Introduction
In recent years, immune checkpoint inhibitors (ICIs)
have revolutionized the treatment landscape for advanced or
metastatic urothelial carcinoma (UC) and renal cell carcinoma
(RCC). In particular, multiple ICIs targeting programmed cell death
1 (PD-1), PD-1 ligand (PD-L1), and cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) have promoted
significantly improved overall survival and responses rates among
patients with these malignancies (1–8).
For advanced or metastatic UC, PD-1 inhibitors such
as nivolumab and pembrolizumab are used as fundamental systemic
therapies, while the PD-L1 inhibitor avelumab is used for
maintenance therapy following first-line chemotherapy In advanced
or metastatic RCC, nivolumab and pembrolizumab are used as backbone
treatments, often in combination with either the CTLA-4 inhibitor
ipilimumab or tyrosine kinase inhibitors like cabozantinib,
axitinib, or lenvatinib (9).
Although the efficacy of ICIs in advanced or metastatic UC and RCC
is well-established, their use has been associated with a unique
spectrum of immune-related adverse events (irAEs), which commonly
include dermatologic, gastrointestinal, endocrine, and pulmonary
toxicities, affecting various organ systems (10,11).
Despite the growing body of knowledge surrounding irAEs, hepatic
irAEs remain less poorly characterized, particularly in the context
of genitourinary cancers. Most large clinical trials have reported
only aggregate rates of hepatotoxicity without detailed
characterizations of the clinical presentations, management
approaches, or outcomes.
Given the liver's crucial role in maintaining
peripheral tolerance, a better understanding of hepatic irAEs in
genitourinary malignancies is imperative. Furthermore, managing
patients with these types of cancer requires a delicate balance
between treatment efficacy and toxicity. As the use of ICIs for
urologic malignancies continues to increase, the incidence of
hepatic irAEs is expected to rise correspondingly, underscoring the
need for improved understanding and management of this
complication. The current study, therefore, aimed to
comprehensively evaluate the incidence, clinical features, and
management of hepatic irAEs among patients with advanced or
metastatic UC and RCC receiving ICIs at three tertiary care
centers.
Materials and methods
Patients and data collection
This multicenter, observational, retrospective study
involving human participants was conducted in accordance with the
ethical standards of the institutional and national research
committees and the ethical principles stated in the Declaration of
Helsinki. The protocol for this study was approved by the
Institutional Review Boards of the three participating
institutions: Inje University Busan Paik Hospital (BPIRB
2024-10-001), Pusan National University Hospital (2408-017-110),
and Dongnam Institute of Radiological & Medical Sciences
(D-2312-021-007). The requirement for informed consent was waived
due to the retrospective nature of this study. All participating
centers obtained approval from their local research ethics boards
prior to data collection. A total of 267 patients aged 20 years or
older with advanced or metastatic UC or RCC, who received ICIs
between February 2018 and September 2023, were retrospectively
identified from three tertiary medical centers in South Korea.
Patients who lacked follow-up data on oncological outcomes after
ICI treatment, had been diagnosed with other malignancies or
received systemic therapy within the past 5 years or had liver
metastases from metastatic UC or RCC were excluded. The final study
cohort comprised 213 patients with a median age of 70 years (IQR,
38–86 years). Demographic, clinical, and laboratory variables prior
to ICI treatment initiation were collected.
ICI treatment
ICI treatment was administered in accordance with
the Korean National Health Insurance regulations and National
Comprehensive Cancer Network guidelines (9,12). The
ICIs used for the treatment of advanced or metastatic UC and RCC
included ipilimumab (a CTLA-4 inhibitor), nivolumab and
pembrolizumab (PD-1 inhibitors), and atezolizumab and avelumab
(PD-L1 inhibitors). The specific drugs and regimens administered to
the patients included herein are summarized in Table I. All participating centers
administered these medications without dose escalation or
reduction, strictly following the regimens outlined in Table I. None of our patients received ICIs
as first-line therapy for advanced or metastatic UC or as adjuvant
treatment for increased risk of RCC recurrence. For advanced UC
patients, second-line treatment included avelumab maintenance
therapy administered after 4–6 cycles of chemotherapy. Patients
continued therapy until disease progression or unacceptable
toxicity occurred. Progression was defined as clinical progression
or fulfillment of radiographic criteria based on the Response
Evaluation Criteria in Solid Tumors version 1.1.
 | Table I.Immune-check point inhibitor regimens
used in the present study for the treatment of advanced and
metastatic RCC and UC. |
Table I.
Immune-check point inhibitor regimens
used in the present study for the treatment of advanced and
metastatic RCC and UC.
| A, Advanced or
metastatic RCC |
|---|
|
|---|
| Drug combination | Regimen | Indication |
|---|
| Pembrolizumab
(monotherapy) | 200 mg IV every 3
weeks or 400 mg IV every 6 weeks | Second-line or later
treatment |
| Nivolumab
(monotherapy) | 240 mg IV every 2
weeks or 480 mg IV every 4 weeks | Second-line or later
treatment |
| Pembrolizumab +
axitinib | Pembrolizumab: 200 mg
IV every 3 weeks or 400 mg IV every 6 weeks; axitinib: 5 mg orally
twice daily | First-line treatment
for IMDC all risk groups |
| Nivolumab +
ipilimumab | Nivolumab: 3 mg/kg IV
every 3 weeks for 4 doses, then 240 mg IV every 2 weeks or 480 mg
IV every 4 weeks; ipilimumab: 1 mg/kg IV every 3 weeks for 4
doses | First-line treatment
for IMDC intermediate or poor-risk |
| Nivolumab +
cabozantinib | Nivolumab: 240 mg IV
every 2 weeks or 480 mg IV every 4 weeks; cabozantinib: 40 mg
orally once daily | First-line treatment
for IMDC all risk groups |
| Pembrolizumab +
lenvatinib | Pembrolizumab: 200 mg
IV every 3 weeks or 400 mg IV every 6 weeks; lenvatinib: 20 mg
orally once daily | First-line treatment
for IMDC all risk groups |
|
| B, Advanced or
metastatic UC |
|
| Drug
combination | Regimen |
Indication |
|
| Pembrolizumab | 200 mg IV every 3
weeks or 400 mg IV every 6 weeks | Second-line or later
treatmentafter progression on platinum-based chemotherapy |
| Atezolizumab | 1,200 mg IV every 3
weeks | Second-line or later
treatment after progression on platinum-based chemotherapy |
| Nivolumab | 240 mg IV every 2
weeks or 480 mg IV every 4 weeks | Second-line or later
treatment after progression on platinum-based chemotherapy |
| Avelumab | 10 mg/kg IV every 2
weeks | Maintenance therapy
post-platinum-based chemotherapy |
IrAEs
IrAEs were defined as adverse events with a probable
immunologic basis that required monitoring and potential
intervention. The irAEs were categorized into dermatologic,
hepatic, renal, gastrointestinal, endocrine, rheumatologic,
pulmonary, hematologic, and other subgroups. Clinical data on
irAEs, including ICI types, duration of ICI therapy, and time to
irAE onset were extracted from outpatient clinic notes,
hospitalization records, and radiological reports. All evaluated
patients had comprehensive clinical documentation of irAEs,
including descriptions of severity and management approaches.
Toxicity was graded according to the Common Terminology Criteria
for Adverse Events (CTCAE) version 5.0 (13).
Characterization of hepatic irAE
Hepatic irAEs were classified into grades 1–4 using
the CTCAE version 5.0, considering that the enrolled patients
presented with normal baseline liver tests. Hepatic irAEs were
defined based on elevations in aspartate aminotransferase (AST),
alanine aminotransferase (ALT), and total bilirubin levels relative
to the upper limit of normal (ULN). Grade 1 events were defined as
those in which AST or ALT was greater than the ULN to 3 times the
ULN and/or total bilirubin was greater than the ULN to 1.5 times
the ULN. Grade 2 events were defined as those in which AST or ALT
was 3–5 times the ULN and/or total bilirubin was greater than 1.5–3
times the ULN. Grade 3 events were defined as those in which AST or
ALT was greater than 5–20 times the ULN and/or total bilirubin was
3–10 times the ULN. Grade 4 events were defined as those in which
AST or ALT was greater than 20 times the ULN and/or total bilirubin
was greater than 10 times the ULN. These criteria were applied to
participants who previously had normal results on their liver
function tests. Given that most patients with hepatic irAEs remain
asymptomatic, liver function tests were performed at baseline and
before each treatment cycle. For patients with liver function test
results outside the ULN prior to ICI treatment, an extensive workup
was performed to rule out other causes of liver enzyme
abnormalities, including viral hepatitis, autoimmune disease,
cancer progression, vascular complications, and other potential
treatments that could cause drug-induced liver injury. Liver
imaging studies were systematically performed in these cases. The
pattern of hepatitis was analyzed using the R value calculated as
(ALT/ULN)/(ALP/ULN) (14). To help
determine the predominant type of liver injury and guide further
management and treatment decisions, hepatic irAE patterns were
categorized as cholestatic (R≤2), mixed (2<R<5), or
hepatocellular (R≥5).
Management of hepatic irAEs
Hepatic irAEs were managed based on the American
Society of Clinical Oncology Clinical Practice Guideline, which
presents practical recommendations according to the hepatotoxicity
grade defined by the CTCAE grading system (4). The management approach utilized in our
study was as follows: patients with grade 1 and 2 hepatic irAEs did
not receive corticosteroids or discontinue ICI therapy in the early
stage of treatment; instead, their hepatic irAEs were managed with
heptatonic agents such as ursodeoxycholic acid (UDCA) and biphenyl
dimethyl dicarboxylate (DDB). However, for patients with grade 2
hepatic irAEs, the use of corticosteroids and discontinuation of
ICI therapy were left to the discretion of the treating physician.
All patients with grade 3 or higher hepatic irAEs discontinued ICI
therapy and received high-dose intravenous corticosteroids (1
mg/kg). In patients with corticosteroid resistance, second-line
immunosuppressive agents were administered.
Statistical analysis
Continuous variables were presented as either mean
and standard deviation or median and interquartile range (IQR),
whereas categorical variables were presented as frequencies and
percentages. Differences between groups were evaluated using
Pearson's chi-squared test, Fisher's exact test, and
linear-by-linear association for categorical variables. For
continuous variables, unpaired Student's t-test, one-way ANOVA with
Tukey's post hoc test and Kruskal-Wallis test with Dunn's post hoc
test were used. The cumulative probability of hepatic irAE
according to ICI regimens was estimated using Kaplan-Meier
analysis. Multiple pairwise comparisons between groups were
performed using log-rank tests with Bonferroni correction to
control for type I error. Hazard ratios (HRs) with 95% confidence
intervals (CIs) were calculated using Cox proportional hazards
regression models for between-group comparisons. Progression-free
survival (PFS) was estimated using the Kaplan-Meier method with
log-rank test. Statistical analysis was performed using SPSS
version 27.0 (IBM Corp.) and MedCalc version 22.0 (MedCalc Software
Ltd.). For all tests, a two-sided P-value of <0.05 indicated
statistical significance.
Results
Patient demographics and irAEs
Throughout the 62-month study period, 213 patients
from three tertiary care centers were included in the analysis. The
cohort comprised 62 (29.1%), 63 (29.6%), and 88 (41.3%) patients
with bladder UC, upper tract UC (UTUC), and RCC, respectively.
Table II summarizes the
characteristics of the study cohort. The median age at ICI
treatment initiation was 70 years (IQR, 38–86 years), with 155
(72.6%) male patients. The median follow-up duration was 16.0
months (IQR, 1–27 months). A total of 141 (66.2%) patients received
at least one prior treatment before an initiating ICI therapy.
Regarding ICI agents, 41.3% (n=88) received PD-L1 inhibitors,
whereas 27.7% (n=59) received PD-1 inhibitors. Additionally, 7%
(n=15) of the patients received PD-1 inhibitors in combination with
tyrosine kinase inhibitors (TKIs), while 23.9% (n=51) received PD-1
inhibitors combined with CTLA-4 inhibitors.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | Total (n=213) | No hepatic irAE
(n=165) | Hepatic irAE
(n=48) | P-value |
|---|
| Median age, years
(IQR) | 70 (38–86) | 71 (43–84) | 68 (35–86) | 0.057 |
| Sex, n (%) |
|
|
|
|
|
Male | 155 (72.8) | 117 (70.9) | 38 (79.2) | 0.258 |
|
Female | 58 (27.2) | 48 (29.1) | 10 (20.8) |
|
| Type of cancer, n
(%) |
|
|
|
|
| Renal
cell carcinoma | 88 (41.3) | 61 (37.0) | 27 (56.3) | 0.046 |
| Upper
tract urothelial carcinoma | 63 (29.6) | 53 (32.1) | 10 (20.8) |
|
| Bladder
urothelial carcinoma | 62 (29.1) | 51 (30.9) | 11 (22.9) |
|
| Type of ICI, n
(%) |
|
|
|
|
|
Anti-PD-L1 monotherapy | 88 (41.3) | 76 (46.0) | 12 (25.0) | 0.014 |
|
Anti-PD-1 monotherapy | 59 (27.7) | 44 (26.7) | 15 (31.3) |
|
|
Anti-PD-1 + TKI | 15 (7.0) | 11 (6.7) | 4 (8.3) |
|
|
Anti-PD-1 + CTLA-4 | 51 (23.9) | 34 (20.6) | 17 (35.4) |
|
| Line of ICI
regimen, n (%) |
|
|
|
|
| First
line ICI | 72 (33.8) | 51 (30.9) | 21 (43.8) | 0.172 |
| Second
line | 111 (52.1) | 90 (54.5) | 21 (43.8) |
|
| ≥Third
line | 30 (14.1) | 24 (14.6) | 6 (12.4) |
|
| Duration of ICI,
months (mean ± SD) | 7.9±8.0 | 7.7±7.0 | 8.8±10.7 | 0.491 |
| ECOG performance
status at ICI initiation, n (%) |
|
|
|
|
| 0 | 203 (95.3) | 160 (97.0) | 43 (89.6) | 0.337 |
| 1 | 6 (2.8) | 1 (0.6) | 5 (10.4) |
|
| ≥2 | 4 (1.9) | 4 (2.4) | 0 (0.0) |
|
| Diabetes mellitus,
n (%) |
|
|
|
|
| No | 179 (84.0) | 144 (87.3) | 35 (72.9) | 0.017 |
|
Yes | 34 (16.0) | 21 (12.7) | 13 (27.1) |
|
| Hypertension, n
(%) |
|
|
|
|
| No | 138 (64.8) | 113 (68.5) | 25 (52.1) | 0.036 |
|
Yes | 75 (35.2) | 52 (31.5) | 23 (47.9) |
|
| Hypothyroidism, n
(%) |
|
|
|
|
| No | 204 (95.8) | 162 (98.2) | 42 (87.5) | 0.005 |
|
Yes | 9 (4.2) | 3 (1.8) | 6 (12.5) |
|
| Pre-existing
chronic liver diseasea, n (%) |
|
|
|
|
| No | 211 (99.1) | 164 (99.4) | 47 (97.9) | 0.401 |
|
Yes | 2 (0.9) | 1 (0.6) | 1 (2.1) |
|
Among the included patients, 76 (35.6%) experienced
at least one type of irAE, and 40 (22.5%) developed hepatic irAEs.
The median time from ICI initiation to the occurrence of any irAE
was 6.5 weeks (IQR, 1–23 weeks). Among those who experienced
hepatic irAEs, 33.3% (n=16) also developed other irAEs. The
temporal sequence of irAE manifestation was as follows:
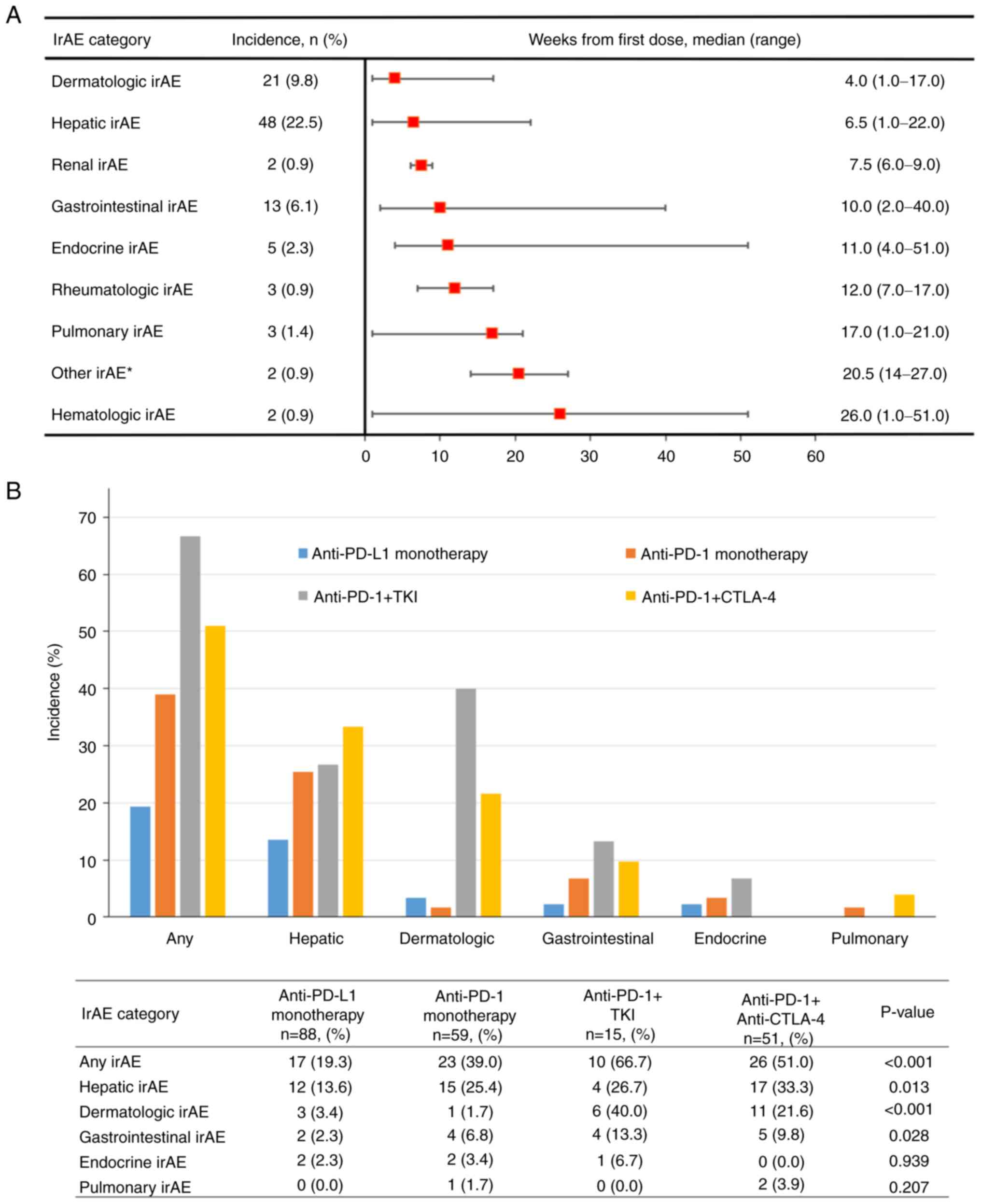
dermatologic, hepatic, renal, and gastrointestinal (Fig. 1A). Two patients succumbed to irAEs,
with the causes of death being pulmonary irAE in one patient and
hepatic failure in the other. Patients receiving combination
therapy involving PD-1 inhibitors and either TKIs or CTLA-4
inhibitors had a significantly higher incidence of any irAE
compared to those who receiving monotherapy (P<0.001), as
illustrated in Fig. 1B.
Hepatic irAE and associated
factors
Hepatic irAEs were observed in 48 (22.5%) patients,
with a median onset time of 6.5 weeks (IQR, 1–22 weeks) following
the initiation of ICI therapy. The prevalence of pre-existing
comorbidities, including diabetes mellitus, hypertension, and
hyperthyroidism, was significantly higher among patients with
hepatic irAEs compared to those without hepatic irAEs (P<0.05
for all). However, the prevalence of pre-existing chronic liver
disease did not differ significantly between the groups (P=0.401).
During ICI treatment, two patients developed liver metastases and
subsequently discontinued ICI therapy due to disease progression.
After discontinuation of ICI treatment, five additional patients
developed liver metastases. None of the seven patients who
developed liver metastases experienced hepatic irAEs. The incidence
of hepatic irAEs was significantly higher in patients receiving
combination therapies than in those on anti-PD-L1 or anti-PD-1
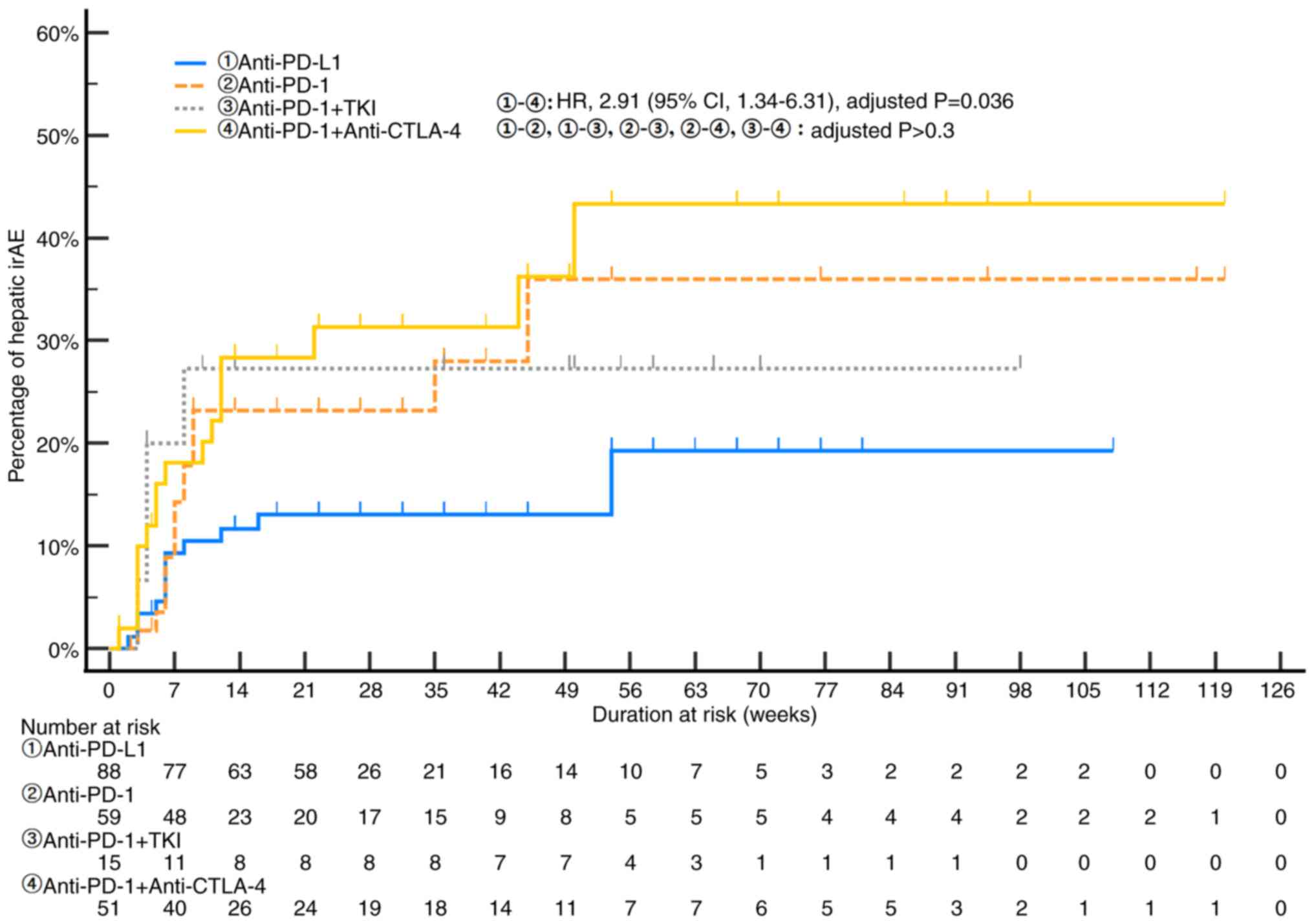
monotherapy (31.8%, n=21 vs. 18.3%, n=27; P=0.014; Table II). Fig. 2 illustrates the temporal dynamics
and comparative risks of hepatic irAE development across different
ICI regimens. Combination therapy with anti-PD-1 and anti-CTLA-4
inhibitors showed the highest cumulative incidence, reaching
approximately 43% by week 56, with an elevated risk compared to
anti-PD-L1 monotherapy (HR 2.91; 95% CI, 1.34–6.31; adjusted
P=0.036). The cumulative incidences of hepatic irAEs for other
treatment regimens, including anti-PD-1 monotherapy (25.4%),
anti-PD-1 plus TKI (26.7%), and anti-PD-L1 monotherapy (13.6%),
showed no statistically significant differences between groups
after adjustment for multiple comparisons (all adjusted P>0.3).
Table III presents the biology
and incidence rates of hepatic irAEs according to ICI regimen. The
median time from ICI initiation to the occurrence of hepatic irAE
was 6.5 weeks (IQR, 1–22 weeks) across all treatment regimens. For
specific treatments, the median times to the occurrence of hepatic
irAE were as follows: anti-PD-L1 (atezolizumab, n=12) 6 weeks (IQR,
2–16 weeks), anti-PD-1 (n=15) 7 weeks (IQR, 3–9 weeks), anti-PD-1 +
TKI (n=4) 4 weeks (IQR: 3–8 weeks), and anti-PD-1 + anti-CTLA-4
(ipilimumab + nivolumab, n=17) 6 weeks (IQR, 1–22 weeks). Among
anti-PD-1 treatments, nivolumab (n=7) showed a median of 8.0 weeks
(IQR: 5–35 weeks) and pembrolizumab (n=8) 7 weeks (IQR: 3–9 weeks).
The difference in median time to hepatic irAE onset among treatment
groups was not statistically significant (P=0.764). Among the 48
patients with hepatic irAEs, 35 (72.9%) experienced grade 1 or 2
events, whereas 13 (27.1%) developed grade 3 or 4 events. Overall,
any-grade and grade ≥3 hepatic irAEs were observed in 87.5 and
12.5% of the patients, respectively. Notably, patients receiving
combination therapy with TKIs or CTLA-4 inhibitors had a
significantly higher frequency of grade ≥3 hepatic irAEs than did
those receiving monotherapy (P=0.011). The distribution of liver
toxicity patterns was as follows: cholestatic in 35.4% (n=17),
mixed in 35.4% (n=17), and hepatocellular in 29.2% (n=14). The
hepatocellular pattern of liver toxicity was significantly more
prevalent in the group receiving combination therapy with PD-1
inhibitors and either TKIs or CTLA-4 inhibitors than in those
receiving monotherapy (P=0.006).
 | Table III.Characteristics of hepatic irAEs
according to ICI regimen in urologic cancer. |
Table III.
Characteristics of hepatic irAEs
according to ICI regimen in urologic cancer.
|
Characteristics | Total (n=48) | Anti-PD-L1
(n=12) | Anti-PD-1
(n=15) | Anti-PD-1 + TKI
(n=4) | Anti-PD-1 +
anti-CTLA-4 (n=17) | P-value |
|---|
| Duration of ICI
treatment, months (mean ± SD) | 7.9±8.0 | 7.0±5.0 | 9.0±11.0 | 7.6±3.4 | 8.4±8.9 | 0.464 |
| Median time to
hepatic irAE, weeks (IQR) | 6.5 (1.0–22.0) | 6.0 (2–16.0);
atezolizumab (n=12): 6.0 (2.0–16.0) | 7.0 (3.0–9.0);
nivolumab (n=7): 8.0 (5.0–35.0); pembrolizumab (n=8): 7.0
(3.0–9.0) | 4.0 (3.0–8.0);
nivolumab + TKI (n=1): 4.0; pembrolizumab + TKI (n=3): 4.0
(3.0–8.0) | 6.0 (1.0–22.0)
ipilimumab + nivolumab (n=17) 6.0 (1.0–22.0) | 0.764 |
| Severity, n
(%) |
|
|
|
|
|
|
| Grade
1 | 27 (56.2) | 7 (58.3) | 10 (66.7) | 3 (75.0) | 7 (41.2) | 0.649 |
| Grade
2 | 8 (16.7) | 2 (16.7) | 3 (20.0) | 0 (0.0) | 3 (17.6) |
|
| Grade
3 | 9 (18.8) | 2 (16.7) | 2 (13.3) | 0 (0.0) | 5 (29.4) |
|
| Grade
4 | 4 (8.3) | 1 (8.3) | 0 (0.0) | 1 (25.0) | 2 (11.8) |
|
| Biology (any grade
hepatic irAE), n (%) |
|
|
|
|
|
|
| AST
elevation | 43 (89.6) | 9 (75.0) | 15 (100.0) | 2 (50.0) | 17 (100.0) | 0.857 |
| ALT
elevation | 42 (87.5) | 12 (100.0) | 12 (80.0) | 3 (75.0) | 15 (88.2) | 0.264 |
|
Bilirubin elevation | 10 (20.8) | 3 (25.0) | 2 (13.3) | 1 (25.0) | 4 (23.5) | 0.911 |
| Biology (hepatic
irAE grade ≥3), n (%) |
|
|
|
|
|
|
| AST
elevation | 11 (22.9) | 2 (16.7) | 2 (13.3) | 1 (25.0) | 6 (35.3) | 0.264 |
| ALT
elevation | 8 (12.5) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 5 (29.4) | 0.011 |
|
Bilirubin elevation | 5 (10.4) | 2 (16.7) | 2 (13.3) | 0 (0.0) | 1 (5.9) | 0.231 |
| Pattern of liver
toxicity, n (%) |
|
|
|
|
|
|
|
Cholestatic | 17 (35.4) | 3 (25.0) | 9 (60.0) | 1 (25.0) | 4 (23.5) | 0.006 |
|
Mixed | 17 (35.4) | 7 (58.3) | 6 (40.0) | 1 (25.0) | 3 (17.6) |
|
|
Hepatocellular | 14 (29.2) | 2 (16.7) | 0 (0.0) | 2 (50.0) | 10 (58.8) |
|
Outcomes of hepatic irAE
treatment
Patients with grade 1 and 2 hepatic irAEs were
managed exclusively with UDCA or DDB, without requiring ICI
discontinuation or steroid use. All patients achieved resolution of
their hepatic irAEs. For the 13 patients who experienced grade 3 or
higher hepatic irAEs, ICI therapy was immediately discontinued, and
high-dose intravenous corticosteroids were administered. This
treatment approach resulted in the recovery of 12 patients, with
only one patient unfortunately succumbing to progressive fulminant
hepatitis. Among the 13 patients with grade 3 or higher hepatic
irAEs, 7 were subsequently rechallenged with ICI therapy after
recovery, with no adverse effects observed following rechallenge.
Among the 213 study participants, 5 (2.3%) permanently discontinued
ICIs due to hepatic irAEs, whereas 11 (5.2%) discontinued ICIs due
to other causes. Notably, neither the occurrence of any irAE nor
hepatic irAEs specifically was associated with improved PFS
(Fig. S1).
Discussion
The present study aimed to evaluate the incidence of
hepatic irAEs in patients with advanced or metastatic RCC and UC
receiving various combinations of ICIs. Our study findings offer a
comprehensive and detailed overview of the entire spectrum of irAEs
associated with ICI therapies among patients with urologic cancer.
The current study presents three key observations: (1) a high proportion of patients
experienced at least one irAE of any grade; (2) the prevalence of grade ≥3 toxicity was
substantial with marked variations across ICI regimens; and
(3) our study provides more
objective clinical data on the impact of ICI therapy on liver
function and toxicity given our inclusion of patients with urologic
cancers and exclusion of those with hepatic metastasis.
Among various solid tumors, we determined that RCC
and UC were the most appropriate solid tumor types for studying
hepatic irAEs, which aligns with our research objectives, based on
two key considerations. First, RCC and UC are prime examples of
solid tumors for which cutting-edge ICI treatments and combinations
are extensively implemented. These malignancies have been at the
forefront of ICI application since its introduction in solid tumor
therapy. Consequently, a substantial body of data has been
accumulated regarding the efficacy, adverse event profiles, and
management strategies of ICI treatments for RCC and UC, surpassing
many other cancer types in this regard. Second, in the absence of
direct hepatic metastases, RCC and UC typically have no
restrictions regarding antineoplastic agent administration due to
liver function concerns during treatment. This characteristic
provides an optimal model for investigating ICI-induced hepatic
irAEs in malignancies with minimal inherent hepatic
involvement.
A previous meta-analysis of clinical trials
involving urologic cancer patients reported that the frequency of
any irAE was approximately 34%, which closely aligns with the 35.6%
observed in our study (11). Only a
few of the original phase 3 trials on ICI regimens for urologic
cancers had reported incidence of hepatic irAEs, with the
proportion of grade 3 or 4 hepatic irAEs varying widely from
approximately 3 to 22% (5–7). For context, other studies on ICI have
reported that the incidence of hepatotoxicity ranged from 2 to 10%
for monotherapies and 25 to 30% for combination therapies (15). This aligns with our findings based
on real-world data, which show a significantly higher incidence
with combination therapy than with ICI monotherapy. However, the
frequency of hepatic irAEs was notably higher in the current study
than in previous ones. This discrepancy likely reflects the reality
of clinical practice settings. Several factors may contribute to
this observed difference. First, 66.2% of the patients included
herein had a history of systemic therapy with other regimes for
advanced or metastatic urologic cancers, unlike those in landmark
clinical trials. The periods in which other antineoplastic agents
were used did not overlap or coincide with ICI treatment.
Therefore, the possibility of liver function abnormalities due to
concurrent use of other antineoplastic agents appears to be low.
Additionally, in patients who received chemotherapy prior to ICI,
we performed a new baseline liver function test and then
investigated the occurrence of hepatic irAEs; hence, related
concerns are not expected to be significant. However, drug-induced
liver injury may promote a multiplicative effect, where previous
damage can feed forward, consequently impairing drug metabolism and
causing further toxicity. Second, hepatic irAEs have clear and
objective diagnostic criteria based on liver function test
abnormalities, allowing for precise grading and early detection,
even among asymptomatic patients. This contrasts with other irAEs,
which may be underreported without proactive testing or specific
patient complaints (16). In this
context, we observed a higher frequency of low-grade hepatic irAEs
compared to high-grade reactions. This finding is consistent with
clinical management protocols that emphasize preventive monitoring
and prompt intervention for low-grade irAEs, which contribute to
limiting the progression to high-grade hepatic irAEs.
To address the potential underestimation of hepatic
irAEs in clinical practice, liver function tests should be
performed at baseline and before each treatment cycle. This
systematic approach enables the detection of low-grade events and
facilitates proactive management to prevent progression to
high-grade irAEs. Close monitoring is therefore essential for
urologic patients given that minimal changes may indicate early
adverse events. It is important to note that drug-induced liver
injury, including hepatic irAE, does not often present with unique
pathological or imaging manifestations in its early stages, making
clinical suspicion and regular monitoring crucial for early
detection and prevention of severe complications. Moreover, when
evaluating the outcomes of hepatic irAE treatment, assessing for
other non-hepatic irAEs is crucial considering their potential for
concurrent or independent occurrence, as noted in our findings.
The mechanisms for the development of hepatotoxicity
have yet to be fully elucidated. However, studies have suggested
the involvement of CD8+ cytotoxic T lymphocytes, CD4+ T cells,
cytokines, and secondary activation of the innate immune system
(17). Considering the frequent use
of ICIs in conjunction with other hepatotoxic drugs, attributing
liver injury solely to ICIs can be challenging. As such, we herein
investigated the R value, a criterion for classifying
hepatotoxicity into hepatocellular, cholestatic, and mixed subtypes
(14). Consistent with previous
research (18), our study found no
significant differences in the distribution of these types. This
finding suggests the limited utility of R value-based
classification for ICI-induced hepatotoxicity, particularly because
it fails to reflect the severity of, prognosis of, or recovery from
hepatic irAEs or guide the alteration of treatment approach.
Clinically, the R value appears useful only for grade III or higher
irAEs, wherein distinguishing hepatotoxicity types may inform
treatment. For grade I or II hepatic irAEs, such classification
offers limited value as their treatment remains consistent. More
critical are the absolute values of AST and ALT, which serve as
reliable indicators of clinical presentation and prognosis.
Our study demonstrated that hepatic irAEs are
dependent on the ICI type and regimen, with the frequency of irAEs
varying significantly across treatment regimens. In fact, a recent
meta-analysis on irAEs in urologic cancers reported that the pooled
incidences for any-grade and grade ≥3 irAEs were 29.1 and 9.1% for
single anti-PD-1 agent monotherapy, 21.1 and 6.9% for single
anti-PD-L1 agent monotherapy, 78.0 and 35.8% for dual ICI therapy,
and 48.8 and 12.4% for ICI combined with TKIs, respectively
(12). Clinical trial evidence
indicates a higher incidence of hepatic irAE among patients
receiving combination therapy than among those receiving
monotherapy, likely due to the synergistic effect of combining
PD-1- or PD-L1-based ICIs with the inherent toxicity of TKIs and
CTLA-4 inhibitors. TKIs alone have been associated with all-grade
hepatotoxicity rates between 25 and 35% (17), with ipilimumab combination therapies
conferring the highest risk of high-grade hepatotoxicity (18). CTLA-4, an inhibitory receptor on
regulatory T cells, plays a crucial role in maintaining immune
tolerance, and its deficiency can induce immune dysregulation
syndromes (10). Our study found a
higher frequency of hepatic irAEs among RCC patients, who actively
received ipilimumab-nivolumab and TKI + PD-1 inhibitor combination
therapies, than among UC patients. This finding aligns with the
results of a recent meta-analysis, which reported any-grade and
grade ≥3 irAE rates of 42.7 and 11.1% for RCC and 24.9 and 7.6% for
UC, respectively (19).
Generally, high-grade hepatic irAEs require drug
discontinuation and high-dose intravenous steroid treatment
(4). However, recent studies have
shown promising results in the management of hepatic irAEs, with
some cases resolving spontaneously with ICI discontinuation alone
(20,21). The key difference between our study
and previous ones in terms of hepatic irAE management is our use of
UDCA or DDB for both low- and high-grade cases. All patients with
low-grade hepatic irAEs in our cohort recovered with UDCA and DDB
alone, while 92.3% of those with grade ≥3 hepatic irAEs resolved
with 1 mg/kg/day of methylprednisolone. Although clear evidence
regarding the use of these agents for liver toxicity is lacking,
their use may be rational given the observed patterns of liver
toxicity in our study (i.e., 35.4% cholestatic, 29.4%
hepatocellular, and 35.4% mixed). The potential benefit of UDCA in
cholestatic patients and the role of DDB in preventing inflammatory
liver damage support their use (22,23).
Nonetheless, further studies are needed to validate these results
and develop tailored management strategies based on both severity
and hepatitis patterns.
A recent meta-analysis indicated that irAEs were
associated with improved treatment responses and survival outcomes
in RCC and UC (11). However, our
study found no difference in survival according to the presence of
any irAE or hepatic irAE, possibly due to the small sample size.
Additionally, the underlying mechanisms linking irAEs and outcomes
remain unclear. The hypothesis that irAEs may reflect enhanced ICI
effectiveness in patients with solid tumors remains controversial,
with unclear mechanisms connecting these events to improved
outcomes. One possible explanation is that ICIs enhance T cell
activation and proliferation, which can also cause the rapid
expansion of cytotoxic T cells, increased inflammation, and
autoimmunity, causing T cells to attack both tumor and normal
tissues. Hence, the presence of irAEs may reflects T cell function,
indicating a robust immune reaction against both tumor and normal
tissues, thereby inducing favorable treatment outcomes (19,24).
Although the precise mechanisms underlying hepatic
irAEs remain elusive, studies have identified several factors that
increase the risk of these events, including younger age (<60
years) (25), high BMI (26), gender-specific responses to
different ICIs (25,27,28),
and chronic smoking (27,29). Notably, one study showed that the
use of acetaminophen increased hepatotoxicity occurrence by 2.1
times, while patients receiving HMG-CoA reductase inhibitors had a
4.7-fold higher risk of grade 3–4 hepatotoxicity (30). We also investigated several other
factors, such as diabetes mellitus, hypertension, hypothyroidism,
and preexisting liver disease, and observed that patients with
underlying conditions showed a slightly increased likelihood of
developing hepatic irAEs. However, it is important to note that
these increased risks are not exclusive to ICI treatments and may
also occur with other types of cancer therapies. As such, although
these factors warrant attention, they are not specific risk factors
for hepatic irAEs. Therefore, the level of caution required appears
to be similar to that typically exercised during general cancer
treatments, with an emphasis on the need for routine monitoring and
vigilance in all patients receiving immunotherapy.
Despite involving multiple centers, our study was
limited by its retrospective and observational design. First, the
relatively small sample size of 213 patients from three tertiary
medical centers may have restricted the generalizability of our
findings to broader populations. Second, unmeasured or immeasurable
confounding factors affecting liver function, including previous
antineoplastic treatment history and treatment interruptions due to
other comorbidities during ICI therapy, may have influenced our
results. In addition, the retrospective design limited our ability
to collect drinking and smoking histories, potentially influencing
our results. However, all patients were strictly prohibited from
smoking and drinking alcohol during the cancer treatment period,
which we believe minimized significant impacts on our findings.
Moreover, the baseline survey of chronic liver disease status
revealed no cases of alcohol-induced cirrhosis or other chronic
liver diseases. Notably, while two patients had pre-existing HCV
infection, neither of these individuals developed hepatic irAEs,
further supporting the limited impact of baseline liver conditions
on our study outcomes. Third, the study's median follow-up duration
of 16.0 months may not have been sufficient to capture long-term
outcomes and late-onset irAEs. However, considering that hepatic
irAEs, which were the primary focus of this study, tend to occur
within 12 months, this follow-up duration was likely adequate for
analyzing these events. Lastly, the management strategies for
hepatic irAEs were based on institutional practices and may not
reflect standardized protocols across all healthcare settings.
Despite these limitations, this study provides valuable insights
into the incidence and management of hepatic irAEs in patients
treated with ICIs.
In conclusion, this multicenter study offers
important information regarding the incidence and management of
hepatic irAEs in patients with advanced urologic cancers receiving
ICIs. Our findings highlight the importance of regular liver
function monitoring, especially in patients receiving combination
therapies. Our study revealed that the majority of hepatic irAEs
were low-grade, with higher grades occurring less frequently.
Although most hepatic irAEs were generally manageable with
appropriate interventions, underscoring the overall tolerability of
the treatments, a significant proportion of patients experienced
high-grade events that required more intensive intervention. These
results highlight the need for tailored management strategies and
close collaboration between oncologists and hepatologists to
optimize patient care and treatment outcomes in the era of
immunotherapy for urologic cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Biomedical Research Institute
Grant, Pusan National University Hospital (grant no. 2018B037).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YJP and CHL contributed to the conception and design
of the study. Data collection and analysis were performed by WIS,
JIC, JYK, KHK, BJK, HKH and YJP. JIC and HKH confirm the
authenticity of all raw data. The first draft of the manuscript was
written by YJP and CHL, and all authors commented on previous
versions of the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki. The protocol for the present study was
approved by the Institutional Review Boards of the three
participating institutions: Inje University Busan Paik Hospital
Institutional Review Board (approval no. BPIRB 2024-10-001; Busan,
South Korea), Pusan National University Hospital Institutional
Review Board (approval no. 2408-017-110; Busan, South Korea), and
Dongnam Institute of Radiological and Medical Sciences
Institutional Review Boards (approval no. D-2312-021-007; Busan,
South Korea). For this type of retrospective and/or observational
study formal consent was not required. Pursuant to the provisions
of the ethics committee and the ethical guidelines of South Korea,
written consent was not required for public disclosure of study
information in the case of a retrospective and/or observational
study using material such as the existing documentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma P, Retz M, Siefker-Radtke A, Baron
A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et
al: Nivolumab in metastatic urothelial carcinoma after platinum
therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 18:312–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced Renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
Renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choueiri TK, Powles T, Burotto M, Escudier
B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U,
Shah AY, et al: Nivolumab plus cabozantinib versus sunitinib for
advanced Renal-Cell carcinoma. N Engl J Med. 384:829–841. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer R, Alekseev B, Rha SY, Porta C, Eto
M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ,
et al: Lenvatinib plus pembrolizumab or everolimus for advanced
renal cell carcinoma. N Engl J Med. 384:1289–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello
BA, et al: NCCN Guidelines® Insights: Kidney Cancer,
Version 2.2024. J Natl Compr Canc Netw. 22:4–16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC,
Carbonara U, Pandolfo SD, Black PC, Paul AK, et al: Adverse events
of immune checkpoint inhibitors therapy for urologic cancer
patients in clinical trials: A collaborative systematic review and
Meta-analysis. Eur Urol. 81:414–425. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flaig TW, Spiess PE, Abern M, Agarwal N,
Bangs R, Buyyounouski MK, Chan K, Chang SS, Chang P, Friedlander T,
et al: NCCN Guidelines® Insights: Bladder Cancer,
Version 3.2024. J Natl Compr Canc Netw. 22:216–225. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Institute NC, . Common Terminology
Criteria for Adverse Events (CTCAE) Version 5.0. 2017.
|
|
14
|
Chalasani NP, Hayashi PH, Bonkovsky HL,
Navarro VJ, Lee WM and Fontana RJ; Practice Parameters Committee of
the American College of Gastroenterology, : ACG Clinical Guideline:
The diagnosis and management of idiosyncratic drug-induced liver
injury. Am J Gastroenterol. 109:950–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwara Y, Horita N, Harrington M,
Namkoong H, Miyashita H and Galsky MD: Incidence of hepatotoxicity
associated with addition of immune checkpoint blockade to systemic
solid tumor therapy: A meta-analysis of phase 3 randomized
controlled trials. Cancer Immunol Immunother. 71:2837–2848. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rini BI, Atkins MB, Plimack ER, Soulières
D, McDermott RS, Bedke J, Tartas S, Alekseev B, Melichar B, Shpary
KY, et al: Characterization and management of Treatment-emergent
hepatic toxicity in patients with advanced renal cell carcinoma
receiving First-line pembrolizumab plus axitinib. Results from the
KEYNOTE-426 Trial. Eur Urol Oncol. 5:225–234. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hountondji L, Ferreira De Matos C, Lebossé
F, Quantin X, Lesage C, Palassin P, Rivet V, Faure S, Pageaux GP,
Assenat É, et al: Clinical pattern of checkpoint inhibitor-induced
liver injury in a multicentre cohort. JHEP Rep. 5:1007192023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YC, Zhu TC, Nie RC, Lu LH, Xiang ZC,
Xie D, Luo RZ and Cai MY: Association between early immune-Related
adverse events and survival in patients treated with PD-1/PD-L1
Inhibitors. J Clin Med. 12:7362023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Martin E, Michot JM, Papouin B,
Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A,
Laghouati S, et al: Characterization of liver injury induced by
cancer immunotherapy using immune checkpoint inhibitors. J Hepatol.
68:1181–1190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gauci ML, Baroudjian B, Zeboulon C, Pages
C, Poté N, Roux O, Bouattour M and Lebbé C: Immune-related
hepatitis with immunotherapy: Are corticosteroids always needed? J
Hepatol. 69:548–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C and Xu YQ: Diphenyl dimethyl
bicarboxylate in the treatment of viral hepatitis, adjuvant or
curative? Gastroenterology Res. 1:2–7. 2008.PubMed/NCBI
|
|
23
|
European Association for the Study of the
Liver, . EASL Clinical Practice Guidelines: The diagnosis and
management of patients with primary biliary cholangitis. J Hepatol.
67:145–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Yao Z, Yang H, Liang N, Zhang X
and Zhang F: Are immune-related adverse events associated with the
efficacy of immune checkpoint inhibitors in patients with cancer? A
systematic review and meta-analysis. BMC Med. 18:872020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asada M, Mikami T, Niimura T, Zamami Y,
Uesawa Y, Chuma M and Ishizawa K: The risk factors associated with
immune checkpoint Inhibitor-Related pneumonitis. Oncology.
99:256–259. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eun Y, Kim IY, Sun JM and Lee J, Cha HS,
Koh EM, Kim H and Lee J: Risk factors for immune-related adverse
events associated with anti-PD-1 pembrolizumab. Sci Rep.
9:140392019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delaunay M, Cadranel J, Lusque A, Meyer N,
Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N,
Guisier F, et al: Immune-checkpoint inhibitors associated with
interstitial lung disease in cancer patients. Eur Respir J.
50:17000502017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Triggianese P, Novelli L, Galdiero MR,
Chimenti MS, Conigliaro P, Perricone R, Perricone C and Gerli R:
Immune checkpoint inhibitors-induced autoimmunity: The impact of
gender. Autoimmun Rev. 19:1025902020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Byrne MM, Lucas M, Pai L, Breeze J and
Parsons SK: Immune-related adverse events in cancer patients being
treated with immune checkpoint inhibitors. Eur J Haematol.
107:650–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho YA, Han JM, Kang SY, Kim DC, Youn YJ,
Choi KH and Gwak HS: Analysis of risk factors for hepatotoxicity
induced by immune checkpoint inhibitors. J Immunother. 44:16–21.
2021. View Article : Google Scholar : PubMed/NCBI
|