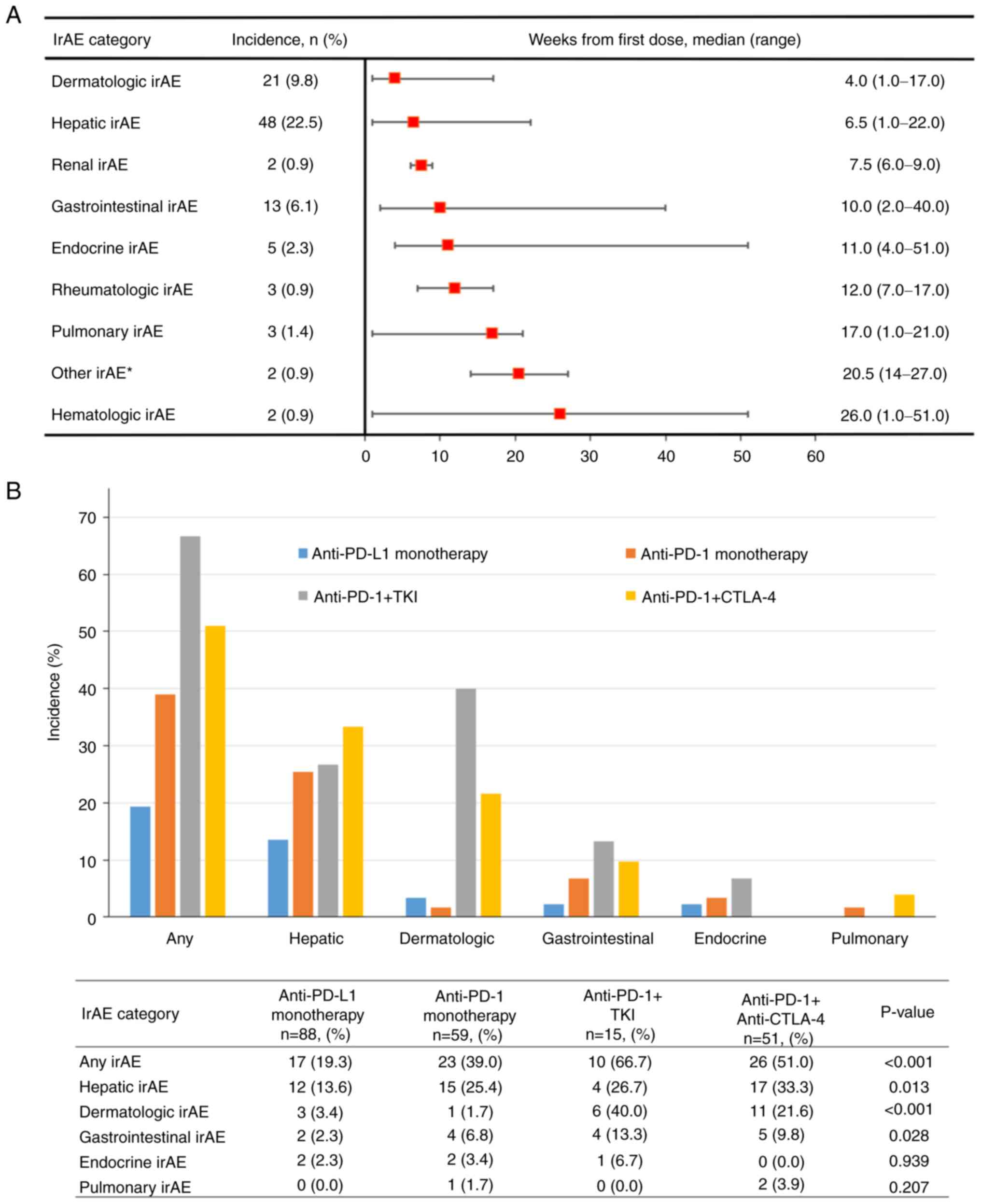
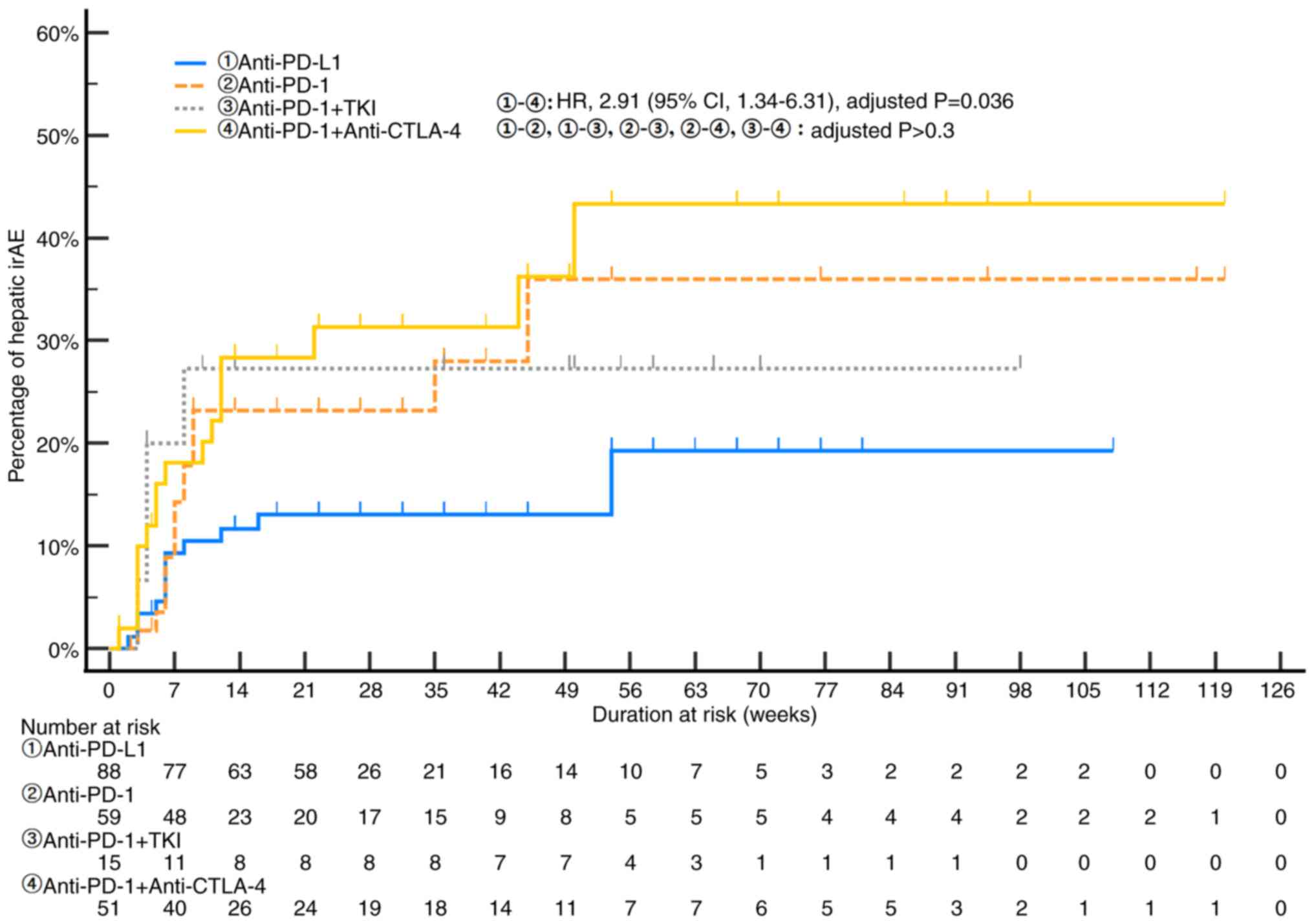
|
1
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma P, Retz M, Siefker-Radtke A, Baron
A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et
al: Nivolumab in metastatic urothelial carcinoma after platinum
therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 18:312–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced Renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
Renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choueiri TK, Powles T, Burotto M, Escudier
B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U,
Shah AY, et al: Nivolumab plus cabozantinib versus sunitinib for
advanced Renal-Cell carcinoma. N Engl J Med. 384:829–841. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer R, Alekseev B, Rha SY, Porta C, Eto
M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ,
et al: Lenvatinib plus pembrolizumab or everolimus for advanced
renal cell carcinoma. N Engl J Med. 384:1289–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello
BA, et al: NCCN Guidelines® Insights: Kidney Cancer,
Version 2.2024. J Natl Compr Canc Netw. 22:4–16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC,
Carbonara U, Pandolfo SD, Black PC, Paul AK, et al: Adverse events
of immune checkpoint inhibitors therapy for urologic cancer
patients in clinical trials: A collaborative systematic review and
Meta-analysis. Eur Urol. 81:414–425. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flaig TW, Spiess PE, Abern M, Agarwal N,
Bangs R, Buyyounouski MK, Chan K, Chang SS, Chang P, Friedlander T,
et al: NCCN Guidelines® Insights: Bladder Cancer,
Version 3.2024. J Natl Compr Canc Netw. 22:216–225. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Institute NC, . Common Terminology
Criteria for Adverse Events (CTCAE) Version 5.0. 2017.
|
|
14
|
Chalasani NP, Hayashi PH, Bonkovsky HL,
Navarro VJ, Lee WM and Fontana RJ; Practice Parameters Committee of
the American College of Gastroenterology, : ACG Clinical Guideline:
The diagnosis and management of idiosyncratic drug-induced liver
injury. Am J Gastroenterol. 109:950–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwara Y, Horita N, Harrington M,
Namkoong H, Miyashita H and Galsky MD: Incidence of hepatotoxicity
associated with addition of immune checkpoint blockade to systemic
solid tumor therapy: A meta-analysis of phase 3 randomized
controlled trials. Cancer Immunol Immunother. 71:2837–2848. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rini BI, Atkins MB, Plimack ER, Soulières
D, McDermott RS, Bedke J, Tartas S, Alekseev B, Melichar B, Shpary
KY, et al: Characterization and management of Treatment-emergent
hepatic toxicity in patients with advanced renal cell carcinoma
receiving First-line pembrolizumab plus axitinib. Results from the
KEYNOTE-426 Trial. Eur Urol Oncol. 5:225–234. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hountondji L, Ferreira De Matos C, Lebossé
F, Quantin X, Lesage C, Palassin P, Rivet V, Faure S, Pageaux GP,
Assenat É, et al: Clinical pattern of checkpoint inhibitor-induced
liver injury in a multicentre cohort. JHEP Rep. 5:1007192023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YC, Zhu TC, Nie RC, Lu LH, Xiang ZC,
Xie D, Luo RZ and Cai MY: Association between early immune-Related
adverse events and survival in patients treated with PD-1/PD-L1
Inhibitors. J Clin Med. 12:7362023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Martin E, Michot JM, Papouin B,
Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A,
Laghouati S, et al: Characterization of liver injury induced by
cancer immunotherapy using immune checkpoint inhibitors. J Hepatol.
68:1181–1190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gauci ML, Baroudjian B, Zeboulon C, Pages
C, Poté N, Roux O, Bouattour M and Lebbé C: Immune-related
hepatitis with immunotherapy: Are corticosteroids always needed? J
Hepatol. 69:548–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C and Xu YQ: Diphenyl dimethyl
bicarboxylate in the treatment of viral hepatitis, adjuvant or
curative? Gastroenterology Res. 1:2–7. 2008.PubMed/NCBI
|
|
23
|
European Association for the Study of the
Liver, . EASL Clinical Practice Guidelines: The diagnosis and
management of patients with primary biliary cholangitis. J Hepatol.
67:145–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Yao Z, Yang H, Liang N, Zhang X
and Zhang F: Are immune-related adverse events associated with the
efficacy of immune checkpoint inhibitors in patients with cancer? A
systematic review and meta-analysis. BMC Med. 18:872020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asada M, Mikami T, Niimura T, Zamami Y,
Uesawa Y, Chuma M and Ishizawa K: The risk factors associated with
immune checkpoint Inhibitor-Related pneumonitis. Oncology.
99:256–259. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eun Y, Kim IY, Sun JM and Lee J, Cha HS,
Koh EM, Kim H and Lee J: Risk factors for immune-related adverse
events associated with anti-PD-1 pembrolizumab. Sci Rep.
9:140392019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delaunay M, Cadranel J, Lusque A, Meyer N,
Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N,
Guisier F, et al: Immune-checkpoint inhibitors associated with
interstitial lung disease in cancer patients. Eur Respir J.
50:17000502017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Triggianese P, Novelli L, Galdiero MR,
Chimenti MS, Conigliaro P, Perricone R, Perricone C and Gerli R:
Immune checkpoint inhibitors-induced autoimmunity: The impact of
gender. Autoimmun Rev. 19:1025902020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Byrne MM, Lucas M, Pai L, Breeze J and
Parsons SK: Immune-related adverse events in cancer patients being
treated with immune checkpoint inhibitors. Eur J Haematol.
107:650–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho YA, Han JM, Kang SY, Kim DC, Youn YJ,
Choi KH and Gwak HS: Analysis of risk factors for hepatotoxicity
induced by immune checkpoint inhibitors. J Immunother. 44:16–21.
2021. View Article : Google Scholar : PubMed/NCBI
|