib concentrations of 30 and 60 µM, carfilzomib
concentrations of 30 and 60 µM were used for subsequent experiments
on MHCC-97H and Huh7 cells, respectively. These observations
collectively suggested that carfilzomib inhibits the viability of
HCC cells.
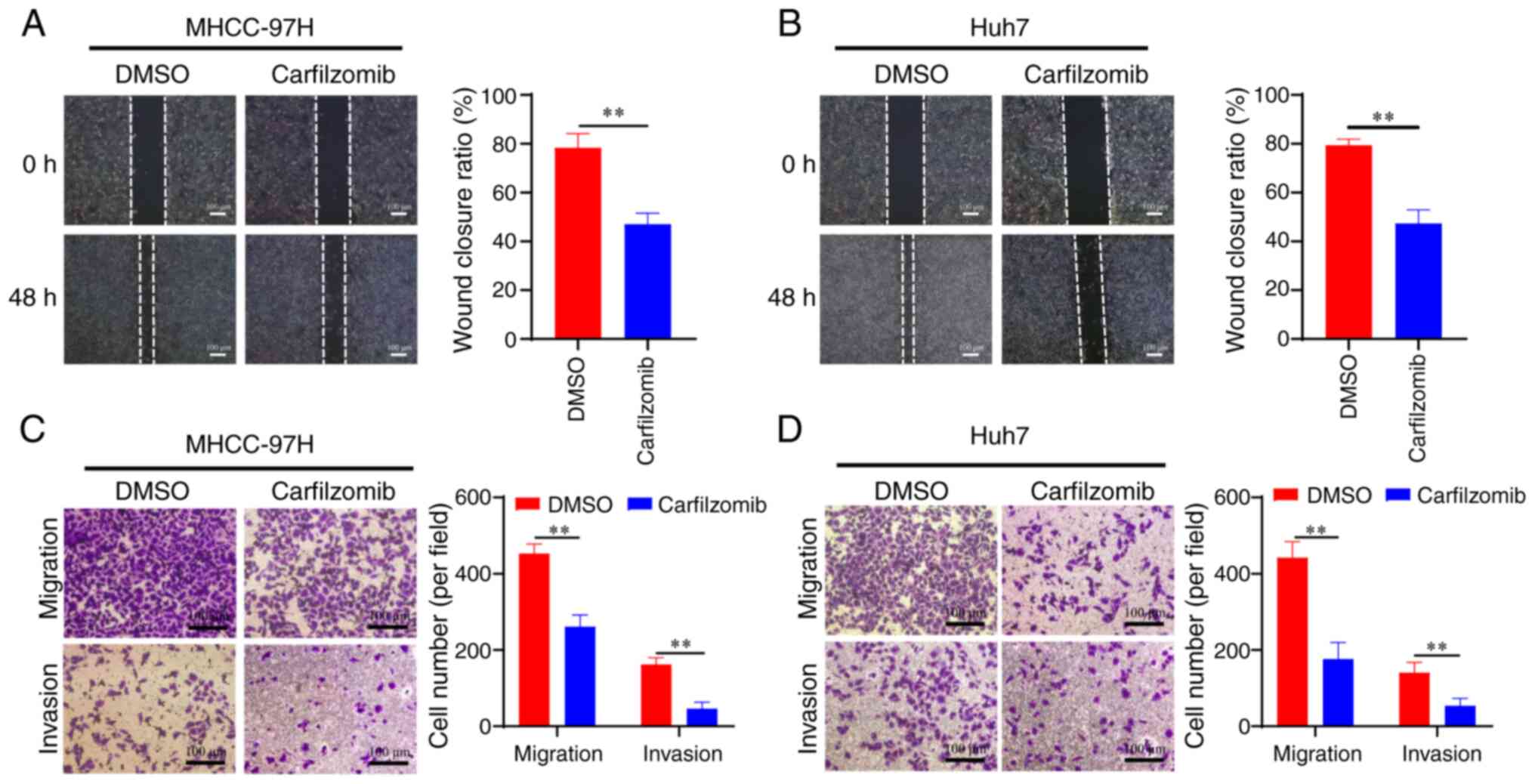
Carfilzomib inhibits HCC cell
migration and invasion
The progression of HCC is characterized by the
increased motility and invasiveness of cancer cells, which
contribute to the high incidence and mortality rate of patients
with HCC (30). Therefore, a series
of experiments were performed to investigate the effect of
carfilzomib on the migration and invasion of HCC cells. The results
of the wound-healing assay showed that carfilzomib inhibited the
migration of MHCC-97H and Huh7 cells compared with that of the
control group (Fig. 2A and B).
Transwell assays with (for invasion) or without (for migration)
Matrigel were used to corroborate the role of carfilzomib in the
migration and invasion of HCC cells. As shown in Fig. 2C and D, carfilzomib significantly
suppressed both invasion and migration in MHCC-97H and Huh7 cells
compared with those of the control groups. These findings indicated
that carfilzomib exhibits strong anti-migratory and anti-invasive
properties against HCC cells.
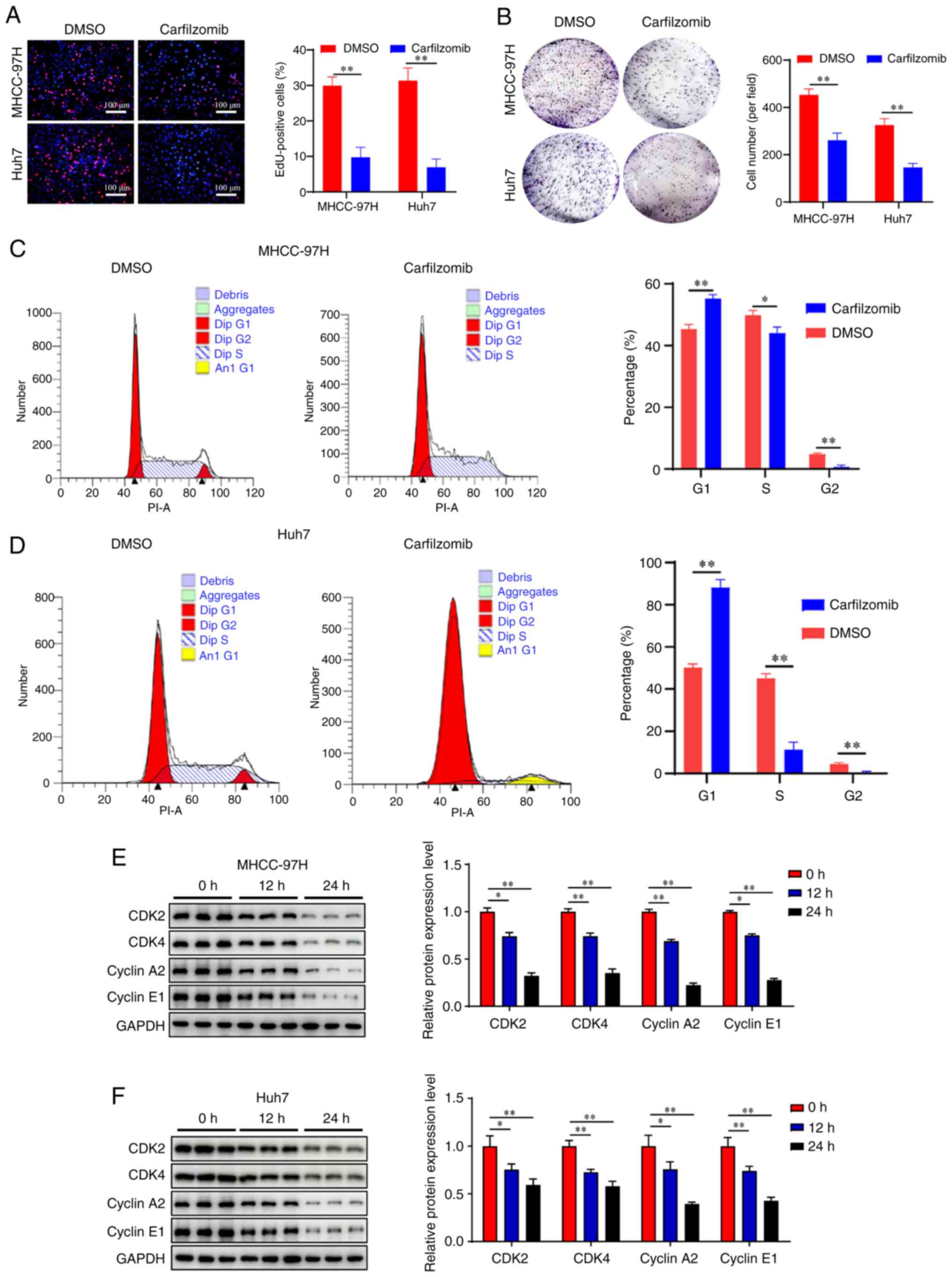
Carfilzomib induces cell cycle arrest
in HCC cells
Uncontrolled cell proliferation caused by cell cycle
dysregulation is a fundamental feature of cancer cells (31,32).
To investigate the anti-proliferative effect of carfilzomib,
MHCC-97H and Huh7 cells were treated with carfilzomib, and
EdU-staining and colony formation assays were performed. The EdU
proliferation assay demonstrated that carfilzomib significantly
decreased the number of EdU-positive MHCC-97H and Huh7 cells
(Fig. 3A). Consistent with these
findings, the colony formation experiments showed that carfilzomib
inhibited colony formation in HCC cells compared with that in the
control group (Fig. 3B).
To investigate the antiproliferative mechanism of
carfilzomib, cell cycle analysis was performed. The results
revealed that carfilzomib increased the percentage of cells in the
G0/G1 phase compared with that in the control
group, indicating that carfilzomib caused cell cycle arrest at the
G0/G1 phase, which reduced entry into the S
and G2 phases, particularly in Huh7 cells (Fig. 3C and D). Additionally, the
expression of cycle-associated proteins was examined by western
blotting. The results demonstrated that carfilzomib significantly
decreased the expression of cyclin A2, cyclin E1, CDK2 and CDK4
(Fig. 3E and F) compared with that
in the control group in both cell lines. These results indicated
that carfilzomib suppressed cell proliferation by inducing
G0/G1 phase arrest in HCC cells.
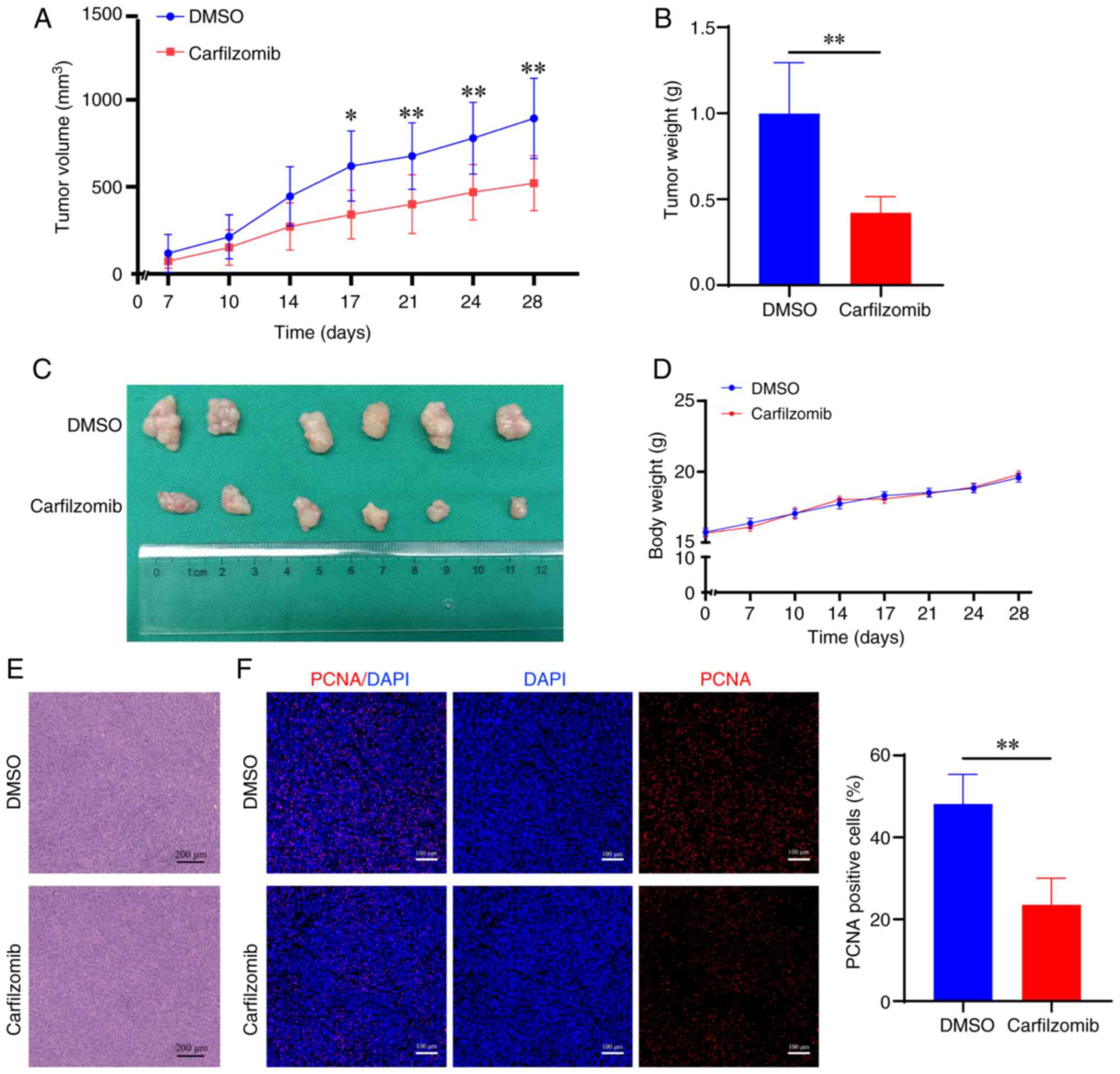
Carfilzomib inhibits the growth of HCC
xenograft tumors
To assess the antitumor effect of carfilzomib in
vivo, a Huh7 ×enograft tumor model using nude mice was
established. In the present study, nude mice were randomly divided
into two groups (n=6 per group), which were treated with vehicle
control or carfilzomib for 4 weeks. Tumor volume was measured to
evaluate the antitumor efficacy of carfilzomib. Carfilzomib
significantly reduced tumor growth, volume and weight compared with
that in the control group after 17, 21, 24 and 28 days of treatment
(Fig. 4A-C). Notably, there was no
significant difference in the body weight of carfilzomib-treated
mice compared with the control mice (Fig. 4D). Furthermore, analysis of the
xenograft tumor tissues using H&E staining, and PCNA
immunofluorescence staining showed that the positive rate of PCNA
in the carfilzomib group was significantly lower compared with that
in the control group (Fig. 4E and
F). These findings indicated that carfilzomib significantly
inhibited tumor cell proliferation in vivo.
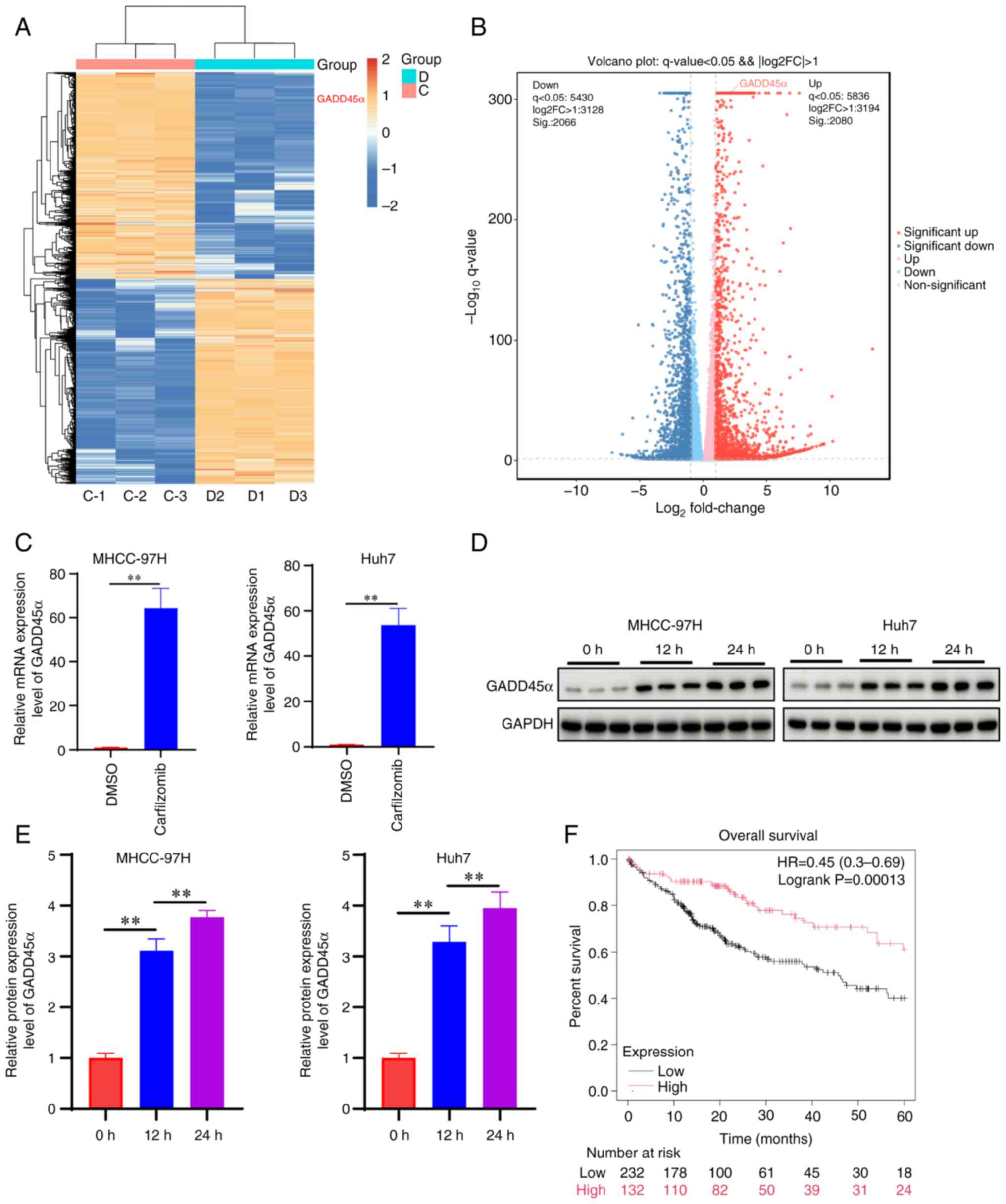
Carfilzomib upregulates GADD45α level
in HCC cells
To investigate the mechanism of carfilzomib action
in HCC cells, transcriptome sequencing was performed in Huh7 cells
treated with or without carfilzomib (Fig. 5A and B). The results showed that
GADD45α exhibited the highest expression among the most significant
top five differentially expressed cell cycle-related genes
(Fig. 5C). Carfilzomib also
exhibited a significant effect on the expression of GADD45β and
GADD45γ (Fig. S1); however,
carfilzomib exhibited the most marked effect on GADD45α expression;
therefore, in the present study the effect of carfilzomib on
GADD45α was investigated. Western blot analysis demonstrated that
carfilzomib upregulated the protein expression level of GADD45α
(Fig. 5D and E). Simultaneously,
analysis of data from The Cancer Genome Atlas showed that higher
expression of GADD45α was associated with improved survival of
patients with HCC compared with low GADD45α expression (Fig. 5F). These findings indicated that
GADD45α may mediate the effects of carfilzomib in HCC cells.
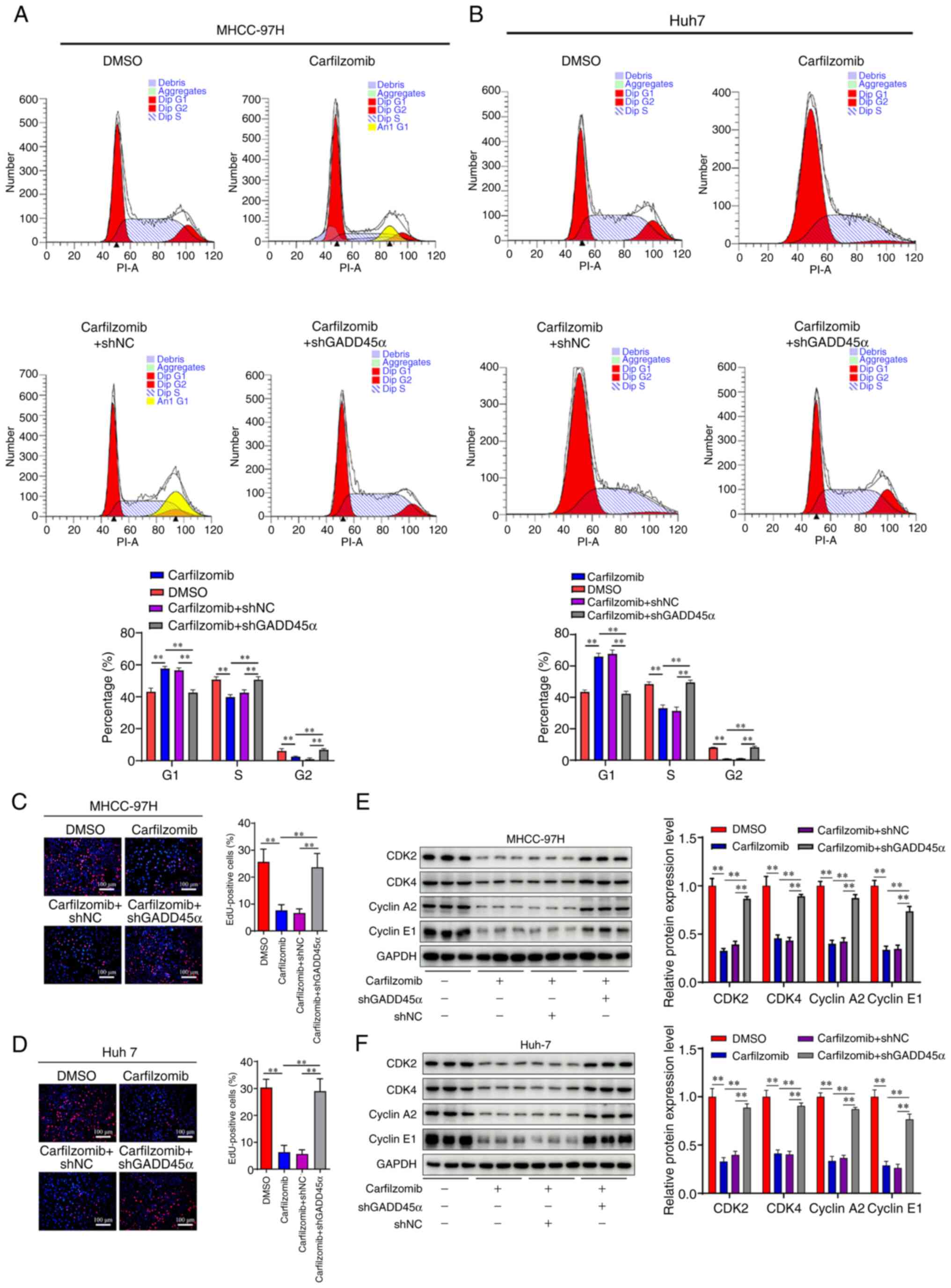
Carfilzomib inhibits cell cycle
progression by upregulating GADD45α expression in HCC cells
To investigate the role of GADD45α in the
carfilzomib-mediated antitumor effect on HCC, stable GADD45α
knockdown cell lines were constructed using lentiviral shRNA
targeting GADD45α (Fig. S2). Based
on the expression of the GADD45α protein, sh2 was the most
effective shRNA tested and was used for the subsequent experiments.
The results demonstrated that knockdown of GADD45α counteracted the
cell cycle inhibitory effects of carfilzomib on the
G0/G1 phase (Fig.
6A and B). Furthermore, the EdU proliferation assay showed that
carfilzomib decreased the number of positive cells, while silencing
GADD45α abolished the anti-proliferative effect of carfilzomib
(Fig. 6C and D). Moreover, the
expression of proteins involved in cell cycle regulation was
examined using western blotting, including cyclin A2, cyclin E1,
CDK2 and CDK4. As shown in Fig. 6E and
F, silencing GADD45α increased the expression levels of cyclin
A2, cyclin E1, CDK2 and CDK4 compared with those in the
carfilzomib-treated group. Collectively, these findings indicated
that GADD45α serves a crucial role in carfilzomib-mediated
antitumor therapy.
GADD45α induces cell cycle arrest by
activating MAPK signaling
The MAPK signaling pathway serves a crucial role in
cell cycle arrest (33). Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis indicated significant enrichment of the ‘MAPK signaling
pathway’ with carfilzomib treatment in Huh7 cells (Fig. 7A). STRING network analysis revealed
a strong association between GADD45α and proteins involved in the
MAPK signaling pathway (Fig. 7B).
To assess the role of GADD45α in the MAPK signaling pathway, the
expression levels of JNK, p-JNK, p38 and p-p38 were examined using
western blotting. As shown in Fig. 7C
and D, carfilzomib upregulated the phosphorylation levels of
JNK and p38, suggesting that carfilzomib activated the MAPK
signaling pathway. Moreover, silencing GADD45α significantly
decreased the levels of p-JNK and p-p38 (Fig. 7C and D). These findings indicated
that carfilzomib may induce cell cycle arrest in HCC cells via
GADD45α-mediated MAPK activation.
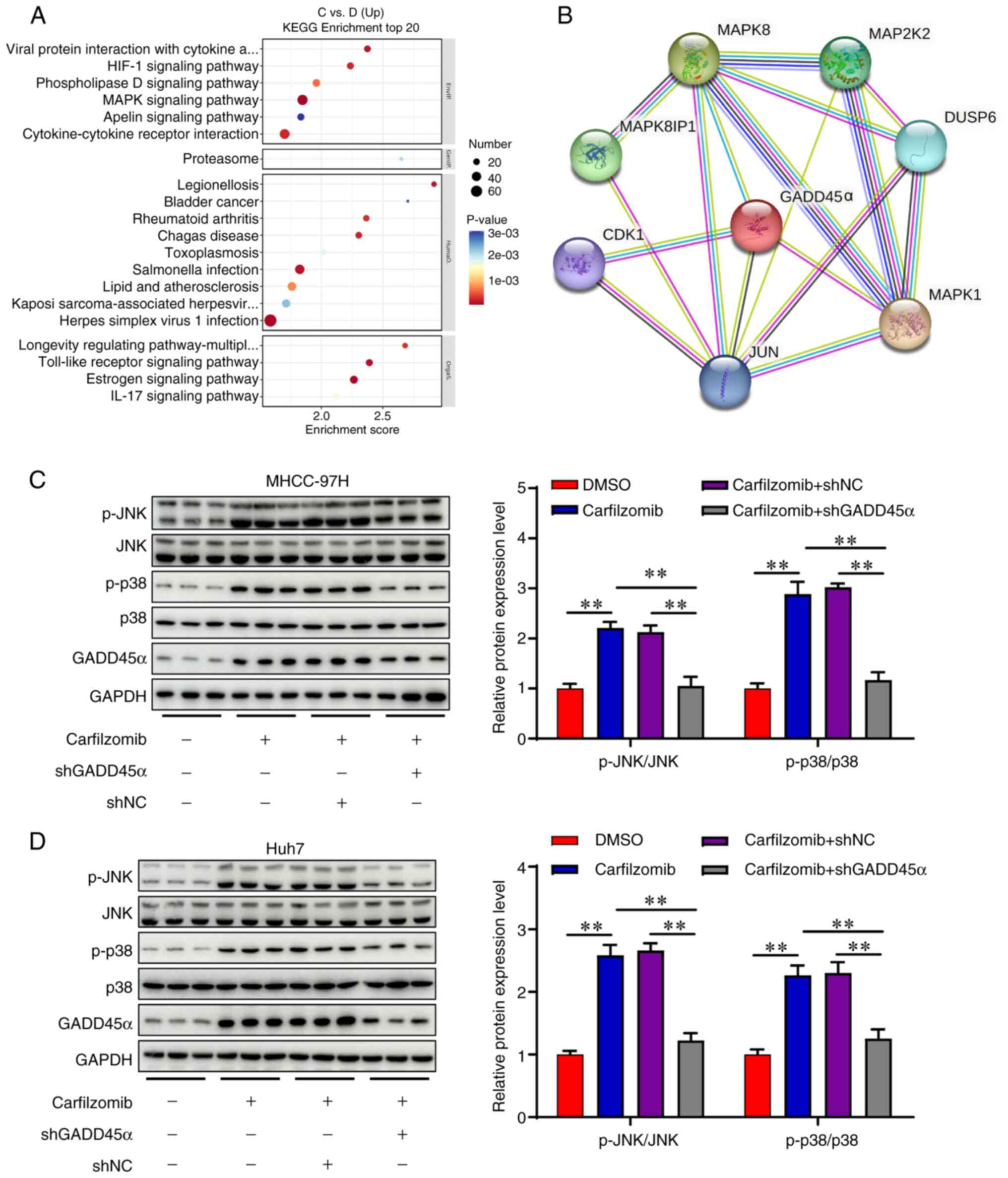 | Figure 7.Carfilzomib inhibits cell
proliferation by activating the MAPK signaling pathway. (A) KEGG
enrichment analysis of the differential expressed genes in Huh7
cells with or without carfilzomib treatment. (B) Functional protein
interaction analysis was performed using the STRING database. The
protein levels of p-JNK, JNK, p-p38, p38 and GADD45α were analyzed
by western blotting in (C) MHCC-97H and (D) Huh7 cells. Data are
presented as the mean ± SD (n=3). **P<0.01. MAPK,
mitogen-activated protein kinase; GADD45α, growth arrest and DNA
damage inducible α; KEGG, Kyoto Encyclopedia of Genes and Genomes;
NC, negative control; sh, short hairpin; p, phosphorylated; C,
carfilzomib; D, DMSO. |
Discussion
Sorafenib was the clinically approved pharmaceutical
agent for unresectable HCC prior to the approval of regorafenib
(34). However, both sorafenib and
regorafenib have demonstrated poor overall survival in certain
patients (35,36). The drug discovery approach has
proven challenging, particularly in HCC. Carfilzomib is a
proteasome inhibitor approved by the Food and Drug Administration
for treating multiple myeloma (37). Targeting the proteasome may be a
promising approach for treating solid tumors. In a phase I clinical
trial, a combination of carfilzomib and irinotecan achieved 20%
partial response and 6% stable disease in patients with small cell
lung cancer (37). However, the
efficacy of carfilzomib in patients with HCC has been poorly
studied. The present results indicated that carfilzomib could delay
tumor cell proliferation, invasion and migration in vitro
and in vivo, thus establishing carfilzomib as a potential
therapeutic agent for HCC.
There is growing consensus that cell cycle
dysregulation has long been described as a hallmark and one of the
causes of cancer (38). Cyclins,
CDKs and CDK inhibitors tightly regulate the cell cycle. CDKs
regulate cell cycle events, including initiation, progression and
completion of the cell cycle (39).
G1/S phase progression is regulated by the binding of
CDKs 4/6 to cyclin D and CDK2 to cyclin E, which eventually
phosphorylates the retinoblastoma protein (40). To determine the effect of
carfilzomib on cell cycle arrest in HCC cells, cell cycle
distribution was analyzed by flow cytometry. The results
demonstrated that carfilzomib notably induced cell cycle arrest at
the G0/G1 phases after 24 h of treatment.
Furthermore, western blot analyses indicated that carfilzomib
significantly downregulated the expression of cyclin A2, cyclin E1,
CDK2 and CDK4, providing further evidence for the
carfilzomib-mediated arrest at the G0/G1
phase. These effects suggested a potential mechanism affecting cell
cycle regulation in HCC cells in response to carfilzomib
treatment.
The cell cycle is regulated by several inhibitory
proteins, including the GADD protein family, which prevent
inappropriate mitosis by regulating the cell cycle checkpoints
(41). GADDs serve an important
role in the DNA damage signal transduction pathway (42). Cell cycle arrest may occur due to
carfilzomib activating signal transduction in HCC cells. The
present study demonstrated that carfilzomib induced GADD45α
expression in HCC cells, which may be associated with cell cycle
arrest at the G0/G1 phases. Previous studies
reported that adenoviral-mediated GADD45α mRNA and protein
overexpression led to apoptosis in pancreatic cancer cells by
activating caspase-9 and inducing cell cycle arrest (43). Moreover, GADD45α shRNA abolished
carfilzomib-mediated cell cycle arrest. Although carfilzomib
appears to induce GADD45α expression and cell cycle arrest, the
underlying mechanisms remain unclear.
MAPK pathways serve a key role in several cellular
processes, including proliferation, differentiation and apoptosis
(44). MAPK signaling has been
demonstrated to promote GADD45α expression by activating p38 and
JNK kinases (45). Specifically,
these kinases activate c-Jun, which binds directly to the GADD45α
promoter and activates its transcription (46). In a number of cases, GADD45α has
been shown to mediate apoptosis via the p38 and JNK pathways
(47,48). GADD45α can also bind to the
N-terminus of mitogen-activated protein kinase kinase kinase 4 and
activate p38 and JNK signaling, which in turn functions as an
upstream activator of GADD45α, forming a positive feedback loop
that increases the levels of the tumor suppressor protein GADD45α
in the event of unresolved DNA damage (49). In addition, GADD45α expression is
critical for maintaining p38 and JNK signaling, resulting in
keratinocyte growth arrest after UV irradiation (50). In lymphocytes, activation of p38 is
essential for apoptosis induced by riboflavin and ultraviolet
light, and GADD45α serves a crucial role in modulating this process
(51). In the present study, the
MAPK signaling pathway was enriched in KEGG pathway analysis
following the treatment of Huh7 cells with carfilzomib. In
addition, knockdown of GADD45α reduced the activation of the MAPK
pathway and abolished the effects of carfilzomib. These data
suggested that carfilzomib-mediated cell cycle arrest may be
associated with GADD45α-induced activation of the MAPK pathway.
To the best of our knowledge, the present study is
the first to explore the possible underlying mechanisms of
carfilzomib in HCC. However, the present study has certain
limitations. While the effect of carfilzomib on HCC has previously
been reported, the present study is an important supplement to
highlight the mechanism of carfilzomib in the treatment of HCC. In
addition, whether GADD45α inhibits HCC growth in
carfilzomib-treated cells by regulating pathways other than MAPK is
still unclear and requires further investigation. Moreover, an
inducible Cre knockout model of GADD45α would be more
physiologically relevant compared with GADD45α knockdown cell lines
to study the GADD45α-mediated anti-HCC progression of carfilzomib,
which will be addressed in future studies, as lentivirus-mediated
GADD45α knockdown still retains a small amount of GADD45α, and this
small amount of GADD45α is still functional.
In summary, the present study demonstrated that
carfilzomib has marked anticancer effects in HCC cells and exerts
its action through the GADD45α-mediated MAPK pathway. Furthermore,
the current study provided a theoretical basis for future clinical
applications of carfilzomib as a potent chemotherapeutic agent for
the treatment of HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Henan Provincial Science
and Technology Research Plan (grant no. 222102310534), the Key
Scientific Research Project of Henan Higher Education Institutions
of China (grant no. 24B320024) and the Foundation of Henan Charity
Federation (grant no. GDXZ2023003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw sequencing data
generated in the present study may be found in the Gene Expression
Omnibus database under accession no. GSE284346 or at the following
URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE284346.
Authors' contributions
MC, XC and WG designed the study. MC, XC, YS and CJ
performed data acquisition and data analysis. MC and XC drafted the
manuscript. MC, YS, CJ and WG edited the manuscript. CJ and WG
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Care and Use Committee of The First Affiliated Hospital of
Zhengzhou University (approval no. 2023-KY-1008-002; Zhengzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eguia E, Baker T and Baker M:
Hepatocellular carcinoma: Surgical management and evolving
therapies. Cancer Treat Res. 192:185–206. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H and Li W: Recent update on
comprehensive therapy for advanced hepatocellular carcinoma. World
J Gastrointest Oncol. 13:845–855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng TR, Weng YF, Wu TW, Wu CC, Chou YC
and Hsu CS: Efficacy and safety of sorafenib or lenvatinib for
advanced hepatocellular carcinoma after failure of first-line
atezolizumab plus bevacizumab: A systematic review and
meta-analysis. Cancers (Basel). 16:28132024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo X, He X, Zhang X, Zhao X, Zhang Y, Shi
Y and Hua S: Hepatocellular carcinoma: Signaling pathways, targeted
therapy, and immunotherapy. MedComm (2020). 5:e4742024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs A, Aidoo-Micah G, Maini MK and
Meyer T: Immunotherapy for hepatocellular carcinoma. JHEP Rep.
6:1011302024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Kuo JC, Huang Y, Hu Y, Deng L,
Yung BC, Zhao X, Zhang Z, Pan J, Ma Y and Lee RJ: Optimized
liposomal delivery of bortezomib for advancing treatment of
multiple myeloma. Pharmaceutics. 15:26742023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wahl K, Siegemund M, Lehner F, Vondran F,
Nüssler A, Länger F, Krech T, Kontermann R, Manns MP,
Schulze-Osthoff K, et al: Increased apoptosis induction in
hepatocellular carcinoma by a novel tumor-targeted TRAIL fusion
protein combined with bortezomib. Hepatology. 57:625–636. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alwahsh M, Farhat J, Talhouni S, Hamadneh
L and Hergenröder R: Bortezomib advanced mechanisms of action in
multiple myeloma, solid and liquid tumors along with its novel
therapeutic applications. EXCLI J. 22:146–168. 2023.PubMed/NCBI
|
|
10
|
Lara PN Jr, Longmate J, Reckamp K, Gitlitz
B, Argiris A, Ramalingam S, Belani CP, Mack PC, Lau DH, Koczywas M,
et al: Randomized phase II trial of concurrent versus sequential
bortezomib plus docetaxel in advanced non-small-cell lung cancer: A
California cancer consortium trial. Clin Lung Cancer. 12:33–37.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ
and Cheng AL: Synergistic interactions between sorafenib and
bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt
inactivation. J Hepatol. 52:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honma Y, Shimizu S, Takehara T and Harada
M: Sorafenib enhances proteasome inhibitor-induced cell death via
inactivation of Akt and stress-activated protein kinases. J
Gastroenterol. 49:517–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan CRC, Abdul-Majeed S, Cael B and Barta
SK: Clinical pharmacokinetics and pharmacodynamics of bortezomib.
Clin Pharmacokinet. 58:157–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimopoulos MA, Moreau P, Palumbo A, Joshua
D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, et
al: Carfilzomib and dexamethasone versus bortezomib and
dexamethasone for patients with relapsed or refractory multiple
myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre
study. Lancet Oncol. 17:27–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mansour MA, Aljoufi MA, Al-Hosaini K,
Al-Rikabi AC and Nagi MN: Possible role of selective, irreversible,
proteasome inhibitor (carfilzomib) in the treatment of rat
hepatocellular carcinoma. Chem Biol Interact. 215:17–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu B, Gao J, Shi J, Wen P, Guo W and Zhang
S: m6A reader YTHDF3 triggers the progression of
hepatocellular carcinoma through the YTHDF3/m6
A-EGFR/STAT3 axis and EMT. Mol Carcinog. 62:1599–1614. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang M, Qi F, Zhang K, Zhang X, Ma J, Xia
S, Chen L, Yu Z, Chen J and Chen D: MARCKSL1-2 reverses
docetaxel-resistance of lung adenocarcinoma cells by recruiting
SUZ12 to suppress HDAC1 and elevate miR-200b. Mol Cancer.
21:1502022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo D, Zhang M, Wei T, Zhang X, Shi X,
Tang H, Ding M, Li J, Zhang S and Guo W: NFKBIZ regulates NFκB
signaling pathway to mediate tumorigenesis and metastasis of
hepatocellular carcinoma by direct interaction with TRIM16. Cell
Mol Life Sci. 81:1672024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang F, Gao J, Liu X, Sun Y, Liu L, Hu B,
Wang Z, Shi J, Guo W and Zhang S: LATS-regulated
nuclear-cytoplasmic translocation of SREBP2 inhibits hepatocellular
carcinoma cell migration and invasion via epithelial-mesenchymal
transition. Mol Carcinog. 62:963–974. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding L, Ren C, Yang L, Wu Z, Li F, Jiang
D, Zhu Y and Lu J: OSU-03012 disrupts Akt signaling and prevents
endometrial carcinoma progression in vitro and in vivo. Drug Des
Devel Ther. 15:1797–1810. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao J, Shi X, Sun Y, Liu X, Zhang F, Shi
C, Yu X, Yan Z, Liu L, Yu S, et al: Deficiency of
betaine-homocysteine methyltransferase activates
glucose-6-phosphate dehydrogenase (G6PD) by decreasing arginine
methylation of G6PD in hepatocellular carcinogenesis. Sci China
Life Sci. 67:1648–1665. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roberts A, Trapnell C, Donaghey J, Rinn JL
and Pachter L: Improving RNA-Seq expression estimates by correcting
for fragment bias. Genome Biol. 12:R222011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo N, Xia Y, He N, Cheng H, Zhang L and
Liu J: IRGM deficiency exacerbates sepsis-induced acute lung injury
by inhibiting autophagy through the AKT/mTOR signaling pathway. J
Inflamm Res. 17:10255–10272. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Kuang J, Li XT, Hu X, Liu YH, Hu
CP, Wang M, Wang Q and Zhang Z: Dimethyl fumarate is repurposed to
ameliorate aortic aneurysm and dissection in mice. Eur J Pharmacol.
988:1772152025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Zhang J, Li J, Song Y, Pan J, Mei C,
Cui M, He Q, Wang H, Li H, et al: Sirtuin 5-mediated
desuccinylation of ALDH2 alleviates mitochondrial oxidative stress
following acetaminophen-induced acute liver injury. Adv Sci
(Weinh). 11:e24027102024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menyhárt O, Nagy Á and Győrffy B:
Determining consistent prognostic biomarkers of overall survival
and vascular invasion in hepatocellular carcinoma. R Soc Open Sci.
5:1810062018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Győrffy B: Integrated analysis of public
datasets for the discovery and validation of survival-associated
genes in solid tumors. Innovation (Camb). 5:1006252024.PubMed/NCBI
|
|
30
|
Lin Y, Zhong W, Lin Q, Ye Y, Li S, Chen H,
Liu H, Xu L, Zhuang W, Chen S, et al: SFPQ promotes the
proliferation, migration and invasion of hepatocellular carcinoma
cells and is associated with poor prognosis. Am J Cancer Res.
13:2269–2284. 2023.PubMed/NCBI
|
|
31
|
Oshima J and Campisi J: Fundamentals of
cell proliferation: Control of the cell cycle. J Dairy Sci.
74:2778–2787. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deng Y, Li Y, Yang M, Gao Y, Luo X, Chen
H, Guo M, Yang X, Liu Y, He J, et al: Carfilzomib activates ER
stress and JNK/p38 MAPK signaling to promote apoptosis in
hepatocellular carcinoma cells. Acta Biochim Biophys Sin
(Shanghai). 56:697–708. 2024.PubMed/NCBI
|
|
34
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther. 5:872020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lv Z, Liu L, You J, Zhou P, Su Y, Zhao K,
Zhang J and Zhu F: Small HBV surface antigen drives regorafenib
resistance in HCC via KIAA1429-dependent m6A modification of CCR9.
J Med Virol. 96:e298942024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leowattana W, Leowattana T and Leowattana
P: Systemic treatment for unresectable hepatocellular carcinoma.
World J Gastroenterol. 29:1551–1568. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arnold SM, Chansky K, Leggas M, Thompson
MA, Villano JL, Hamm J, Sanborn RE, Weiss GJ, Chatta G and
Baggstrom MQ: Phase 1b trial of proteasome inhibitor carfilzomib
with irinotecan in lung cancer and other irinotecan-sensitive
malignancies that have progressed on prior therapy (Onyx IST
reference number: CAR-IST-553). Invest New Drugs. 35:608–615. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu H, Li L, Ai Z, Yin J and Chen L:
Pristimerin induces apoptosis of oral squamous cell carcinoma cells
via G1 phase arrest and MAPK/Erk1/2 and Akt signaling
inhibition. Oncol Lett. 17:3017–3025. 2019.PubMed/NCBI
|
|
39
|
Wang B, Li R, Wu S, Liu X, Ren J, Li J, Bi
K, Wang Y and Jia H: Breast cancer resistance to cyclin-dependent
kinases 4/6 inhibitors: Intricacy of the molecular mechanisms.
Front Oncol. 11:6515412021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Desvoyes B and Gutierrez C: Roles of plant
retinoblastoma protein: Cell cycle and beyond. EMBO J.
39:e1058022020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Humayun A and Fornace AJ Jr: GADD45 in
stress signaling, cell cycle control, and apoptosis. Adv Exp Med
Biol. 1360:1–22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Palomer X, Salvador JM, Griñán-Ferré C,
Barroso E, Pallàs M and Vázquez-Carrera M: GADD45A: With or without
you. Med Res Rev. 44:1375–1403. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Qian H, Li X, Wang H, Yu J, Liu Y,
Zhang X, Liang X, Fu M, Zhan Q and Lin C: Adenoviral-mediated gene
transfer of Gadd45a results in suppression by inducing apoptosis
and cell cycle arrest in pancreatic cancer cell. J Gene Med.
11:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang M, Tian B, Shen J, Xu S, Liu C, Guan
L, Guo M and Dou J: Bavachin induces apoptosis in colorectal cancer
cells through Gadd45a via the MAPK signaling pathway. Chin J Nat
Med. 21:36–46. 2023.PubMed/NCBI
|
|
46
|
Wang Y, Gao H, Cao X, Li Z, Kuang Y, Ji Y
and Li Y: Role of GADD45A in myocardial ischemia/reperfusion
through mediation of the JNK/p38 MAPK and STAT3/VEGF pathways. Int
J Mol Med. 50:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang HH, Chang TY, Lin WC, Wei KC and Shin
JW: GADD45A plays a protective role against temozolomide treatment
in glioblastoma cells. Sci Rep. 7:88142017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peretz G, Bakhrat A and Abdu U: Expression
of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg
asymmetric development that is mediated by the c-Jun N-terminal
kinase pathway. Genetics. 177:1691–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geifman-Holtzman O, Xiong Y and Holtzman
EJ: Gadd45 stress sensors in preeclampsia. Adv Exp Med Biol.
793:121–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hildesheim J, Bulavin DV, Anver MR, Alvord
WG, Hollander MC, Vardanian L and Fornace AJ Jr: Gadd45a protects
against UV irradiation-induced skin tumors, and promotes apoptosis
and stress signaling via MAPK and p53. Cancer Res. 62:7305–7315.
2002.PubMed/NCBI
|
|
51
|
Yang P, Wen H, Zhong T, Hu H, Zhu B, Xia
K, Xu M and Bian M: GADD45α is involved in the apoptosis of
lymphocytes induced by riboflavin and ultraviolet light.
Transfusion. 57:646–656. 2017. View Article : Google Scholar : PubMed/NCBI
|