Introduction
Non-Hodgkin lymphoma (NHL) is the most prevalent
form of malignant lymphoma and 40% of patients with NHL have
diffuse large B-cell lymphoma (DLBCL) (1). DLBCL is an aggressive malignancy
characterized by rapid progression and diverse clinical
manifestations, depending on the site of origin and extent of
disease dissemination (2). Gingival
DLBCL is rare and occurs in ~1% of patients with primary extranodal
lymphoma. Its rarity poses significant diagnostic and therapeutic
challenges, as the initial clinical features often mimic benign
conditions (3). Gingival DLBCL
commonly manifests as a localized mass or ulcerative lesion in the
oral mucosa, frequently accompanied by painless and progressive
lymphadenopathy. These non-specific symptoms can lead to delayed
diagnosis and disease progression. Most studies of gingival DLBCL
have described histopathological and imaging features of this type
of blood cancer; however, to the best of our knowledge, therapeutic
strategies for gingival DLBCL with muscle invasion have not been
described (4,5). The present study describes the
clinical presentation, diagnostic approach, and therapeutic
management of a patient with gingival DLBCL involving local muscle
tissue. This case highlights the importance of a multidisciplinary
approach, integrating oncology, maxillofacial surgery, and
radiation therapy to achieve optimal outcomes. We aim to provide
insights into the complexities of managing this rare but clinically
significant presentation.
Case report
The physical examination of a 49-year-old man who
initially presented at Yingshan County People's Hospital (Nanchong,
China) in August 2017 revealed a firm mass in the lower left
gingiva that measured ~3 cm, with tenderness and swelling. The
Eastern Cooperative Oncology Group Performance Status score of the
patient was 2 (3); and their blood
test results were negative for the hepatitis B and C viruses, the
human immunodeficiency virus (HIV) and the Epstein-Barr virus
(EBV), although their lactate dehydrogenase (LDH) level was
elevated (577 U/l; normal range: 120–250 U/l). The patient received
a positron emission tomography computed tomography (PET-CT) scan,
but these images became unavailable after an upgrade of the
hospital's imaging system in 2019. The contrast-enhanced CT scans
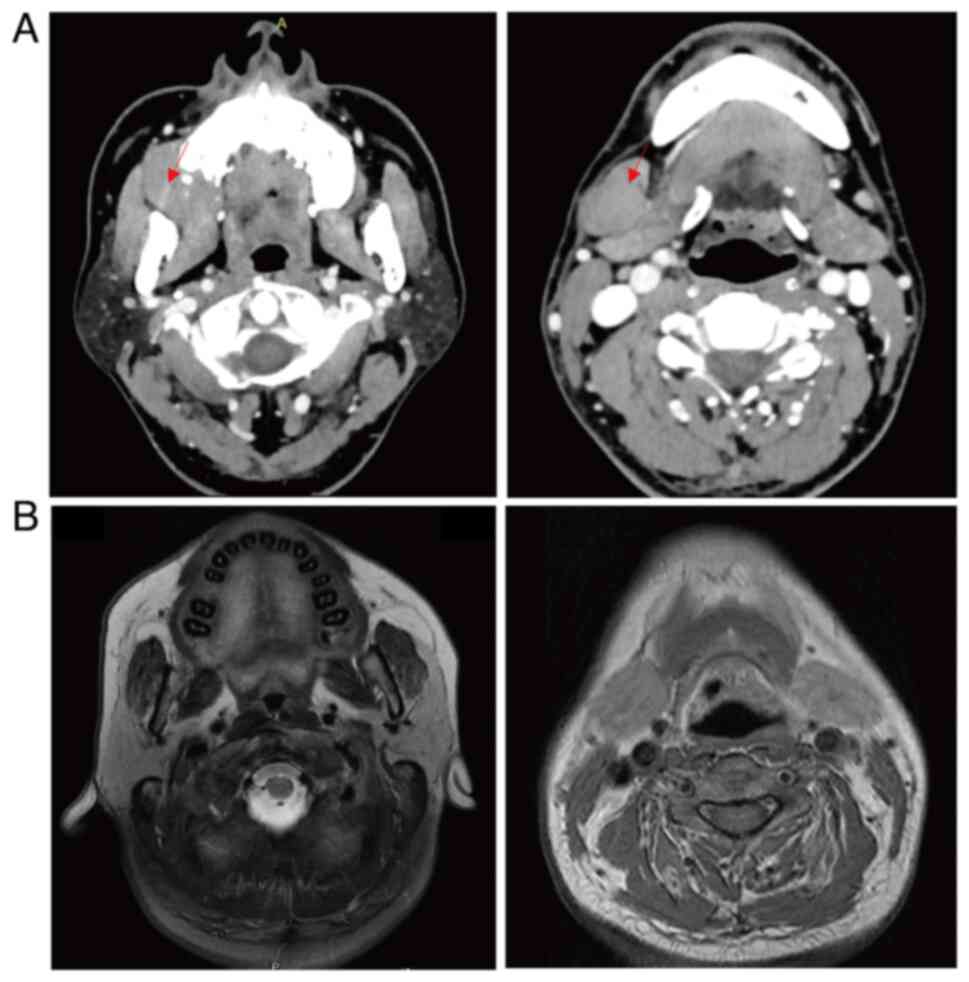
revealed the same findings as the PET-CT scans. Contrast-enhanced
CT scans demonstrated a mass in the gingiva, and enlarged lymph
nodes in the cervical, mediastinal and gastro-hepatic ligaments
(Fig. 1). Bone marrow aspiration
was performed and the specimens were subsequently analyzed using
immunohistochemistry and a bone marrow smear, all of which
demonstrated normal findings. The patient underwent gingival
excision biopsy in August 2017, the diagnosis was established
through immunohistochemical staining of the gingival biopsy
specimen. The following markers were evaluated: CD20 (+), BCL-6
(+), BCL-2 (+), C-MYC (+), MUM1(+), NF-κB (+) and Ki-67 (+, 50–60%;
data not shown). However, the patient declined next-generation
sequencing (NGS) and fluorescence in situ hybridization
(FISH) testing due to financial constraints, preventing
determination of double-hit lymphoma (DHL) or triple-hit lymphoma
(THL). These findings led to a diagnosis of DLBCL stage III (Ann
Arbor staging system) (2) and an
International Prognostic Index score of 4 (high risk) (3).
A total of six cycles of rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP,
rituximab 600 mg + cyclophosphamide 800 mg + doxorubicin 110 mg +
vincristine 2 mg + prednisone 500 mg) was administered as
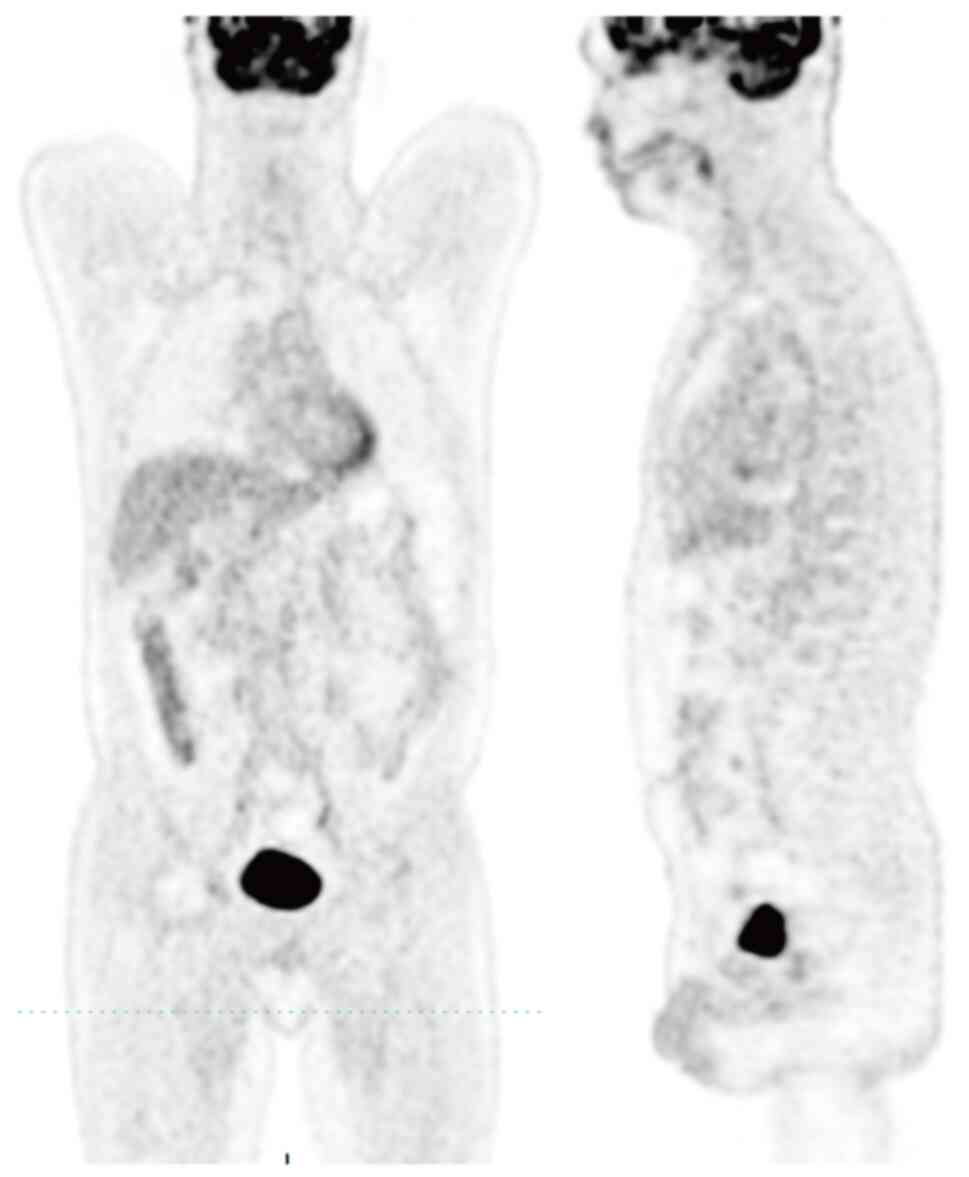
first-line chemotherapy. PET-CT showed no evidence of disease for
40 months (Fig. 2). A follow-up
contrast-enhanced CT scan in December 2020 showed tumor progression
with invasion of the left orbital region (data not shown).
According to the National Comprehensive Cancer Network guidelines
(2023, version 6) for relapsed/refractory (R/R) DLBCL, a regimen
that is not cross-resistant to R-CHOP should be used, and
autologous stem cell transplantation (ASCT) or CAR-T therapy should
also be considered (6). However,
due to the financial burden of ASCT or CAR-T, the patient did not
undergo these treatments; therefore, the patient received four
cycles of etoposide (400 mg), prednisone 500 mg, vincristine 2 mg,
cyclophosphamide 1200 mg and doxorubicin 110 mg with rituximab 600
mg as second-line chemotherapy. The patient again achieved complete
response until December 2021, when another contrast-enhanced CT
scan detected retroperitoneal lymph node metastases (data not
shown).
The patient then enrolled in a clinical trial at
Sichuan Cancer Hospital (Chengdu, China): ‘A multicenter,
single-arm, open phase I/II clinical trial to evaluate the safety
and tolerability of SYHX1903 (a cyclin-dependent kinase-9
inhibitor) in patients with relapsed/refractory malignant lymphoma’
(trial no. CTR20212017). The patient achieved partial response
after 1 month, but developed shortness of breath, tiredness and
palpitations at 15 months after enrollment. Echocardiography at
that time showed a massive pericardial effusion, and there was a
large increase in the serum level of troponin (7.8 ng/ml; normal
range, 0–0.1 ng/ml; data not shown). The patient was diagnosed with
heart failure (New York Heart Association Class III) (6), and the cardiologist considered the
high doses of doxorubicin and the study drug (SYHX1903) as
potential causes. Thus, the patient withdrew from the clinical
trial and regular follow-up was conducted.
In January 2024, the patient injured their left
forearm in a car accident and presented with limb swelling.
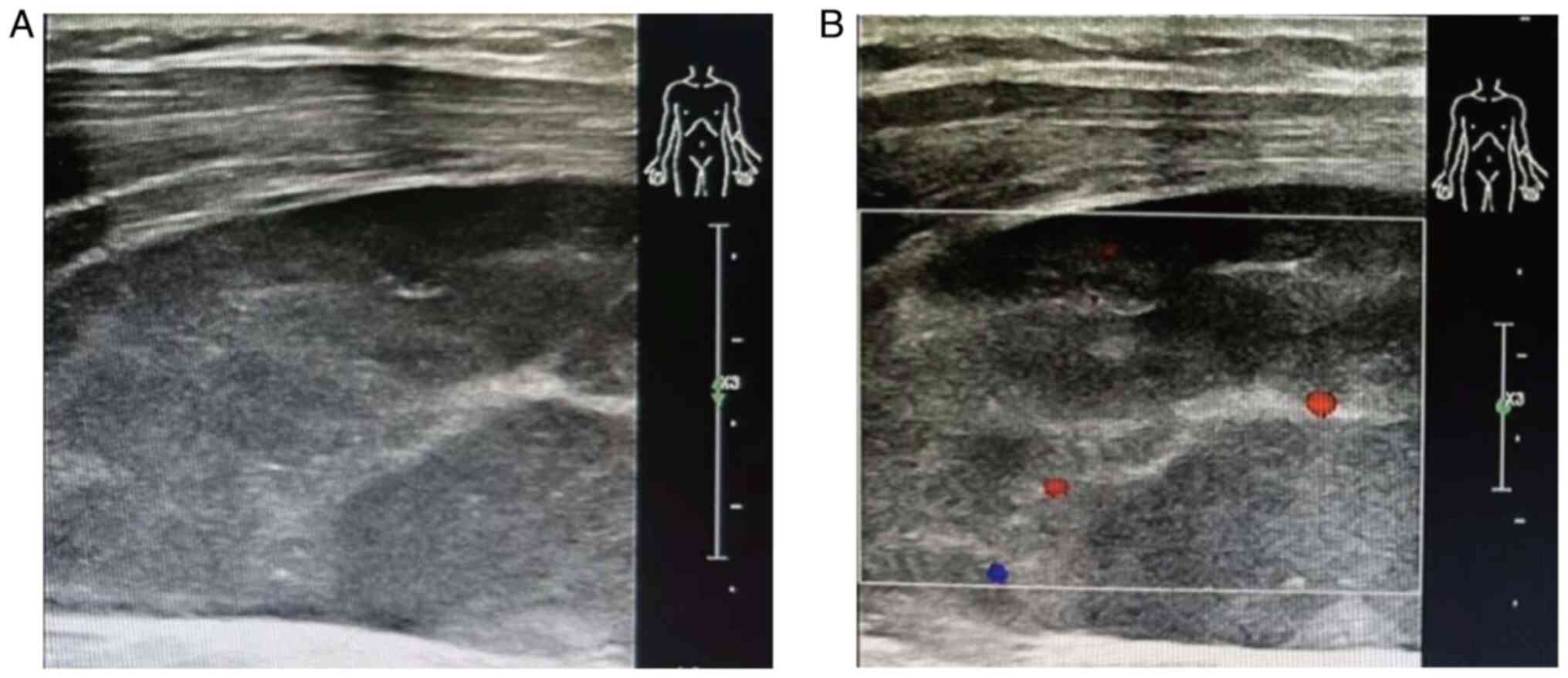
Examination by color Doppler imaging (CDI) revealed a hypoechoic
mass with a clear boundary and regular shape that was ~8.0×3.0 cm.
Color Doppler flow imaging showed a spot blood flow signal around
the mass and the suggested diagnosis was ‘hematoma considered’
(Fig. 3). The results from
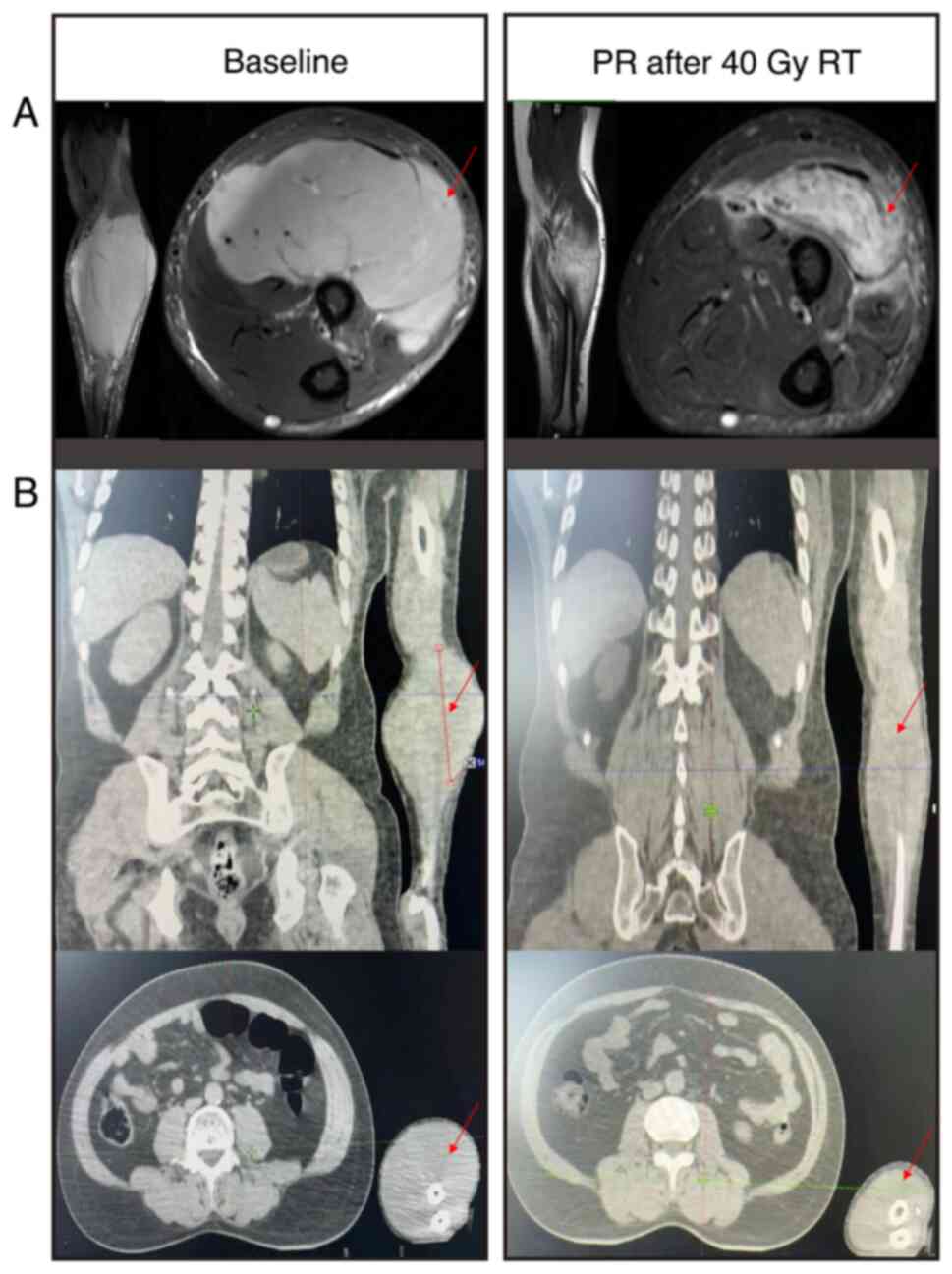
subsequent magnetic resonance imaging (MRI) demonstrated a signal
with intermediate intensity on the T1-weighted images and a signal
with slightly higher intensity than the normal surrounding muscle
on T2-weighted images. These results demonstrated a spindle-shaped
mass in the brachioradialis and supinator that was ~4.5×9.6 cm.
Contrast-enhanced CT of the chest and abdomen indicated no evidence
of abnormality (data not shown). These results led to prompt
administration of a CT-guided biopsy of the forearm muscle. The
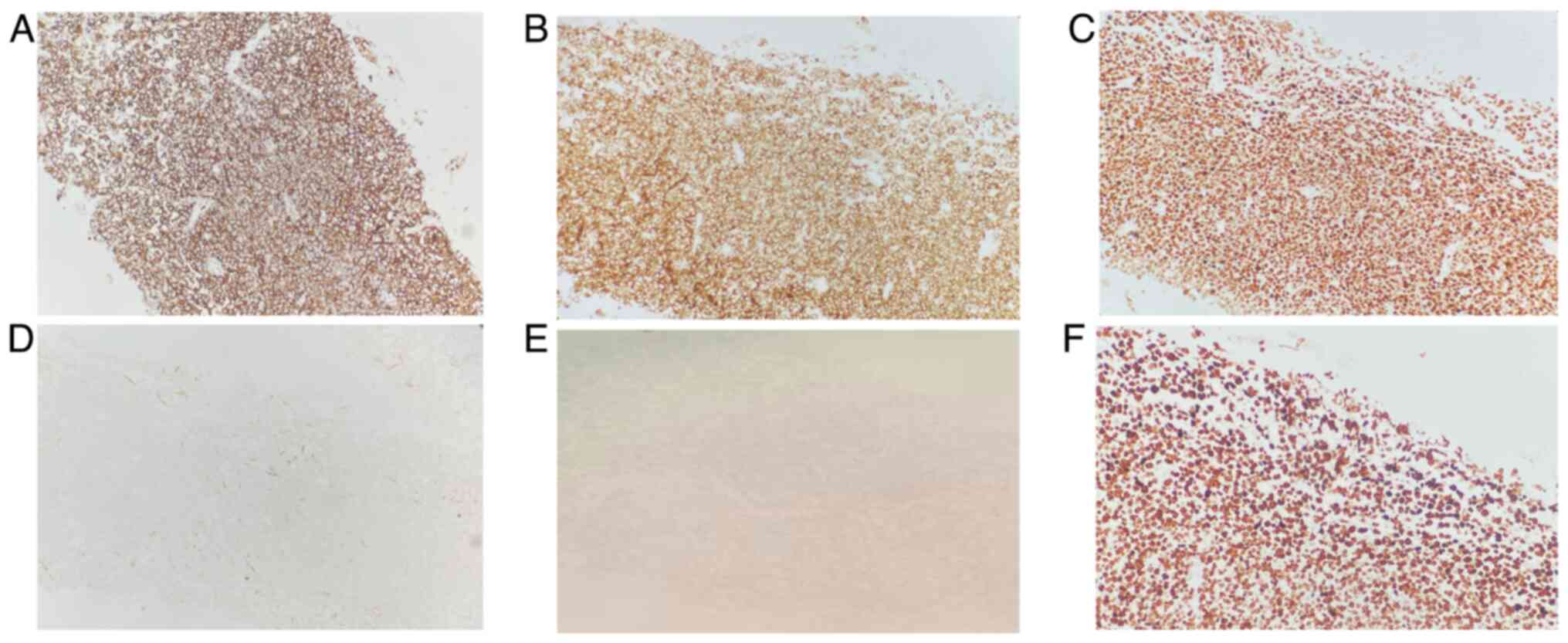
results of immunohistochemical staining were CD20 (+), CD79a (+),
BCL-2 (−), C-MYC (−), CD10 (−), BCL-6 (−), MUM1 (+) and Ki-67 (+,
90%) (Fig. 4). These results
confirmed a diagnosis of muscle invasion by gingival DLBCL. After a
multidisciplinary consultation, local radiotherapy (RT) was
administered, due to the patient suffering from heart failure
during the third line of the previous chemotherapy. The RT dose was
40 Gy in 20 fractions and led to partial response after 4 weeks
(Fig. 5). The irradiated field was
then decreased and an additional dose of 10 Gy was administered in
5 fractions. The last RT dose was in April 2024, and at the most
recent follow-up (August 2024) the patient had a good
quality-of-life and a survival time beyond 84 months.
Discussion
In most patients with extranodal NHL the digestive
system is affected, and this type of cancer is rare in the gingiva
(7). Notably, there is a paucity of
data regarding primary gingival DLBCL in patients who are
HIV-negative (3), although most
patients who present with gingival DLBCL have tumors in the maxilla
(8,9). A review of the literature identified
22 other cases of primary gingival DLBCL in HIV-negative patients
between 2008 and 2024 (Table I).
The median age of the patients was 63.2 years (range, 31–87 years),
and most of these patients had normal LDH levels, stage I–II
cancer, received R-CHOP or RT, and had a favorable prognosis.
Notably, it is possible that immune dysregulation may be involved
in primary gingival DLBCL. For example, Shapiro (10) reported on the case of an 81-year-old
patient with gingival DLBCL who was EBV-positive and was taking
methotrexate for 50 years as treatment for psoriasis; this patient
experienced tumor remission after discontinuing methotrexate. By
contrast, in the present case report, the patient was EBV-negative,
they did not use any immunosuppressive agent, and the initial
R-CHOP regimen had a long-term and stable curative effect.
 | Table I.Clinical characteristics of patients
with primary DLBCL in the gingiva. |
Table I.
Clinical characteristics of patients
with primary DLBCL in the gingiva.
| First author,
year | Case no. | Sex/Age, years | Elevated serum
LDH | Tumor size (maximum
diameter), cm | AA stage | Treatment | Survival time,
months | (Refs.) |
|---|
| Sato et al,
2009 | 1 | F/57 | - | 2.5 | IE | RT | 79 | (26) |
| Sato et al,
2009 | 2 | F/65 | + | 3.5 | IE | R-CHOP + RT | 13 | (26) |
| Sato et al,
2009 | 3 | F/68 | - | 3.5 | IE | CHOP + RT | 22 | (26) |
| Sato et al,
2009 | 4 | M/60 | - | 2 | IE | R-CHOP + RT | 16 | (26) |
| Sato et al,
2009 | 5 | F/68 | + | >5 | IE | NA | NA | (26) |
| Sato et al,
2009 | 6 | M/62 | - | 2.0 | IE | R-CHOP | 19 | (26) |
| Sato et al,
2009 | 7 | M/76 | - | 4 | IE | R-THP-COP + RT | 33 | (26) |
| Sato et al,
2009 | 8 | F/72 | - | 3 | IE | NA | NA | (26) |
| Sato et al,
2009 | 9 | F/62 | + | 1.5 | IE | CHOP + RT | 177 | (26) |
| Sato et al,
2009 | 10 | F/77 | - | 3.5 | IE | PR + CHOP | 31 | (26) |
| Sato et al,
2009 | 11 | M/57 | - | 3 | IIE | R-CHOP | 52 | (26) |
| Sugimoto et
al, 2014 | 12 | M/73 | NA | 1.5 | IE | R-THP-COP | 36 | (3) |
| Ürün et al,
2012 | 13 | M/53 | - | NA | IE | R-CHOP | 18 | (27) |
| Angiero et
al, 2006 | 14 | M/56 | - | 2 | NA | CHOP | 59 | (28) |
| Sepúlveda et
al, 2016 | 15 | F/40 | NA | 3 | NA | CHOP + RT | 60 | (29) |
| Li et al,
2024 | 16 | M/79 | NA | NA | IIE | None | 12 | (30) |
| Li et al,
2024 | 17 | F/83 | NA | NA | NA | None | 1 | (30) |
| Kaibuchi et
al, 2015 | 18 | M/87 | NA | 3.5 | NA | None | 30 | (31) |
| Aoki et al,
2022 | 19 | M/84 | - | 0.4 | IE | None | 24 | (32) |
| Flatow-Trujillo
et al, 2019 | 20 | F/61 | - | NA | NA | None | 22 | (33) |
| Wong et al,
2014 | 21 | M/50 | - | 2.9 | IE | R-CHOP | 15 | (34) |
| Wong et al,
2014 | 22 | F/31 | - | 1.6 | IE | R-CHOP | 4 | (34) |
| Deng et al,
2024 | 23 | M/49 | + | 3.0 | III | R-CHOP + RT | 84 | The present
study |
DHL and THL are highly aggressive variants of B-cell
lymphoma that are characterized by simultaneous rearrangements of
MYC and one or more additional oncogenes, such as
BCL2 or BCL6 (11).
Relative to other lymphoma subtypes, these genetic abnormalities
contribute to a more unfavorable prognosis due to increased tumor
cell proliferation, greater resistance to treatment and a higher
likelihood of relapse (12). These
more aggressive types of lymphoma often necessitate more intensive
therapeutic approaches and are associated with significantly lower
survival rates (13). Identifying
these genetic mutations is critical for achieving precise
prognostication and tailoring individualized treatment regimens,
because DHL and THL tend to be more treatment-resistant and
associated with less favorable responses to standard therapies
(14). However, in the present
case, the patient was unable to bear the financial burden and thus
refused further NGS and FISH testing. However, based on the
aggressive disease progression, this patient may have had DHL or
THL.
A rare feature of the present patient was invasion
of the gingival DLBCL to muscle in the forearm. Notably, two
possible mechanisms could be suggested for this: i) Disease
dissemination via a hematogenous or lymphatic pathway, or ii)
disease extension from adjacent organs, such as the bones or lymph
nodes (15). The most frequently
encountered clinical symptoms in patients with gingival DLBCL are
painful swelling and local edema. Muscle lymphoma has certain
distinctive characteristics on MRI that allow it to be
distinguished from other soft-tissue tumors (16). In particular, the signal intensity
of a T1-weighted image has a similar or slightly increased signal
intensity compared with normal muscle, and the T2-weighted signal
has a high intensity relative to normal fat tissue (17,18).
The MRI of the present patient clearly demonstrated these features.
The identification of tumor recurrence in the forearm was
fortuitous and only occurred because the patient required imaging
following a car accident. The CDI results of lymphoma in skeletal
muscle are nonspecific and often make it difficult to distinguish
lymphoma from hematoma, sarcoma, metastases or myositis (19). This emphasizes the need for a biopsy
and pathological evaluation for the diagnosis of skeletal lymphoma.
The immunohistochemical staining results of the forearm muscle:
CD10 (−), BCL-6 (−) and MUM1 (+), led to the diagnosis of
non-germinal center B-cell (non-GCB) type DLBCL, and suggested a
poor prognosis (20).
Previous studies have reported that R-CHOP can
significantly decrease disease relapse and progression in patients
with non-GCB type DLBCL (21,22).
For example, Nyman et al (23) reported that the R-CHOP regimen
eliminated differences in prognosis for patients with
immunohistochemically defined GCB and non-GCB phenotypes of DLCBL.
However, in the present case, the patient suffered from severe
cardiac problems following a previous chemotherapy regimen and was
therefore administered palliative RT to relieve symptoms and delay
local disease progression. The guidelines of the International
Lymphoma Radiation Oncology Group suggest the use of RT for R/R
aggressive DLBCL, with salvage doses up to 55 Gy (24). Wong et al (25) described 217 patients with R/R DLBCL
who altogether received 370 courses of palliative RT. The median
equivalent dose was 19 Gy (range, 2–42 Gy) and the rate of local
control at 6 months was 66.7%. In the present case, 40 Gy in 20
fractions was initially administered, and the patient achieved a
partial response. To minimize radiation damage, the irradiated
field was then reduced and an additional dose of 10 Gy in 5
fractions was administered.
In conclusion, invasion of gingival DLBCL to the
muscle is rare, and the diagnosis requires consideration of medical
history and a combination of procedures, including imaging and
histological examination. Individualized treatment of these
patients is necessary, although determination of the most
appropriate treatment regimen can be challenging.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WX, XD and YL conceived and designed the study. WP
and JZ collected the data. QY and BC analyzed and interpretated the
results. WX and XD wrote the manuscript. WG and YL confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethical approval and consent to
participate
The patient provided written informed consent for
the publication of this case report.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michi Y, Harada H, Oikawa Y, Okuyama K,
Kugimoto T, Kuroshima T, Hirai H, Mochizuki Y, Shimamoto H, Tomioka
H, et al: Clinical manifestations of diffuse large B-cell lymphoma
that exhibits initial symptoms in the maxilla and mandible: A
single-center retrospective study. BMC Oral Health. 22:202022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugimoto KJ, Shimada A, Sakurai H,
Wakabayashi M, Imai H, Sekiguchi Y, Nakamura N, Sawada T, Izumi H,
Ota Y, Komatsu N and Noguchi M: Primary gingival diffuse large
B-cell lymphoma: A case report and a review of the literature. Int
J Clin Exp Pathol. 7:418–424. 2013.PubMed/NCBI
|
|
4
|
Bhattacharyya I, Chehal HK, Cohen DM and
Al-Quran SZ: Primary diffuse large B-cell lymphoma of the oral
cavity: Germinal center classification. Head Neck Pathol.
4:181–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silva TD, Ferreira CB, Leite GB, de
Menezes Pontes JR and Antunes HS: Oral manifestations of lymphoma:
A systematic review. Ecancermedicalscience. 10:6652016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zelenetz AD, Gordon LI, Abramson JS,
Advani RH, Andreadis B, Bartlett NL, Budde LE, Caimi PF, Chang JE,
Christian B, et al: NCCN Guidelines® Insights: B-Cell
Lymphomas, Version 6.2023. J Natl Compr Canc Netw. 21:1118–1131.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gommier A and Radoi L: Primary Extra-nodal
diffuse large B-cell lymphoma of the gingiva mimicking a dental
abscess: A diagnostic challenge. Cureus. 16:e733492024.PubMed/NCBI
|
|
8
|
Siqueira JM, Fernandes PM, de Oliveira
ACF, Vassallo J, Alves FA and Jaguar GC: Primary diffuse large
B-cell lymphoma of the mandible. Autops Case Rep. 9:e20191092019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pekiner FN, Borahan MO, Keser G and
Bozkurt SU: Diffuse large B-cell lymphoma: A case report. Clin Exp
Health Sci. 8:150–153. 2018.
|
|
10
|
Shapiro N: Diffuse Large B-cell lymphoma
of the gingiva in a patient on long-term use of methotrexate being
treated for psoriasis. Compend Contin Educ Dent. 36:426–428.
2015.PubMed/NCBI
|
|
11
|
Rosenthal A and Younes A: High grade
B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6:
Double hit and triple hit lymphomas and double expressing lymphoma.
Blood Rev. 31:37–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petrich AM, Nabhan C and Smith SM:
MYC-associated and double-hit lymphomas: A review of pathobiology,
prognosis, and therapeutic approaches. Cancer. 120:3884–3895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunleavy K: Aggressive B cell lymphoma:
optimal therapy for MYC-positive, double-hit, and triple-hit DLBCL.
Curr Treat Options Oncol. 16:582015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li LR, Wang L, He YZ and Young KH: Current
perspectives on the treatment of double hit lymphoma. Expert Rev
Hematol. 12:507–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hatem J and Bogusz AM: An unusual case of
extranodal diffuse large B-cell lymphoma infiltrating skeletal
muscle: A case report and review of the literature. Case Rep
Pathol. 2016:91048392016.PubMed/NCBI
|
|
16
|
Burton E, Schafernak K, Morgan E and Samet
J: Skeletal muscle involvement in B-cell lymphoma: Two cases
illustrating the contribution of imaging to a clinically
unsuspected diagnosis. Case Rep Radiol. 2017:20689572017.PubMed/NCBI
|
|
17
|
Gao S, Shu H and Yang H: Imaging features
of skeletal muscle lymphoma: A case report and literature review.
BMC Med Imaging. 21:1362021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Lin Q, Zhang L, Dong L and Li Y:
Primary skeletal muscle diffuse large B cell lymphoma: A case
report and review of the literature. Oncol Lett. 10:2156–2160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong W, Zhou H, Piao Z, Wu J, Wang X and
Zhou X: Ultrasound and Contrast-enhanced ultrasound patterns of
primary psoas major muscle diffuse large B-cell lymphoma: A Case
Report. J Clin Med Images Case Rep. 22022.
|
|
20
|
Leonard JP, Kolibaba KS, Reeves JA,
Tulpule A, Flinn IW, Kolevska T, Robles R, Flowers CR, Collins R,
DiBella NJ, et al: Randomized phase II study of R-CHOP with or
without bortezomib in previously untreated patients with
non-germinal center B-cell-like diffuse large B-cell lymphoma. J
Clin Oncol. 35:3538–3546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He XH, Li B, Yang S, Lu N, Zhang X, Zou
SM, Li YX, Song YW, Zheng S, Dong M, et al: R-CHOP regimen can
significantly decrease the risk of disease relapse and progression
in patients with non-germinal center B-cell subtype diffuse large
B-cell lymphoma. Chin J Cancer. 31:306–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho MC, Chung Y, Jang S, Park CJ, Chi HS,
Huh J, Suh C and Shim H: Prognostic impact of germinal center
B-cell-like and non-germinal center B-cell-like subtypes of bone
marrow involvement in patients with diffuse large B-cell lymphoma
treated with R-CHOP. Medicine (Baltimore). 97:e130462018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nyman H, Adde M, Karjalainen-Lindsberg ML,
Taskinen M, Berglund M, Amini RM, Blomqvist C, Enblad G and Leppä
S: Prognostic impact of immunohistochemically defined germinal
center phenotype in diffuse large B-cell lymphoma patients treated
with immunochemotherapy. Blood. 109:4930–4935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng AK, Yahalom J, Goda JS, Constine LS,
Pinnix CC, Kelsey CR, Hoppe B, Oguchi M, Suh CO, Wirth A, et al:
Role of radiation therapy in patients with Relapsed/Refractory
diffuse large B-Cell lymphoma: Guidelines from the international
lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys.
100:652–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong J, Pickles T, Connors J,
Aquino-Parsons C, Sehn L, Freeman C, DeVries K and Lo A: Efficacy
of palliative radiation therapy (RT) for chemotherapy relapsed or
refractory diffuse large B-Cell lymphoma: A Population-based
retrospective review. Pract Radiat Oncol. 11:e203–e209. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato Y, Onishi N, Morito T, Takata K,
Mizobuchi K, Nagatsuka H, Ichimura K, Tanaka T, Tamura M and
Yoshino T: Patients with localized primary non-tonsillar oral
diffuse large B-cell lymphoma exhibit favorable prognosis despite a
non-germinal center B-cell-like phenotype. Cancer Sci. 100:42–46.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ürün Y, Can F, Bariş E, Akbulut H, Utkan G
and İçli F: Primary extranodal non-Hodgkin's lymphoma presenting as
painful gingival swelling. Exp Oncol. 34:134–135. 2012.PubMed/NCBI
|
|
28
|
Angiero F, Stefani M and Crippa R: Primary
non-Hodgkin's lymphoma of the mandibular gingiva with maxillary
gingival recurrence. Oral Oncol Extra. 42:123–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sepúlveda I, Schorwer M, Platin E, Yañez M
and Fredes F: Primary non-Hodgkin's lymphoma of the gingiva: A case
report and review of the literature. Memo-Magazine Eur Med Oncol.
9:183–186. 2016. View Article : Google Scholar
|
|
30
|
Li G, Lou J, Tan N and Zheng H: Gingival
diffuse large B-cell lymphoma: Aeport of 2 cases. Hua Xi Kou Qiang
Yi Xue Za Zhi. 42:256–261. 2024.(In English, Chinese). PubMed/NCBI
|
|
31
|
Kaibuchi N, Okamoto T, Kataoka T, Kumasaka
A and Ando T: A case of spontaneous regression of lymphoma in the
mandibular gingiva after biopsy. Oral Maxillofacial Surg Cases.
1:33–37. 2015. View Article : Google Scholar
|
|
32
|
Aoki Y, Hasegawa S, Miyabe S and Nagao T:
Spontaneous regression of malignant lymphoma of the maxillary
gingiva following biopsy. Int J Oral Maxillofac Surg. 51:1145–1148.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Flatow-Trujillo L, Win K, Jencks A,
Andritsos L and Arana Yi C: Spontaneous resolution of untreated
diffuse large B-cell lymphoma of maxillary bone after incisional
biopsy. Clin Case Rep. 7:2082–2086. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong GB, Spadafora S, Barbon N and Caputo
M: Primary extranodal B-cell non-Hodgkin lymphoma mimicking an
endodontic lesion: Report of 2 cases. J Can Dent Assoc.
79:d932013.PubMed/NCBI
|