Introduction
The worldwide incidence of skin neoplasms has
notably increased over the past few decades, particularly among
white individuals (1,2). It is estimated that globally the
incidence of melanoma will increase by ~50% and melanoma-associated
mortalities will increase by 68% by the year 2040 (1). The number of new non-melanoma skin
cancer (NMSC) cases is projected to increase ~20-fold for men and
15-fold for women over the next 25 years (2). Despite the high global incidence of
non-melanocytic neoplasms, their comorbidity with melanomas is
uncommon. A study by Neale et al (3) reported a 7% prevalence of NMSC in
melanoma settings. By contrast, several patients with melanoma
never experience NMSC, even basal cell carcinoma (BCC), which
shares an analogous pattern of ultraviolet exposure
(non-occupational or recreational). Currently, skin cancer
comorbidity also results from rapidly increasing host
susceptibility factors, such as sun-sensitive skin phenotype caused
by migration, older age (extended lifespan), a growing number of
chronically immunosuppressed patients (of iatrogenic or
haematological origin) and infection with human papillomavirus or
human immunodeficiency virus (4).
Melanoma with NMSC comorbidity affects not only individuals at
general population-level risk, but also those with the highest risk
of developing multiple skin neoplasms caused by germline mutations
[such as cyclin-dependent kinase inhibitor 2A, melanocyte inducing
transcription factor (MITF-E318K variant), BRAC1-associated
protein, p53], red hair colour phenotype (melanocortin-1 receptor
polymorphism), chronic immunosuppression, chronic lymphocytic
leucaemia, allogeneic hematopoietic stem cell transplant proceeded
by total-body irradiation or solid organ transplantation (5–24).
Common features among these high-risk patients are the difficulty
of skin examination and multistep treatment approach when the
dermoscopic surveillance was not implemented (23,24).
Therefore, it is essential to characterize patients
with melanoma and NMSC comorbidity to facilitate clinical
practitioners performing the dermoscopic total skin examination.
Publications including patients with both types of these tumours
have described the epidemiological aspects of this comorbidity
(3,4). The dermoscopic patterns of melanoma in
this setting of patients were not evaluated in the studies.
Therefore, the aim of the present study was to analyse the
association between the dermoscopic features of melanomas and the
presence of clinically expressed risk factors in patients with NMSC
comorbidity to identify simple and fast practical implications for
screening and follow-up.
Materials and methods
The present study enrolled consecutive adult
patients (≥18 years of age) who were referred for a dermoscopic
skin examination to a dermatology clinic of the Military Institute
of Medicine-National Research Institute (Warsaw, Poland) between
January 2015 and October 2023. The Bioethics Commission at the
Military Institute of Medicine (Warsaw, Poland) approved the study
protocol (#21/WIM/2021, May 2021; no. 65/24, Dec 2024). Patients
signed consent permitting the publication of anonymised
photographs.
The retrospective analysis of the patient's medical
records consisted of epidemiological data, including previous
diagnoses of melanoma (personal and/or among close relatives), NMSC
(personal) and melanoma pathological report, including the
topology, histological subtype and invasiveness according to the
tumour-node-metastasis (TNM) staging system upon 8th American Joint
Committee on Cancer classification (25). Patients diagnosed with lesions of
uncertain malignant potential (melanocytic tumour of uncertain
malignant potential, superficial atypical melanocytic
proliferations of unknown significance or atypical spitzoid tumour)
were also identified. Patient age was regarded as that on the
pathological report; where there were multiple melanomas, the age
at first diagnosis was considered.
The evaluated melanoma risk factors included those
manifested clinically. The atypical nevus syndrome (ANS) or
numerous acquired nevi (NAN; defined as >50 lesions on the body
surface), skin phototype (I or II), solar lentiginosis (SL) located
on the trunk and upper arms and previous/concomitant/subsequent
basal or squamous cell carcinoma (SCC; described as NMSC) were
evaluated as the most common risk factors. Patients with
genodermatoses and associated types of skin cancer, such as
Gorlin-Goltz syndrome (GGS), were considered eligible.
The dermoscopic features of melanoma, particularly
the characteristic pattern and presence of regression structures,
were evaluated based on the videodermoscopic documentation that was
captured in polarised light and at 20-fold magnification. The
images were captured using Fotofinder HD 800 or Medicam 1000
(FotoFinder Systems GmbH) or Mole Max (Derma Medical Systems
Handels u. Entwicklungs GmbH).
The exclusion criteria were as follows: i) Lack of
videodermoscopic images of primary cutaneous melanoma or clinically
manifested melanoma risk factors such as number of nevi, SL, types
of skin cancer and skin phototype; ii) lack or incomplete
pathological report of primary melanoma and NMSC; and iii)
recurrent melanomas, metastatic melanomas after skin tumour
excision, melanomas of unknown primary location or melanomas unable
to be examined with videodermoscopy due to technical reasons.
The control group consisted of adult patients (≥18
years of age) who were referred for a dermoscopic skin examination
to a dermatology clinic of the Military Institute of
Medicine-National Research Institute (Warsaw, Poland) between
January 2015 and October 2023, and were diagnosed with NMSC, whose
detailed medical documentation was available, including anamnesis
proving the absence of previous/concomitant/subsequent melanoma, a
histopathological report confirming the diagnosis of BCC and/or
SCC, videodermoscopic images of NMSC and trunk and/or arms skin
area that allowed for the assessment of SL and melanocytic nevi.
NMSC lesions were regarded as multiple when ≥2 BCC and/or SCC
lesions were diagnosed.
Statistical analysis
Statistical analysis was performed using the R
software (version 4.3.1; RStudio, version 2023.09.1+494; R
software, version 4.4.1; RStudio, version 2024.04.2+764; Posit
Software, PBC) and R packages (26).
The frequencies of count data were determined using
cross tables. Fisher's exact test was employed to assess
differences in frequencies as the expected counts in certain groups
were <5. Meanwhile, differences in the means of continuous
numerical data were evaluated using the Kruskal-Wallis rank test
due to the non-normal distribution of the numerical data in both
the entire dataset and the subgroups. The normality of distribution
was evaluated with Shapiro-Wilk test. The statistical analysis
involved computing the differences in the means between multiple
groups using Dunn's test and Bonferroni's P-value correction
[adjusted P-value (P-adj.)]. For detailed count data statistics for
tables >2×2, row-wise Fisher's test and Bonferroni's P-adj. were
used. In addition, crude odds ratios (OR) were computed with 95%
confidence intervals (CIs) for pairs of binomial variables in
groups using conditional maximum likelihood estimation.
Furthermore, ORs adjusted for age and sex with 95% CIs were
calculated using multivariable logistic regression. P<0.05 was
considered to indicate a statistically significant difference.
Results
Population characteristics
Data from consecutive dermoscopic examinations of
patients admitted between January 2015 and October 2023 to the
dermatological clinic were analysed, identifying 295 melanomas in
264 patients (63.3% women and 36.7% men), aged 18–90 years. The
mean age of patients with melanoma was 52.2 and the median age was
49 years. A total of 63 patients with an NMSC comorbidity exhibited
69 melanomas. The mean age of these patients was 67.3 and the
median age was 70.0 years (P<0.0001). BCC was present in all
cases; SCC was also present in 10/63 (15.9%) patients. In 53/63
(84.1%) patients, NMSC was diagnosed before or concomitantly with
melanoma and in 10/63 (15.9%) patients it was diagnosed as a second
primary tumour. Thin melanomas [lentigo maligna (LM), LM melanoma
(LMM), pTis and pT1] comprised 257/295 (87.1%) lesions and 57/69
(82.6%) melanomas coexisting with NMSC. A total of 37/264 patients
(14.0%) were diagnosed with ≥2 melanomas, and 4/264 (1.5%) had 3
melanomas. In 24/264 (9.0%) patients, melanoma occurred in an
immediate family member. Among the 63 patients with an NMSC
comorbidity, 6 (9.5%) were diagnosed with ≥2 melanomas and 8
(12.7%) reported a familial melanoma. Non-cutaneous types of cancer
were found sporadically; mainly prostate cancer (6 cases in total,
3 coexisting with NMSC), breast cancer (3 cases in total, no
coexisting with NMSC) and chronic lymphocytic leukaemia (2 cases in
total, both coexisting with NMSC). No genetic syndromes and organ
transplant recipients were detected among the patients. Out of the
264 patients, 249 (94.3%) had II skin phototype, 9 (3.4%) had
phototype I and 6 (2.3%) had phototype III. The recapitulation of
the analysed data is presented in Table
I.
 | Table I.Epidemiological, clinical,
histopathologic, topographic and dermoscopic data of patients
diagnosed with melanoma stratified by NMSC comorbidity. |
Table I.
Epidemiological, clinical,
histopathologic, topographic and dermoscopic data of patients
diagnosed with melanoma stratified by NMSC comorbidity.
| Factor | Melanoma, n
(%) | Melanoma without
NMSC, n (%) | Melanoma with NMSC
comorbidity, n (%) | P-value | P-value, within the
group | Bonferroni adjusted
P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 167 (63.3) | 127 (63.2) | 40 (63.5) | NS |
|
|
|
Male | 97 (36.7) | 74 (36.8) | 23 (36.5) |
|
|
|
| Age |
|
|
|
|
|
|
|
Range | 18-90 | 18-88 | 19-90 |
|
|
|
|
Mean | 52.2 | 47.5 | 67.3 |
|
|
|
|
Median | 49.0 | 45.0 | 70.0 | <0.0001 |
|
|
|
Standard deviation | 16.5 | 13.9 | 14.9 |
|
|
|
| History of
personal/familial melanoma |
|
|
|
|
|
|
|
Yes | 38 (14.4) | 27 (13.4) | 11 (17.5) | NS |
|
|
| No | 226 (85.6) | 174 (86.6) | 52 (82.5) |
|
|
|
| NAN/ANS |
|
|
|
|
|
|
|
Yes | 157 (59.5) | 127 (63.2) | 30 (47.6) | <0.05 |
|
|
| No | 107 (40.5) | 74 (36.8) | 33 (52.4) |
|
|
|
| Solar
lentiginosis |
|
|
|
|
|
|
|
Yes | 133 (50.4) | 79 (39.3) | 54 (85.7) | <0.0001 |
|
|
| No | 131 (49.6) | 122 (60.7) | 9 (14.3) |
|
|
|
| Melanoma
location |
|
|
| <0.01 | <0.01 |
|
| Head
and neck | 40 (13.5) | 22 (9.7) | 18 (26.1) |
| 0.001 | <0.01 |
|
Trunk | 106 (35.9) | 84 (37.2) | 22 (31.8) |
| NS | NS |
| Upper
limb | 48 (16.3) | 34 (15.0) | 14 (20.3) |
| NS | NS |
| Lower
limb | 94 (31.9) | 79 (35.0) | 15 (21.7) |
| <0.05 | NS |
| Nail
apparatus | 1 (0.3) | 1 (0.4) | 0 (0.0) |
| NS | NS |
| Mucous
membrane | 6 (2.0) | 6 (2.6) | 0 (0.0) |
| NS | NS |
| Histopathological
type |
|
|
| NS | NS |
|
| LM | 35 (11.9) | 22 (9.7) | 13 (18.9) |
|
|
|
|
LMM | 17 (5.8) | 10 (4.4) | 7 (10.1) |
|
|
|
|
Superficial spreading | 227 (76.9) | 179 (79.2) | 48 (69.6) |
|
|
|
|
Spitzoid | 4 (1.3) | 4 (1.8) | 0 (0.0) |
|
|
|
|
Nevoid | 2 (0.7) | 2 (0.9) | 0 (0.0) |
|
|
|
|
Desmoplastic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
|
| Acral
lentiginous | 2 (0.7) | 2 (0.9) | 0 (0.0) |
|
|
|
|
MELTUMP | 8 (2.7) | 7 (3.1) | 1 (1.4) |
|
|
|
| Histopathological
report |
|
|
|
|
| NS |
| LM
(facial/extra-facial) | 35 (11.8) | 22 (9.7) | 13 (18.8) | <0.05 | <0.05 |
|
| LMM
(facial) | 17 (5.7) | 10 (4.4) | 7 (10.1) |
|
|
|
|
pTis | 76 (25.7) | 64 (28.3) | 12 (17.4) |
|
|
|
|
pT1 | 129 (43.7) | 104 (46.0) | 25 (36.2) |
|
|
|
|
pT2 | 17 (5.7) | 10 (4.4) | 7 (10.1) |
|
|
|
|
pT3 | 7 (2.4) | 5 (2.2) | 2 (2.9) |
|
|
|
|
pT4 | 6 (2.0) | 4 (1.7) | 2 (2.9) |
|
|
|
|
MELTUMP/SAMPUS | 8 (2.7) | 7 (3.1) | 1 (1.4) |
|
|
|
| Dermoscopic pattern
of melanoma |
|
|
| <0.0005 | <0.005 |
|
|
Multicomponent asymmetric | 113 (38.3) | 90 (39.8) | 23 (33.3) |
| NS | NS |
|
Spitzoid | 51 (17.3) | 48 (21.2) | 3 (4.3) |
| <0.001 | <0.01 |
|
Melanoma on sun damaged
skin | 40 (13.6) | 27 (11.9) | 13 (18.8) |
| NS | NS |
|
Hypomelanotic/amelanotic | 16 (5.4) | 13 (5.7) | 3 (4.3) |
| NS | NS |
| Dermoscopic pattern
of melanoma |
|
|
| <0.0005 | <0.005 |
|
|
Homogenous | 7 (2.4) | 6 (2.6) | 1 (1.4) |
| NS | NS |
|
Reticular | 10 (3.4) | 8 (3.5) | 2 (2.9) |
| NS | NS |
|
Nodular | 17 (5.8) | 9 (4.0) | 8 (11.6) |
| <0.05 | NS |
|
Melanoma on face | 34 (11.5) | 19 (8.4) | 15 (21.7) |
| <0.005 | <0.05 |
|
Melanoma in special location
(nail apparatus/acral/mucous membranes) | 7 (2.4) | 6 (2.6) | 1 (1.4) |
| NS | NS |
| Dermoscopic
structures of regression |
|
|
|
|
|
|
|
Yes | 93 (31.5) | 59 (26.1) | 34 (49.3) | <0.001 |
|
|
| No | 202 (68.5) | 167 (73.9) | 35 (50.7) |
|
|
|
Analysis of differences between groups
of patients with melanoma depending on NMSC comorbidity
The median age of patients with melanoma with NMSC
was ~25 years higher compared with that of patients with melanoma
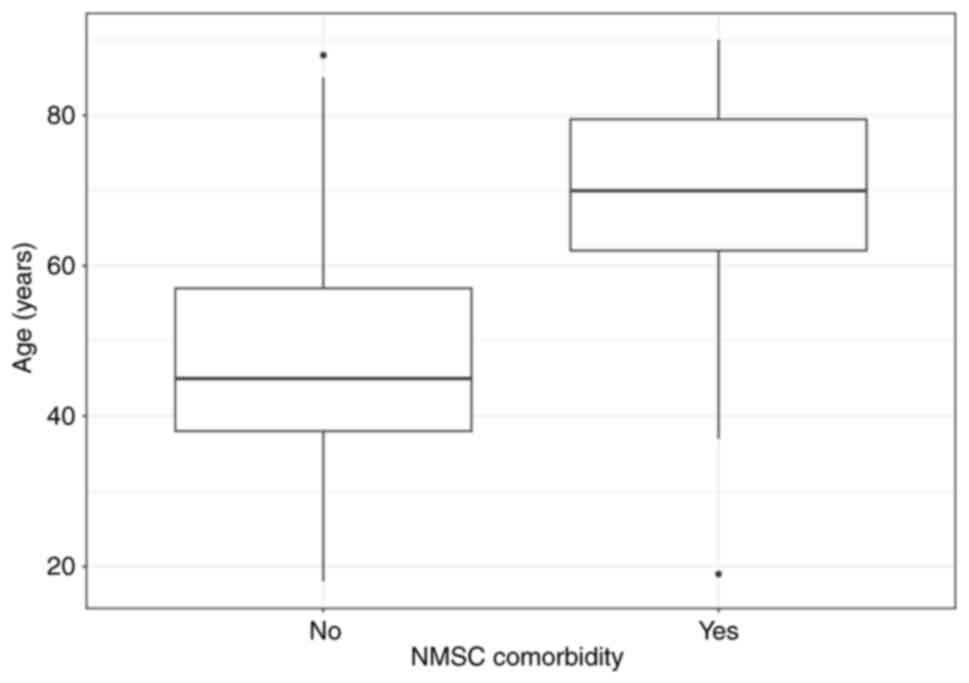
without NMSC comorbidity (P<0.0001) (Fig. 1). No difference in sex was observed.
Among the analysed melanoma risk factors, ANS/NAN were observed
significantly more often in patients with melanoma without NMSC
comorbidity (127 patients vs. 30 patients; 63.2% vs. 47.6%,
respectively; P<0.05). By contrast, SL was a characteristic risk
factor in patients with NMSC comorbidity (54 patients vs. 79
patients; 85.7% vs. 39.3%, respectively; P<0.0001).
The comparison of melanoma locations revealed
statistically significant differences (P<0.005) in the group
NMSC comorbidity compared with that of the melanoma without NMSC
group; melanomas were detected significantly more frequently within
the head and neck region (P<0.05; P-adj.<0.01) and less
frequently on lower limbs (P<0.05; P-adj.>0.05). Despite the
similar predominance of thin melanomas in both groups, the
histological report in the NMSC group showed an increased incidence
of LM (in facial and extra-facial locations) and LMM (P<0.05)
compared with that of melanoma patients without NMSC.
The aforementioned findings were also complemented
by data regarding the dermoscopic pattern of melanoma (P<0.0005;
Table I). The asymmetric
multicomponent pattern was the most frequent dermoscopic pattern of
melanoma among those identified during dermoscopic examination in
patients with melanoma without NMSC and with NMSC comorbidity (39.8
and 33.3%, respectively). This finding was associated with the most
commonly identified type of melanoma in pathological reports, the
superficial spreading melanoma in patients with melanoma without
NMSC and with NMSC comorbidity (79.2 and 69.6%, respectively). The
second in frequency was the dermoscopic melanoma on face, when NMSC
was present (21.7%; P<0.005; P-adj.<0.05) or the spitzoid
pattern when NMSC was absent (21.2%; P<0.001; P-adj.<0.01).
The melanoma on chronically sun-damaged skin was more frequently
presented with NMSC coexistence (18.8% vs. 11.9%), though the
difference was statistically insignificant. The dermoscopic
regression structures within melanomas were characteristic of NMSC
(49.3% vs. 26.1%; P<0.001). Common dermoscopic patterns of
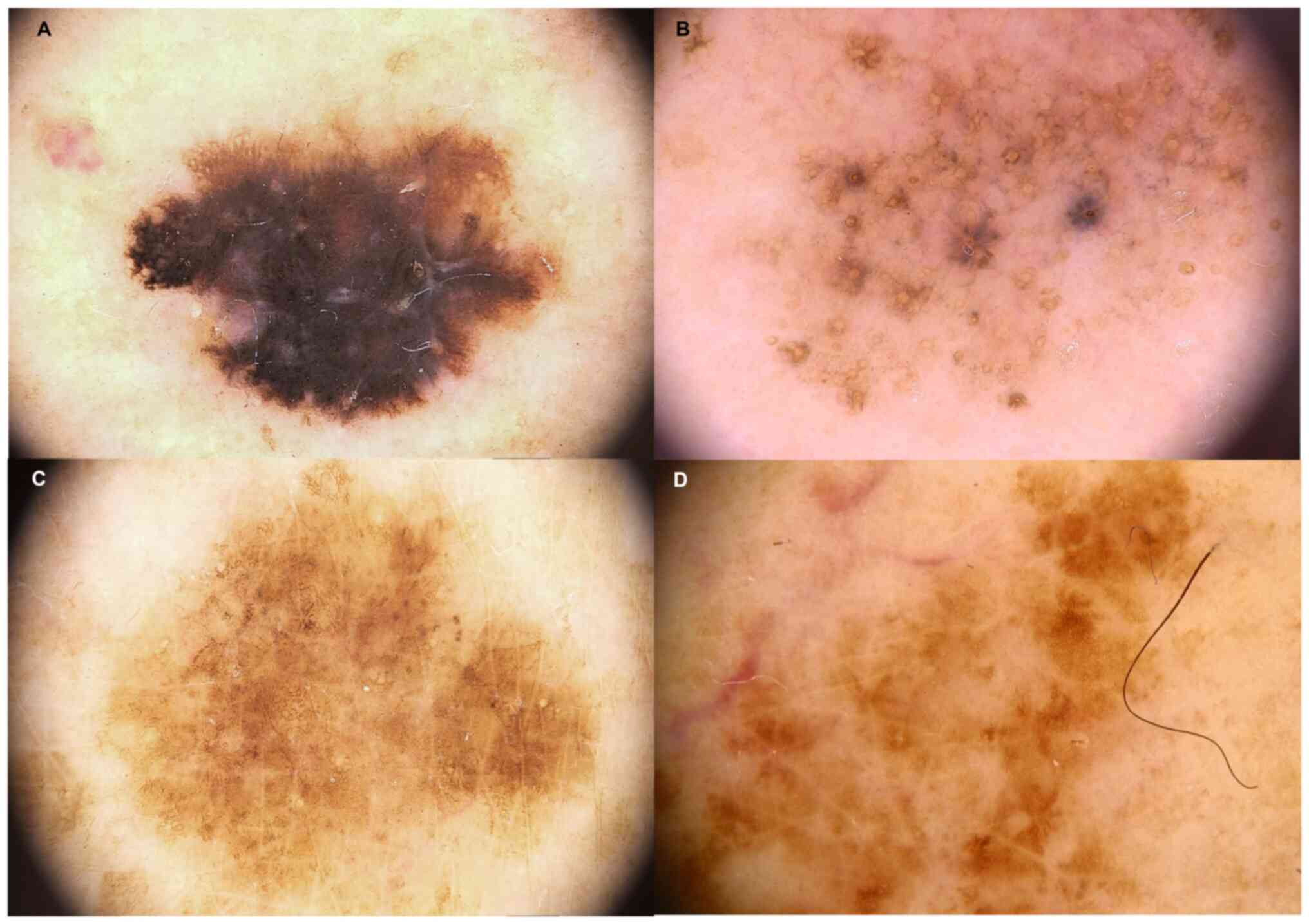
melanoma in this setting of patients are shown in Fig. 2.
In the case of patients with field cancerisation,
the enhanced regression of melanoma, particularly within the scalp
area, might be responsible for the delayed diagnosis due to
difficulties in recognising the melanoma extension despite its
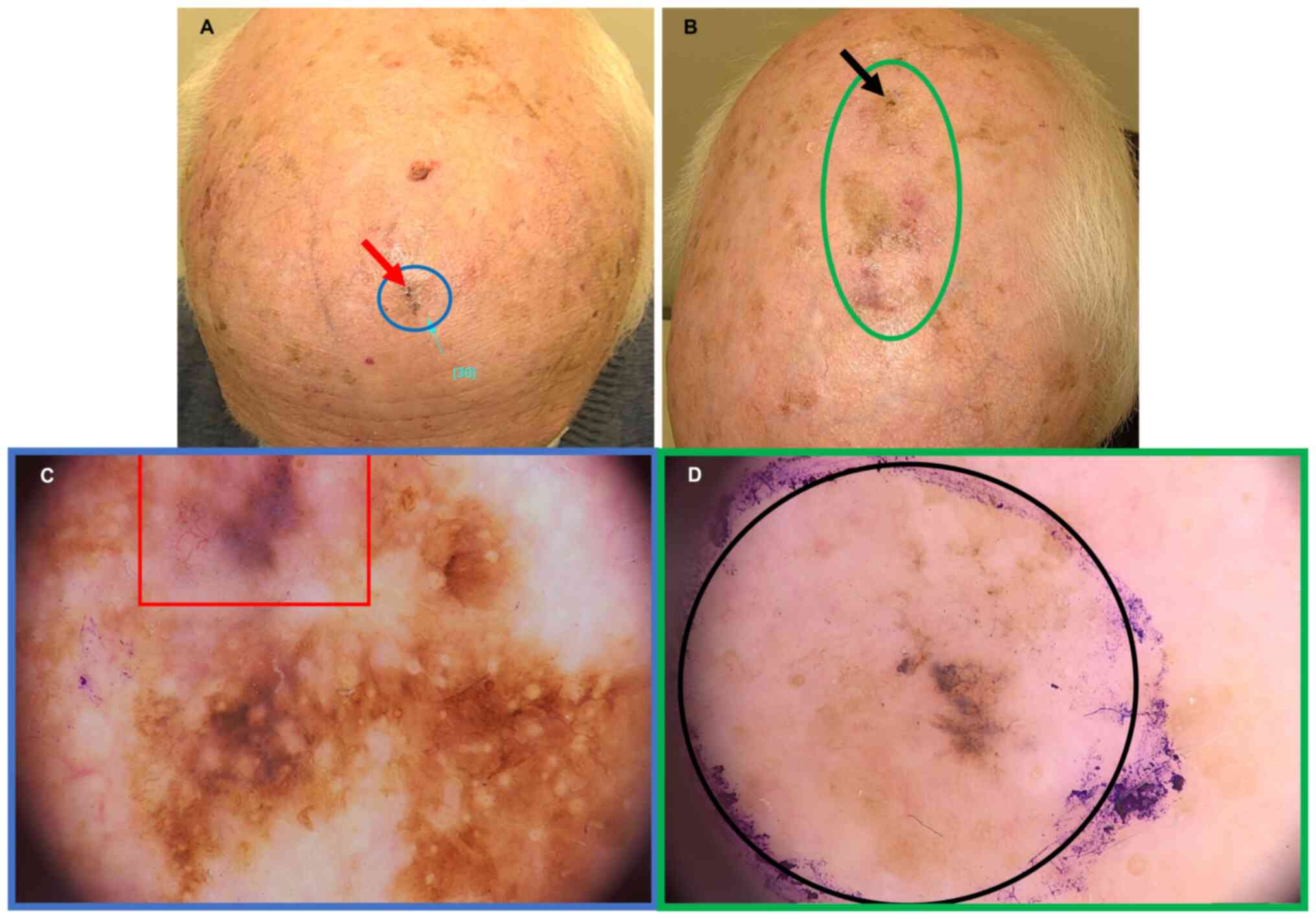
already advanced stage. For example, the case of an elderly patient
(85 years old) included in the present study demonstrated the
complexity of skin examination in this setting (Fig. 3) as the diagnosis and margins of
advanced melanoma (pT3b) on the scalp (Fig. 3B-D) were obtained after detailed,
multistep and profound non-invasive examinations with
videodermoscopy and reflectance confocal microscopy (RCM). In
addition, this workflow enabled revealing the collision tumour -
the overlap with pigmented BCC and lentigo maligna melanoma (pT1)
within the forehead (Fig. 3A-C) in
this patient. Among younger (<65 years) patients with NMSC
comorbidity, the diagnosis of melanoma remains challenging,
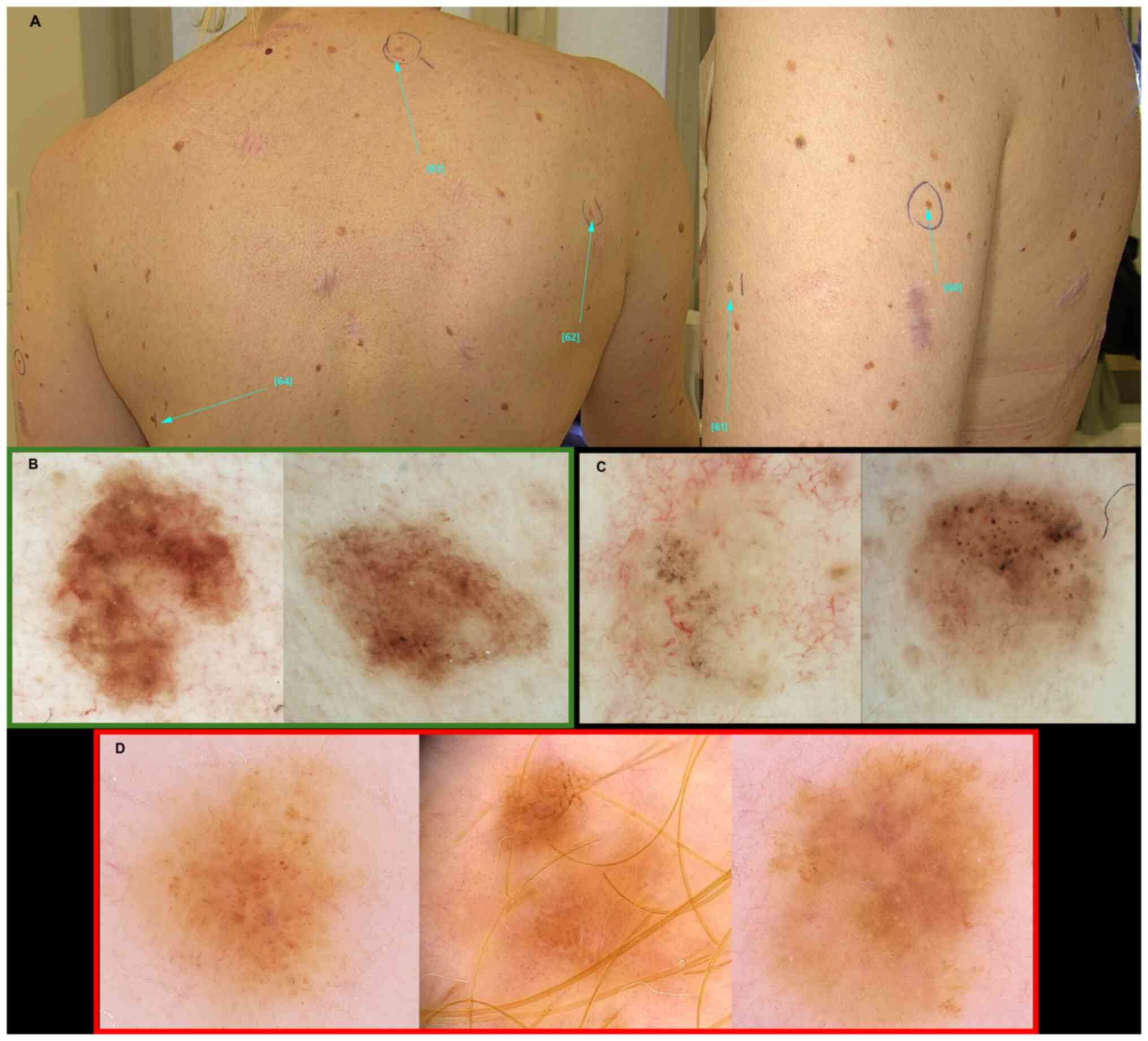
particularly when numerous melanocytic nevi are present. Fig. 4A presents images of a 43-year-old
patient who exhibited 3 melanomas (Fig.
4D) within 1 year after >12 excisions of BCCs. Though GGS
was excluded, some of the BCCs were pigmented (Fig. 4C) with dermoscopic features similar
to those observed in GGS. Numerous melanocytic nevi were not
clinically atypical, but many simulated patterns of melanoma on
sun-damaged skin under dermoscopy (Fig.
4B). Therefore, RCM was also performed for both aforementioned
patients (presented in Figs. 3 and
4), leading to the identification
of melanoma and reducing unnecessary excisions of benign lesions.
This had an additional positive impact on the second patient, who
was heavily surgically pretreated, and implementation of the
two-step diagnostics, videodermoscopic and RCM, enabled us to
achieve more precise qualification of lesions for excision or
videodermoscopic monitoring.
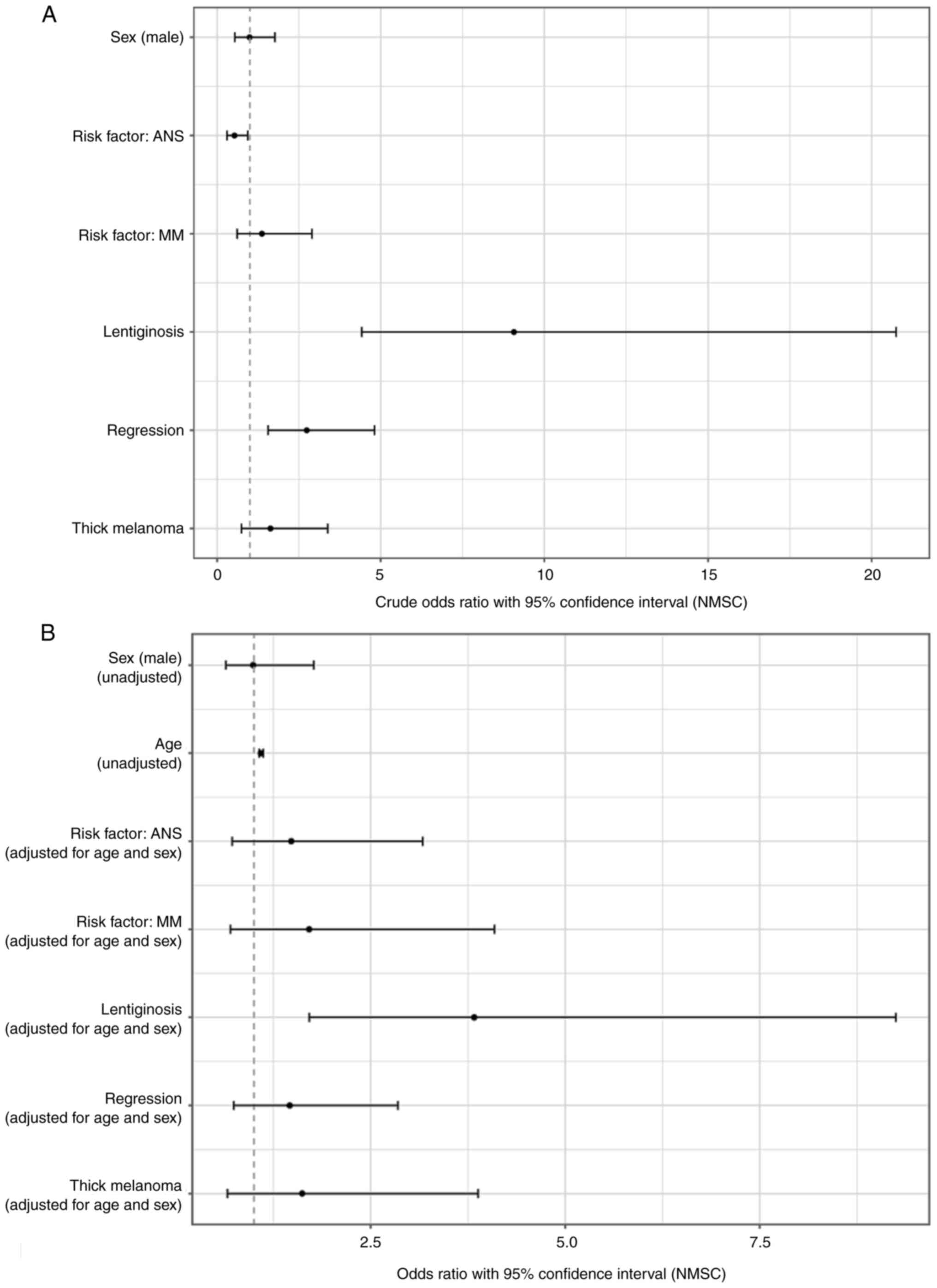
OR of groups depending on NMSC
comorbidity
Table II and
Fig. 5 summarise the ORs (crude and
adjusted for age and sex), 95% CIs and P-values for the clinical,
dermoscopic and epidemiological characteristics of the patients
with melanoma depending on NMSC comorbidity.
 | Table II.Summary of OR, 95% CI and P-value
results for clinical, dermoscopic and epidemiologic characteristics
of patients with melanoma with NMSC comorbidity. |
Table II.
Summary of OR, 95% CI and P-value
results for clinical, dermoscopic and epidemiologic characteristics
of patients with melanoma with NMSC comorbidity.
| A, Characteristics
of patients with melanoma with NMSC comorbidity (unadjusted) |
|---|
|
|---|
| Factor | OR | 95% CI | P-value |
|---|
| Male | 0.99 | 0.54–1.77 | NS |
| Median age | 1.09 | 1.07–1.10 | <0.0001 |
| Melanoma
(previous/concomitant or in family history) | 1.37 | 0.61–2.9 | NS |
| ANS/NAN | 0.53 | 0.30–0.94 | <0.05 |
| Regression under
dermoscopy | 2.74 | 1.56–4.81 | <0.001 |
| Solar
lentiginosis | 9.07 | 4.42–20.75 | <0.0001 |
|
| B,
Characteristics of patients with melanoma with NMSC comorbidity
adjusted for age and sex |
|
| Factor | OR | 95% CI | P-value |
|
| Melanoma
(previous/concomitant or in family history) | 1.71 | 0.70–4.09 | NS |
| ANS/NAN | 1.48 | 0.72–3.17 | NS |
| Regression under
dermoscopy | 1.46 | 0.74–2.85 | NS |
| Solar
lentiginosis | 3.83 | 1.71–9.25 | <0.001 |
| Melanoma
thickness | 1.62 | 0.66–3.88 | NS |
The unadjusted ORs of investigated factors in the
group of patients with NMSC comorbidity demonstrated a high risk
for occurrence of SL (OR, 9.07; 95% CI, 4.42–20.75; P<0.0001),
regression structures in melanoma under dermoscopy (OR, 2.74; 95%
CI, 1.56–4.81; P<0.001) and with absence of ANS or NAN (OR,
0.53; 95% CI, 0.3–0.94, P<0.05). The crude ORs for sex
(P>0.05) and age (OR, 1.09; 95% CI, 1.07- 1.12, P<0.0001) of
patients with NMSC comorbidity are shown in Fig. 5A.
The ORs adjusted for age and sex of investigated
factors in a group of patients with NMSC comorbidity demonstrated a
statistically significant risk for occurrence only for SL (OR,
3.83; 95% CI, 1.71–9.25; P<0.001; Fig. 5B).
Analysis of differences in NMSC
comorbidity group depending on age
Differences in investigated factors were analysed
between younger (age, <65 years) and older (age ≥65 years)
patients with NMSC comorbidity (Table
III). The clinical findings in the younger subgroup
demonstrated a significantly lower frequency of SL (68.4% vs.
93.2%; P<0.05), although it was still found to be ~30% more
common compared with that of patients with melanoma without NMSC
comorbidity (39.3%). Another clinical factor, NAN/ANS, was observed
notably less frequently in older patients (P>0.05). The analysis
of differences in the topography of melanoma with NMSC comorbidity
demonstrated a statistically significant trend in the occurrence of
melanoma in the head and neck area in the elderly group (34.7% vs.
5%; P<0.05; P-adj.>0.05). In the analysis of dermoscopic
factors, regression features were present independently of the
patients' age and the pattern of facial melanoma was predominant in
the elderly patients (28.5% vs. 5.0%; P<0.05; P-adj.>0.05),
but the multicomponent asymmetric pattern was more frequent in
younger patients (60.0% vs. 22.4%; P<0.005; P-adj.<0.05).
 | Table III.Clinical, topographic and dermoscopic
data of patients diagnosed with melanoma and NMSC comorbidity
comparing younger (<65 years) and older (≥65 years)
patients. |
Table III.
Clinical, topographic and dermoscopic
data of patients diagnosed with melanoma and NMSC comorbidity
comparing younger (<65 years) and older (≥65 years)
patients.
| Factor | NMSC, comorbidity n
(%) | NMSC comorbidity
aged <65 years, n (%) | NMSC comorbidity
aged ≥65 years, n (%) | P-value | P-value within the
group | Bonferroni adjusted
P-value |
|---|
| Total patients | 63 (100.0) | 19 (30.1) | 44 (69.9) |
|
|
|
| Total melanoma | 69 (100.0) | 20 (29.0) | 49 (71.0) |
|
|
|
| Solar
lentiginosis |
|
|
| <0.05 |
|
|
|
Yes | 54 (85.7) | 13 (68.4) | 41 (93.2) |
|
|
|
| No | 9 (14.3) | 6 (31.6) | 3 (6.8) |
|
|
|
| NAN/ANS |
|
|
| NS |
|
|
|
Yes | 30 (47.6) | 13 (68.4) | 17 (38.6) |
|
|
|
| No | 33 (52.4) | 6 (31.6) | 27 (61.4) |
|
|
|
| Melanoma
location |
|
|
| <0.05 |
|
|
| Head
and neck | 18 (26.1) | 1 (5.0) | 17 (34.7) |
| <0.05 | NS |
|
Trunk | 22 (31.8) | 9 (45.0) | 13 (26.5) |
| NS | NS |
| Upper
limb | 14 (20.3) | 3 (15.0) | 11 (22.4) |
| NS | NS |
| Lower
limb | 15 (21.7) | 7 (35.0) | 8 (16.3) |
| NS | NS |
| Nail
apparatus | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NS | NS |
| Mucous
membrane | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NS | NS |
| Dermoscopic pattern
of melanoma |
|
|
| <0.05 |
|
|
|
Multicomponent asymmetric | 23 (33.3) | 12 (60.0) | 11 (22.4) |
| <0.005 | <0.05 |
|
Spitzoid | 3 (4.3) | 2 (10.0) | 1 (2.0) |
| NS | NS |
|
Melanoma on sun-damaged
skin | 13 (18.8) | 1 (5.0) | 12 (24.5) |
| NS | NS |
|
Hypomelanotic/amelanotic | 3 (4.3) | 1 (5.0) | 2 (4.0) |
| NS | NS |
|
Homogenous | 1 (1.4) | 0 (0.0) | 1 (2.0) |
| NS | NS |
|
Reticular | 2 (2.9) | 0 (0.0) | 2 (4.0) |
| NS | NS |
|
Nodular | 8 (11.6) | 2 (10.0) | 6 (12.2) |
| NS | NS |
|
Melanoma on face | 15 (21.7) | 1 (5.0) | 14 (28.5) |
| <0.05 | NS |
|
Melanoma in a special location
(nail apparatus/acral/mucous membranes) | 1 (1.4) | 1 (5.0) | 0 (0.0) |
| NS | NS |
| Dermoscopic
structures of regression |
|
|
| NS |
|
|
|
Yes | 34 (49.3) | 8 (40.0) | 26 (53.0) |
|
|
|
| No | 35 (50.7) | 12 (60.0) | 23 (47.0) |
|
|
|
Analysis of SL comorbidity differences
between patients with melanoma and patients with NMSC without
melanoma
The control group containing 233 patients with NMSC
without melanoma comorbidity was included in the present study to
evaluate the impact of SL. The control group consisted of 209
patients with BCC, 51 patients with SCC, 27 patients with BCC and
SCC, and 3 patients with multiple BCC and SCC (Table IV). The comparison of SL
comorbidity between patients with melanoma and the control group
demonstrated statistically significant differences in the mean and
median age; the patients with melanoma with SL were younger
compared with the patients with NMSC (mean, 52.2 years vs. 64.2
years; median, 49.0 years vs. 66.0 years; P<0.0001; Table V). SL was found more frequently
among patients with melanoma (P<0.01) and in selected
subcategories of patients with NMSC: i) with multiple BCC
(P<0.0001); ii) multiple SCC (P<0.005) or single SCC
(P<0.01); and iii) the comorbidity of BCC and SCC (P<0.0001).
No statistically significant differences in the sex distribution
between patients with and without melanoma, were observed,
regardless of the diagnosed skin cancer.
 | Table IV.Summary of data analysed in patients
diagnosed with melanoma or NMSC without melanoma with division into
lentiginosis comorbidity. |
Table IV.
Summary of data analysed in patients
diagnosed with melanoma or NMSC without melanoma with division into
lentiginosis comorbidity.
| Factor | Total patients, n
(%) | Patients with
lentiginosis, n (%) | Patients without
lentiginosis, n (%) | P-value | OR (95% CI) |
|---|
| Melanoma |
|
|
| <0.02 | 1.58 |
| No | 233 (46.9) | 91 (40.6) | 142 (52.0) |
| (1.11–2.67) |
|
Yes | 264 (53.1) | 133 (59.4) | 131(48.0) |
|
|
|
Total | 497 (100.0) | 224 (100.0) | 273 (100.0) |
|
|
| BCC |
|
|
| NS | 0.73 |
| No | 24 (10.3) | 11 (12.1) | 13 (9.2) |
| (0.31–1.76) |
|
Yes | 209 (89.7) | 80 (87.9) | 129 (90.9) |
|
|
|
Total | 233 (100.0) | 91 (100.0) | 142 (100.0) |
|
|
| SCC |
|
|
| <0.01 | 2.28 |
| No | 182 (78.1) | 63 (69.2) | 119 (83.8) |
| (1.22–4.34) |
|
Yes | 51 (21.9) | 28 (30.8) | 23 (16.2) |
|
|
|
Total | 233 (100.0) | 91 (100.0) | 142 (100.0) |
|
|
| Multiple BCC |
|
|
| <0.0001 | 3.98 |
| No | 166 (71.2) | 49 (53.9) | 117 (82.4) |
| (2.20–7.32) |
|
Yes | 67 (28.8) | 42 (46.1) | 25 (17.6) |
|
|
|
Total | 233 (100.0) | 91 (100.0) | 142 (100.0) |
|
|
| Multiple SCC |
|
|
| <0.01 | 10.41 |
| No | 225 (96.6) | 84 (92.3) | 141 (99.3) |
| (1.76–268.44) |
|
Yes | 8 (3.4) | 7 (7.7) | 1 (0.7) |
|
|
|
Total | 233 (100.0) | 91 (100.0) | 142 (100.0) |
|
|
| Multiple NMSC |
|
|
| <0.0001 | 3.96 |
| No | 164 (70.4) | 48 (52.8) | 116 (82.7) |
| (2.20–7.25) |
|
Yes | 69 (29.6) | 43 (47.2) | 26 (18.3) |
|
|
|
Total | 233 (100.0) | 91 (100.0) | 142 (100.0) |
|
|
 | Table V.Summary of age and sex data analysed
in patients diagnosed with melanoma or NMSC without melanoma with
stratified by lentiginosis comorbidity. |
Table V.
Summary of age and sex data analysed
in patients diagnosed with melanoma or NMSC without melanoma with
stratified by lentiginosis comorbidity.
| Factor | Total patients | Patients with
melanoma | Patients with NMSC
without melanoma | P-value | OR (95% CI) |
|---|
| Sex, n (%) |
|
|
|
|
|
|
Female | 299 (60.2) | 167.0 (55.9) | 132.0 (44.1) | NS | 0.76
(0.53–1.09) |
|
Male | 198 (39.8) | 97.0 (49.0) | 101.0 (51.0) |
|
|
|
Total | 491 (100.0) | 264.0 (100.0) | 2330 (100.0) |
|
|
| Age, years |
|
|
|
|
|
|
Mean |
| 52.2 | 64.2 | <0.0001 |
|
|
Median |
| 49.0 | 66.0 |
|
|
|
Standard deviation |
| 16.5 | 15.6 |
|
|
|
Range |
| 18.0–90.0 | 18.0–96.0 |
|
|
Discussion
While the prevalence of NMSC in patients with
melanoma has been previously reported as 7% by Neale et al
(3), the incidence reached 23.8% in
the present study. Based on results of multivariable logistic
regression presented in Table II
the likelihood of patients with melanoma developing NMSC increased
by 9% for each year of life (OR 1.09). Furthermore, while BCC was
found in every patient, 83.4% of patients with melanoma exhibited
NMSC mainly before, rather than concomitantly with, the diagnosis
of melanoma, which offers insights into the comorbidity of NMSC. To
the best of our knowledge, this finding has not been reported thus
far.
The present study demonstrated a significant
(P<0.0001) association between SL in the trunk and upper limbs,
and NMSC in patients who presented with melanoma concurrently or
after the initial presentation. Further analysis of the presence of
SL demonstrated an independent association both with melanoma and
multiple NMSC in the control group. The differentiation factor was
the mean and median age, as the patients with melanoma with SL were
>10 years younger compared with that of the non-melanoma group.
The SL comorbidity was insignificant among patients with a
diagnosis of BCC, but BCC was found in all patients with melanoma
with NMSC comorbidity. By contrast, SCC was associated with SL
irrespectively of their burden (single or multiple) but was present
only in 15.9% of patients with melanoma with NMSC comorbidity.
Therefore, SL may potentially be used as a marker of comorbidity in
different types of skin cancer, as well as an indicator of their
multiplicity.
Drawing the clinicians' attention to the presence of
lentiginosis may be valuable, as it could affect the risk
assessment of patients during clinical/dermoscopic skin
examination. Lentiginosis also indicates the possibility of
comorbid melanoma, which is difficult to identify due to excessive
regression or its similarity to SL on the face (27–29).
Previous studies have provided evidence linking SL to sun exposure
in various types (intense and intermittent or chronic-occupational
and everyday solar irradiation) and photodamage to the skin
(30–33). Certain studies have made
distinctions within SL, classifying those on the face as associated
with cumulative lifetime sun exposure (30–33).
By contrast, those on the trunk and arms were considered to be
associated with cumulative sun exposure and a history of sunburns
before the age of 20 (33). Thus,
in light of these findings, the present study focused on evaluating
the presence of SL on the upper arms and trunk, considering that
the presence of SL only on the face and dorsum of hands may be
age-related. By applying this approach, the present study
identified and described a subgroup of patients who are likely to
demonstrate NMSC before melanoma, without the need to conduct
complex sun exposure calculations or rely on potentially unreliable
questionnaires. A number of patients may not accurately recall
their sun exposure history or the number of sunburns experienced,
making it challenging to obtain accurate information through
self-reports. Therefore, the present approach allowed for a more
straightforward and practical method of identifying patients at
higher risk. At the same time, it should be considered that
dermatologists or healthcare providers performing routine screening
visits should ask patients about their sun exposure history and
number of sunburn episodes.
Considering the median age of patients with NMSC
comorbidity (70 years), it was found that individual factors
associated with those melanomas differ from the previously
described characteristics of melanomas in older patients (34–37).
Though facial melanoma is commonly found in the elderly population,
melanomas occurring in ‘special locations’ were not found in the
group of patients with NMSC, except for 1 case of acral melanoma.
Due to the mean age of onset for acral (63.1 years) or mucosal
(64±15 years) melanomas more cases might be expected, especially
considering that the NMSC comorbidity group was ~25 years older
compared with that of the comparator group (38,39).
Despite the rarity of acral and mucosal melanomas, the small sample
size of the present study could be a possible explanation. In
addition, a previous study proved that the most common dermoscopic
patterns among individuals aged >60 years were melanomas on
chronically sun-damaged skin, including the extra-facial LM type,
and multicomponent asymmetric or homogenous melanomas (37). Those results were consistent with
previous observations (37,40–42).
In the present study the distinguishing patterns of the NMSC
comorbidity appeared to be other dermoscopic patterns,
predominantly melanoma on the face and nodular melanomas, while
spitzoid melanomas were rare.
Therefore, to investigate whether the patients' age
affected the characteristics of the melanoma with NMSC comorbidity
setting, patients were compared between two subgroups: younger (age
<65), and older (age ≥65 years). Among the clinical aspects, the
younger subgroup demonstrated a significantly lower frequency of SL
compared with that of the elderly group, but SL frequency was still
more common compared with that in patients with melanoma without
NMSC comorbidity (68.4% vs. 39.3%). The analysis of differences in
the topography of melanoma with NMSC comorbidity demonstrated a
trend in the predominance of melanoma in the head and neck region
in the elderly group (34.7% vs. 5%; P<0.05; P-adj.>0.05). The
topography of melanoma in the younger group was similar to that of
patients without NMSC. The dermoscopic regression structures of
melanoma were present independently of the patient's age, and
therefore can be regarded as the characteristic feature of NMSC
comorbidity. The dermoscopic pattern of facial melanoma was
predominant in elderly patients (28.5% vs. 5.0%; P<0.05);
however, this was insignificant due to the small sample size
following Bonferroni's correction. The multicomponent asymmetric
pattern was the most frequently described in younger patients (60%
vs. 22.4%; P<0.005; P-adj. <0.05), similar to patients
without NMSC.
Melanomas on the face and scalp often pose notable
diagnostic difficulties under dermoscopy, particularly in
amelanotic/hypomelanotic LMM, and regressed or recurrent LMM
(27,28,43–46).
In such situations, RCM may indicate an adequately representative
area for biopsy and suggest the primary diagnosis. Furthermore, LM
can cause diagnostic problems for pathologists; LM is often
misdiagnosed as junctional melanocytic nevi, which may result in a
delay of diagnosis for years (29).
The present study demonstrated no statistically
significant differences between the groups regarding NMSC
comorbidity in the histological type or stage of melanomas. A
possible explanation is that the comparator group (patients with
melanoma without NMSC; median age, 45.0 years) undergo dermoscopic
screening tests more often, which results in a higher frequency of
early-stage or micro-melanomas diagnoses (47). Among patients with NMSC comorbidity,
melanomas arising within the photodamaged skin are most often
characterised by the primary horizontal type of growth (LM, LMM,
extra facial LM and superficial spreading melanoma) (27,36,40–42,48).
The differences between the aforementioned
individual patient groups partly indicate clinicians' possible
difficulties during the skin screening. Hence, to reflect real
clinical situations based on two cases, the present study described
different features of melanomas in patients with NMSC comorbidity,
independent of their age, which influenced the diagnostic workflow.
Elderly patients usually exhibit enhanced SL, which can overlap
with pigmented actinic keratosis or LM lesions, particularly in the
head area. The presence of melanoma simulators such as pigmented
BCC or pigmented actinic keratosis, collision tumours (that consist
of NMSC and melanoma tissue overlap) and features of wide
regression can make these melanomas go unnoticed despite their
large size or may lead to false-negative results. This, in turn,
may result in the delayed diagnosis of advanced melanomas. Younger
patients with NMSC comorbidity will present different diagnostic
difficulties, mainly due to the common presence of the ANS/NAN
(63.2%) and the multicomponent asymmetric pattern of melanomas
(60%) with features of regression (40%), which are also present in
dysplastic nevi of ANS. As a result, patients with NMSC comorbidity
require detailed dermoscopic skin examinations, preferably
accompanied by complementary RCM, and multiple punch biopsies to
enhance the specificity of the presurgical diagnostic process.
Therefore, simple descriptive features of unique patients'
characteristics might help physicians identify patients with NMSC
who may present or already have a difficult-to-diagnose melanoma,
thus preventing potential diagnostic pitfalls in clinical
practice.
For future exploration of the characteristics of
melanoma and NMSC comorbidity, haematological patients or organ
transplant recipients may provide data regarding the role of the
patient-related risk factors such as ANS, NAN, SL and degree of
skin photodamage, as these patients are known to be at a high risk
of developing multiple skin neoplasms (11–23).
For organ transplant recipients, Rizvi et al (17) reported standardised incidence ratios
of 51.9 for SCC (95% CI, 48.4–55.5), 54.9 for Kaposi sarcoma (95%
CI, 27.4–98.2) and 2.4 for melanoma (95% CI, 1.9–3.0). A study by
Omland et al (18) on
allogeneic hematopoietic stem cell transplant (HSCT) recipients
demonstrated an increased risk (hazard ratio, HR) of BCC (HR, 3.1;
95% CI, 1.9–5.2), SCC (HR, 18.3; 95% CI, 4.1–81.8) and melanoma
(HR, 5.5; 95% CI, 1.7–17.7). Morbidity varied depending on the type
of transplant, with SCC being most common in renal transplant
recipients (RTRs) and allogeneic HSCT recipients having a higher
risk of melanoma. The risk of BCC following allogeneic HSCT has
only been reported in patients treated with total-body irradiation
(HR, 3.9; 95% CI, 2.6–6.8), where it was found to be similar to
that of RTRs (18). A complex and
not fully explored group is that of patients who underwent
hematopoietic stem cell or solid organ transplantation in childhood
or have been under chronic immunosuppressive treatment since then
(20). It is crucial to address
several aspects related to their ongoing care, such as the
longevity of follow-ups, adherence to screening, education on
photoprotection and the importance of self-examination of the skin
(21). Silverberg and Ratner
(22) reported that patients with a
history of NMSC and melanoma are at an increased risk of developing
extra-cutaneous cancer (single or multiple), particularly at a
younger age (18–49 years), with a smoking history or of Caucasian
origin (P<0.0001). A possible explanation of melanoma and NMSC
comorbidity is polymorphisms of genes involved in DNA repair or
T-lymphocyte pathways observed in subsets of patients prone to
develop multiple types of cancer (22). Zheng et al (49) reported an increased risk of types of
cancer associated with CTLA-4 +49G>A variant genotypes (OR,
1.24; 95% CI, 1.18–1.32; P<0.05). These findings were also
consistent with those of other studies with common types of skin
cancer such as melanoma, BCC and SCC (OR, 1.30; 95% CI, 1.10–1.52;
P=0.001) and were more predominant in Caucasian patients (OR, 1.29;
95% CI, 1.13–1.47; P<0.005) (49–51).
A limitation of the present study was its
retrospective nature. The analysis of melanoma risk factors was
primarily based on empirical data gathered from medical procedures.
The differential analysis of the NMSC comorbidity group depending
on age (<65 years vs. ≥65 years) may have been influenced by the
small sample size of the younger population (the Bonferroni
correction was applied). Based on the retrospective analysis of the
medical records data regarding patient history and number of
sunburn episodes, and patient compliance with the rules of
photoprotection could not be obtained; hence, SL was considered as
the objective marker of sunburns.
In conclusion, the present study highlighted the
importance of closely monitoring patients who show signs of SL on
their trunk and upper arms, in terms of the high-risk of developing
multiple NMSC and melanoma comorbidity. Understanding the
differentiation features may increase the precision of dermoscopic
examination of difficult-to-diagnose melanomas by modifying the
diagnostic workflow, such as performing RCM rather than multiple
biopsies, and more detailed skin examination in this setting.
Given the expected global increase of skin neoplasm
and an increasing population of chronically immunosuppressed and
hemato-oncological patients in the coming decades, the diagnostic
difficulties described in the present study may become increasingly
common in everyday practice. The present study highlights the
importance of performing skin examinations to avoid an increase in
mortality due to late-diagnosed melanomas developing with time in
an increasingly younger population of patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS contributed to the conceptualization,
methodology, writing and original draft preparation and project
administration. RC performed software handling. MS and RC performed
data visualisation and formal analysis. MS, IC and ANG performed
data validation. MS, IC, PT, ANG, ML, JK and WO undertook the study
investigation. MS and IC acquired resources. MS, IC and PT
performed data curation. MS, IC, RC and WO participated in
manuscript writing, review and editing. MS and WO were responsible
for supervision. MS was responsible for funding acquisition. All
authors read and agreed the final version of the manuscript. MS and
IC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The Bioethics Commission at the Military Institute
of Medicine (Warsaw, Poland) approved the study protocol
(#21/WIM/2021, 19 May 2021; No. 65/24, 18 Dec 2024).
Patient consent for publication
Patients signed consent permitting the publication
of anonymised photographs.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Monika Słowińska, ORCID ID 0000-0002-7875-7383;
Iwona Czarnecka, ORCID ID 0000-0001-6229-5236; Robert Czarnecki,
ORCID ID 0000-0001-5291-2376; Anna Nasierowska-Guttmejer, ORCID ID
0000-0001-7182-5841; Witold Owczarek, ORCID ID
0000-0003-2049-942X.
References
|
1
|
Arnold M, Singh D, Laversanne M, Vignat J,
Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC and Bray
F: Global burden of cutaneous melanoma in 2020 and projections to
2040. JAMA Dermatol. 158:495–503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Fang L, Ni R, Zhang H and Pan G:
Changing trends in the disease burden of non-melanoma skin cancer
globally from 1990 to 2019 and its predicted level in 25 years. BMC
Cancer. 22:8362022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neale RE, Forman D, Murphy MF and Whiteman
DC: Site-specific occurrence of nonmelanoma skin cancers in
patients with cutaneous melanoma. Br J Cancer. 93:597–601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Small J, Barton V, Peterson B and Alberg
AJ: Keratinocyte carcinoma as a marker of a high cancer-risk
phenotype. Adv Cancer Res. 130:257–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zocchi L, Lontano A, Merli M, Dika E,
Nagore E, Quaglino P, Puig S and Ribero S: Familial melanoma and
susceptibility genes: A review of the most common clinical and
dermoscopic phenotypic aspect, associated malignancies and
practical tips for management. J Clin Med. 10:37602021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toussi A, Mans N, Welborn J and Kiuru M:
Germline mutations predisposing to melanoma. J Cutan Pathol.
47:606–616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ciccarese G, Dalmasso B, Bruno W, Queirolo
P, Pastorino L, Andreotti V, Spagnolo F, Tanda E, Ponti G, Massone
C, et al: Clinical, pathological and dermoscopic phenotype of MITF
p.E318K carrier cutaneous melanoma patients. J Transl Med.
18:782020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sturm RA, Fox C, McClenahan P, Jagirdar K,
Ibarrola-Villava M, Banan P, Abbott NC, Ribas G, Gabrielli B, Duffy
DL and Soyer PH: Phenotypic characterization of nevus and tumor
patterns in MITF E318K mutation carrier melanoma patients. J Invest
Dermatol. 134:141–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vergani E, Frigerio S, Dugo M, Devecchi A,
Feltrin E, De Cecco L, Vallacchi V, Cossa M, Di Guardo L, Manoukian
S, et al: Genetic variants and somatic alterations associated with
MITF-E318K germline mutation in melanoma patients. Genes (Basel).
12:14402021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang JM, Chikeka I and Hornyak TJ:
Melanocytic nevi and the genetic and epigenetic control of
oncogene-induced senescence. Dermatol Clin. 35:85–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jobson D, McCormack CJ, Mar V, Tam C and
Henderson MA: Impact of chronic lymphocytic leukaemia on melanoma
outcomes: A retrospective case-control study. Br J Haematol.
197:320–325. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olsen CM, Lane SW and Green AC: Increased
risk of melanoma in patients with chronic lymphocytic leukaemia:
Systematic review and meta-analysis of cohort studies. Melanoma
Res. 26:188–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishdorj G, Beiggi S, Nugent Z, Streu E,
Banerji V, Dhaliwal D, Mahmud SM, Marshall AJ, Gibson SB, Wiseman
MC and Johnston JB: Risk factors for skin cancer and solid tumors
in newly diagnosed patients with chronic lymphocytic leukemia and
the impact of skin surveillance on survival. Leuk Lymphoma.
60:3204–3213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Besson C, Moore A, Wu W, Vajdic CM, de
Sanjose S, Camp NJ, Smedby KE, Shanafelt TD, Morton LM, Brewer JD,
et al: Common genetic polymorphisms contribute to the association
between chronic lymphocytic leukaemia and non-melanoma skin cancer.
Int J Epidemiol. 50:1325–1334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins L, Quinn A and Stasko T: Skin
cancer and immunosuppression. Dermatol Clin. 37:83–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mittal A and Colegio OR: Skin cancers in
organ transplant recipients. Am J Transplant. 17:2509–2530. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rizvi SMH, Aagnes B, Holdaas H, Gude E,
Boberg KM, Bjørtuft Ø, Helsing P, Leivestad T, Møller B and
Gjersvik P: Long-term change in the risk of skin cancer after organ
transplantation: A population-based nationwide cohort study. JAMA
Dermatol. 153:1270–1277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Omland SH, Gniadecki R, Hædersdal M,
Helweg-Larsen J and Omland LH: Skin cancer risk in hematopoietic
stem-cell transplant recipients compared with background population
and renal transplant recipients: A population-based cohort study.
JAMA Dermatol. 152:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szlauer-Stefańska A, Kamińska-Winciorek G,
Giebel S and Bagłaj M: Secondary skin neoplasms in patients after
autologous and allogeneic hematopoietic stem cell transplantation
procedures. Adv Clin Exp Med. 29:1221–1230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keslova P, Formankova R, Riha P, Sramkova
L, Snajderova M, Malinova B, Luks A, Sterba J, Stary J and Sedlacek
P: Total body irradiation is a crucial risk factor for developing
secondary carcinomas after allogeneic hematopoietic stem cell
transplantation in childhood. Neoplasma. 67:1164–1169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehrhardt MJ, Brazauskas R, He W, Rizzo JD
and Shaw BE: Survival of patients who develop solid tumours
following hematopoietic stem cell transplantation. Bone Marrow
Transplant. 51:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silverberg JI and Ratner D: Associations
of non-melanoma skin cancer and melanoma, extra-cutaneous cancers
and smoking in adults: A US population-based study. J Eur Acad
Dermatol Venereol. 29:1389–1397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kearney L, Hogan D, Conlon P, Roche M,
O'Neill JP and O'Sullivan JB: High-risk cutaneous malignancies and
immunosuppression: Challenges for the reconstructive surgeon in the
renal transplant population. J Plast Reconstr Aesthet Surg.
70:922–930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossi M, Pellegrini C, Cardelli L,
Ciciarelli V, Di Nardo L and Fargnoli MC: Familial melanoma:
Diagnostic and management implications. Dermatol Pract Concept.
9:10–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keung EZ and Gershenwald JE: The eighth
edition American Joint Committee on Cancer (AJCC) melanoma staging
system: Implications for melanoma treatment and care. Expert Rev
Anticancer Ther. 18:775–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wickham H, Averick M, Bryan J, Chang W,
McGowan LDA, François R, Grolemund G, Hayes A, Henry L, Hester J,
et al: Welcome to the tidyverse. J Open Source Softw. 4:16862019.
View Article : Google Scholar
|
|
27
|
Gouda G, Pyne J and Dicker T: Pigmented
macules on the head and neck: A systematic review of dermoscopy
features. Dermatol Pract Concept. 12:e20221942022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carapeba MOL, Alves Pineze M and Nai GA:
Is dermoscopy a good tool for the diagnosis of lentigo maligna and
lentigo maligna melanoma? A meta-analysis. Clin Cosmet Investig
Dermatol. 12:403–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moscarella E, Guitera P, Scolyer RA, Rocha
L, Thomas L, Ronchi A, Scharf C, Brancaccio G and Argenziano G:
Junctional nevus and early melanoma on sun-damaged skin of the
head/neck: A clinico-pathologic challenge. Dermatol Pract Concept.
13:e20231222023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Praetorius C, Sturm RA and Steingrimsson
E: Sun-induced freckling: Ephelides and solar lentigines. Pigment
Cell Melanoma Res. 27:339–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monestier S, Gaudy C, Gouvernet J, Richard
MA and Grob JJ: Multiple senile lentigos of the face, a skin ageing
pattern resulting from a life excess of intermittent sun exposure
in dark-skinned caucasians: A case-control study. Br J Dermatol.
154:438–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bastiaens M, Hoefnagel J, Westendorp R,
Vermeer BJ and Bouwes Bavinck JN: Solar lentigines are strongly
related to sun exposure in contrast to ephelides. Pigment Cell Res.
17:225–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Derancourt C, Bourdon-Lanoy E, Grob JJ,
Guillaume JC, Bernard P and Bastuji-Garin S: Multiple large solar
lentigos on the upper back as clinical markers of past severe
sunburn: A case-control study. Dermatology. 214:25–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagore E, Hueso L, Botella-Estrada R,
Alfaro-Rubio A, Serna I, Guallar J, González I, Ribes I and Guillen
C: Smoking, sun exposure, number of nevi and previous neoplasias
are risk factors for melanoma in older patients (60 years and
over). J Eur Acad Dermatol Venereol. 24:50–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Demierre MF: Thin melanomas and
regression, thick melanomas and older men: Prognostic implications
and perspectives on secondary prevention. Arch Dermatol.
138:678–682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tiodorovic-Zivkovic D, Argenziano G,
Lallas A, Thomas L, Ignjatovic A, Rabinovitz H, Moscarella E, Longo
C, Hofmann-Wellenhof R and Zalaudek I: Age, gender, and topography
influence the clinical and dermoscopic appearance of lentigo
maligna. J Am Acad Dermatol. 72:801–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Słowińska M, Czarnecka I, Czarnecki R,
Tatara P, Nasierowska-Guttmejer A, Lorent M, Cierniak S and
Owczarek W: Clinical, dermoscopic, and histological characteristics
of melanoma patients according to the age groups: A retrospective
observational study. Life (Basel). 13:13692023.PubMed/NCBI
|
|
38
|
Teramoto Y, Keim U, Gesierich A, Schuler
G, Fiedler E, Tüting T, Ulrich C, Wollina U, Hassel JC, Gutzmer R,
et al: Acral lentiginous melanoma: A skin cancer with unfavourable
prognostic features. A study of the German central malignant
melanoma registry (CMMR) in 2050 patients. Br J Dermatol.
178:443–451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keller DS, Thomay AA, Gaughan J, Olszanski
A, Wu H, Berger AC and Farma JM: Outcomes in patients with mucosal
melanomas. J Surg Oncol. 108:516–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jaimes N, Marghoob AA, Rabinovitz H, Braun
RP, Cameron A, Rosendahl C, Canning G and Keir J: Clinical and
dermoscopic characteristics of melanomas on nonfacial chronically
sun-damaged skin. J Am Acad Dermatol. 72:1027–1035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeWane ME, Kelsey A, Oliviero M,
Rabinovitz H and Grant-Kels JM: Melanoma on chronically sun-damaged
skin: Lentigo maligna and desmoplastic melanoma. J Am Acad
Dermatol. 81:823–833. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Massone C, Hofman-Wellenhof R, Chiodi S
and Sola S: Dermoscopic criteria, histopathological correlates and
genetic findings of thin melanoma on non-volar skin. Genes (Basel).
12:12882021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Navarrete-Dechent C, Cordova M, Liopyris
K, Rishpon A, Aleissa S, Rossi AM, Lee E, Chen CJ, Busam KJ,
Marghoob AA and Nehal KS: Reflectance confocal microscopy and
dermoscopy aid in evaluating repigmentation within or adjacent to
lentigo maligna melanoma surgical scars. J Eur Acad Dermatol
Venereol. 34:74–81. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Licata G, Scharf C, Ronchi A, Pellerone S,
Argenziano G, Verolino P and Moscarella E: Diagnosis and management
of melanoma of the scalp: A review of the literature. Clin Cosmet
Investig Dermatol. 14:1435–1447. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pizzichetta MA, Polesel J, Perrot JL,
Rubegni P, Fiorani D, Rizzo A, Stanganelli I, Magi S, Mazzoni L,
Medri M, et al: Amelanotic/hypomelanotic lentigo maligna:
Dermoscopic and confocal features predicting diagnosis. J Eur Acad
Dermatol Venereol. 37:303–310. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spyridis I, Papageorgiou C, Apalla Z,
Manoli SM, Eftychidoy P, Gkentsidi T, Bobos M, Boutis A, Vakirlis
E, Sotiriou E, et al: The peculiar dermatoscopic pattern of scalp
melanoma. J Eur Acad Dermatol Venereol. 36:1564–1567. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Slowinska M, Kaminska-Winciorek G,
Kowalska-Oledzka E, Czarnecka I, Czarnecki R, Nasierowska-Guttmejer
A, Paluchowska E and Owczarek W: Dermoscopy of small diameter
melanomas with the diagnostic feasibility of selected algorithms-A
clinical retrospective multicenter study. Cancers (Basel).
13:60952021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferrari B, Pupelli G, Farnetani F, De
Carvalho NT, Longo C, Reggiani C, Argenziano G and Pellacani G:
Dermoscopic difficult lesions: An objective evaluation of
reflectance confocal microscopy impact for accurate diagnosis. J
Eur Acad Dermatol Venereol. 29:1135–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng J, Yu X, Jiang L, Xiao M, Bai B, Lu
J and Zhou Y: Association between the Cytotoxic T-lymphocyte
antigen 4 +49G > A polymorphism and cancer risk: A
meta-analysis. BMC Cancer. 10:5222010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bouwhuis MG, Gast A, Figl A, Eggermont
AMM, Hemminki K, Schadendorf D and Kumar R: Polymorphisms in the
CD28/CTLA4/ICOS genes: Role in malignant melanoma susceptibility
and prognosis? Cancer Immunol Immunother. 59:303–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Welsh MM, Applebaum KM, Spencer SK, Perry
AE, Karagas MR and Nelson HH: CTLA4 variants, UV-induced tolerance,
and risk of non-melanoma skin cancer. Cancer Res. 69:6158–6163.
2009. View Article : Google Scholar : PubMed/NCBI
|