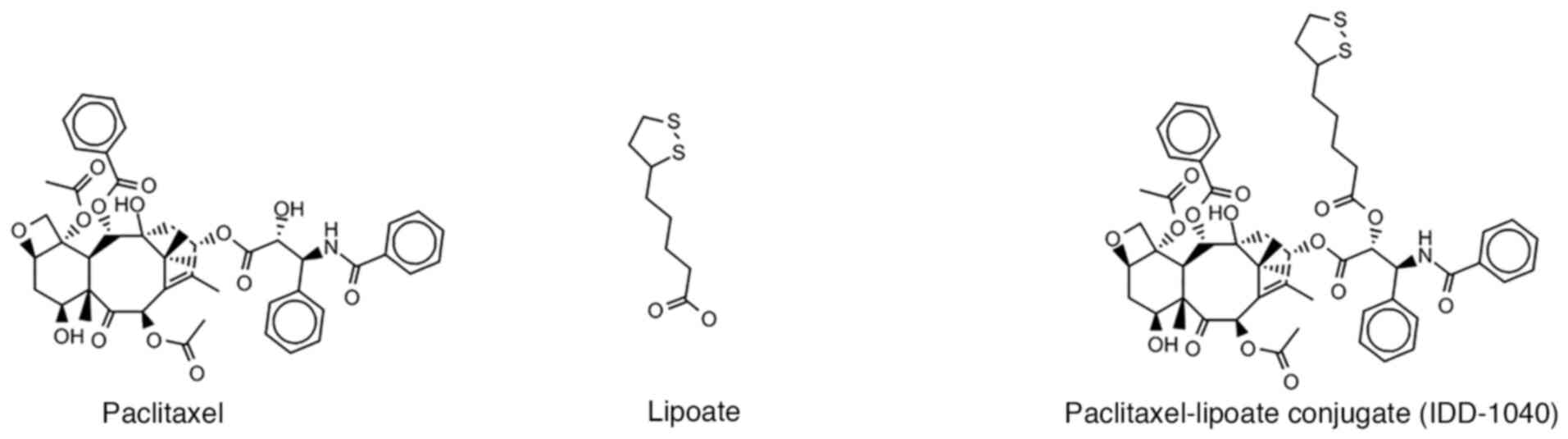
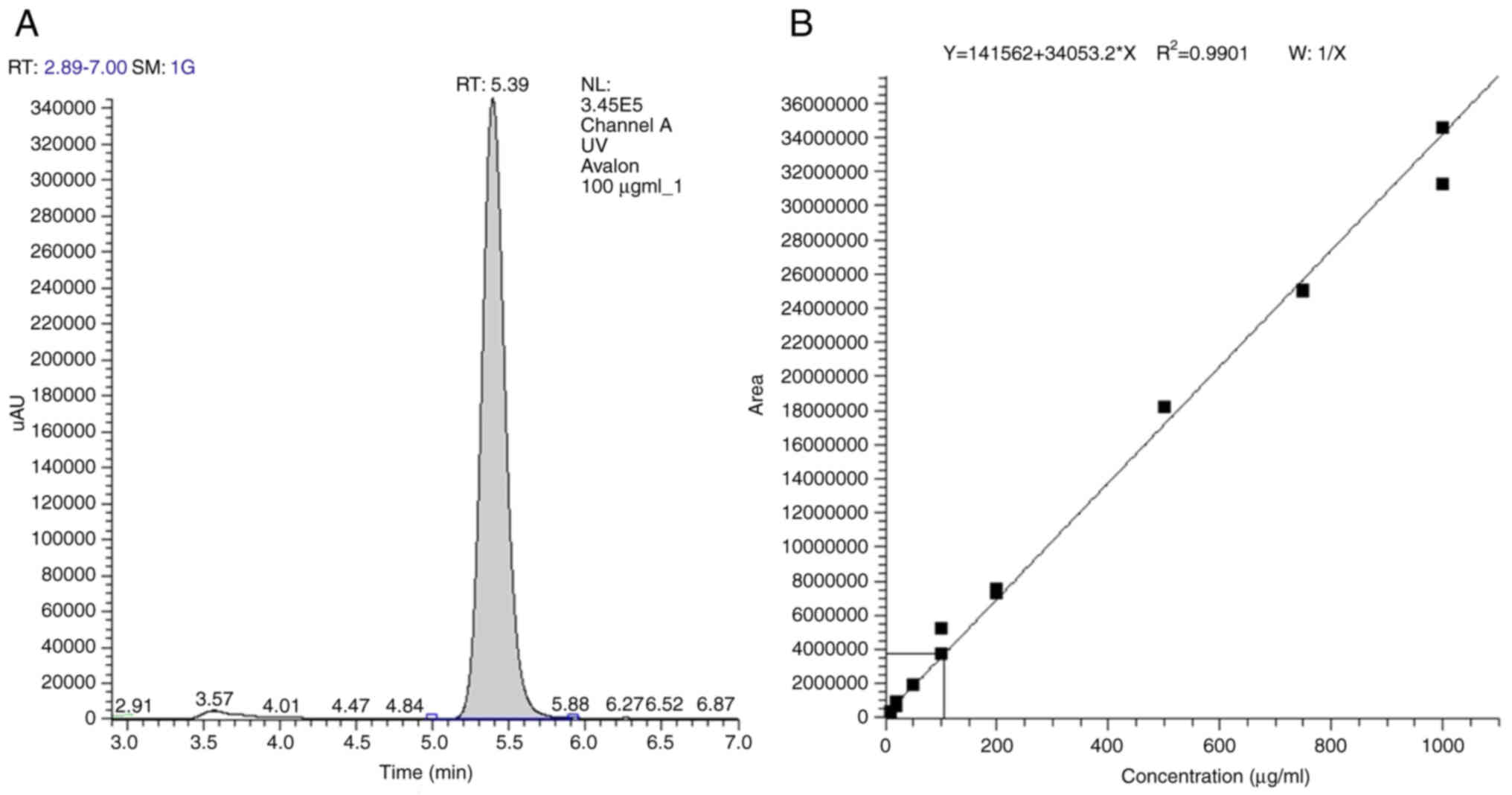
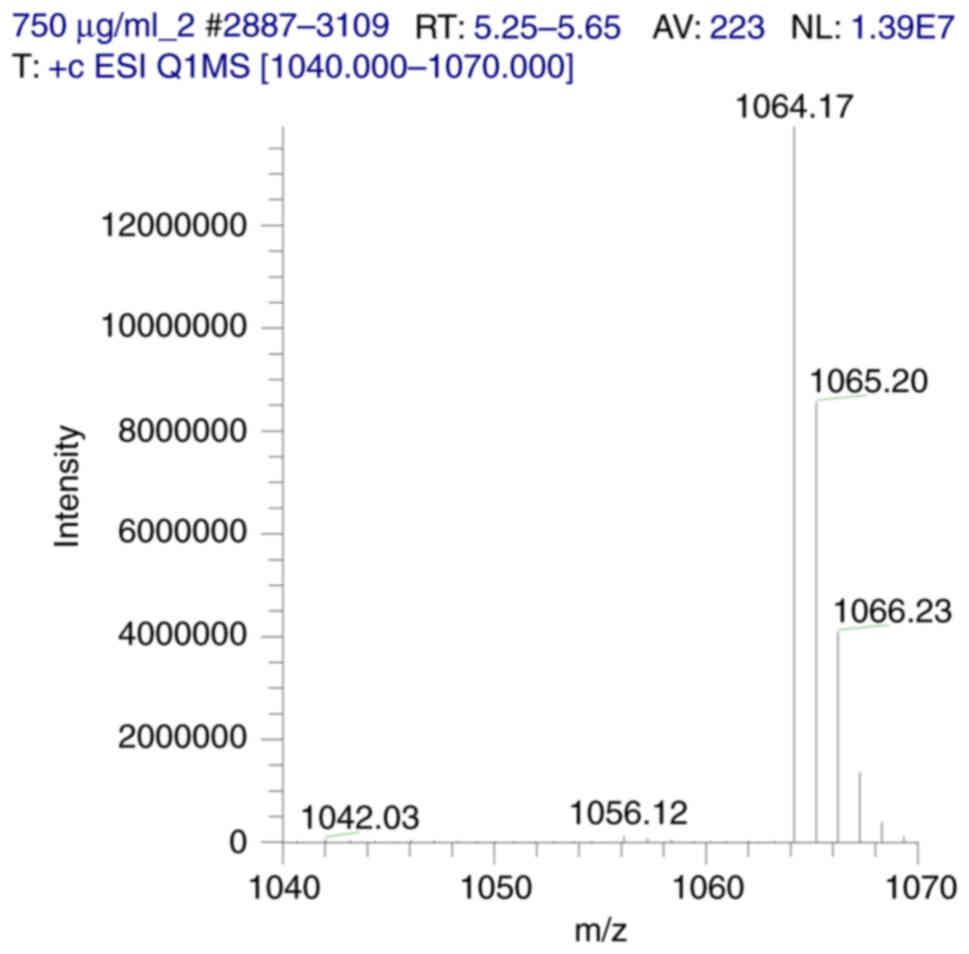
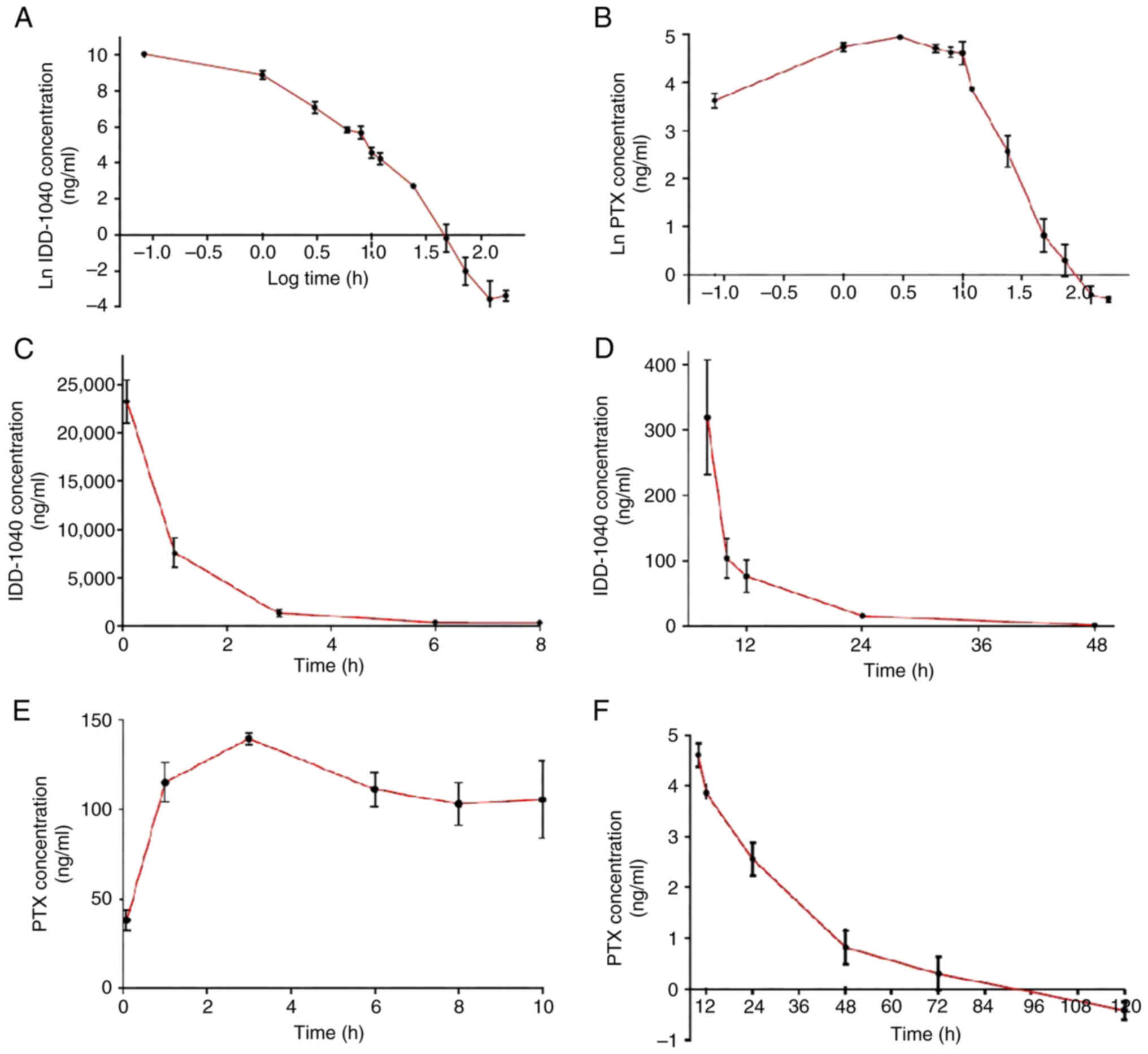
|
1
|
Reyes-Farias M and Carrasco-Pozo C: The
anti-cancer effect of quercetin: Molecular implications in cancer
metabolism. Int J Mol Sci. 20:31772019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alipour V, Rezapour A, Adel A, Pourtaleb
A, Bazrafshan M, Ghaem Mohammadi MS and Jahangiri R: Economical
evaluation of cancer types using intensity-modulated radiation
therapy compared to 3d conformal radiation therapy: A systematic
review. Iran J Public Health. 52:1355–1366. 2023.PubMed/NCBI
|
|
3
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manthalkar L, Ajazuddin and Bhattacharya
S: Evidence-based capacity of natural cytochrome enzyme inhibitors
to increase the effectivity of antineoplastic drugs. Discov Oncol.
13:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tauro S, Dhokchawle B, Mohite P, Nahar D,
Nadar S and Coutinho E: Natural anticancer agents: Their
therapeutic potential, challenges,s and promising outcomes. Curr
Med Chem. May 2–2023.(Epub ahead of print). PubMed/NCBI
|
|
6
|
Ahmed Khalil A, Rauf A, Alhumaydhi FA,
Aljohani ASM, Javed MS, Khan MA, Khan IA, El-Esawi MA, Bawazeer S,
Bouyahya A, et al: Recent developments and anticancer therapeutics
of paclitaxel: An update. Curr Pharm Des. 28:3363–3373. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seidman AD: Single-agent use of Taxol
(paclitaxel) in breast cancer. Ann Oncol. 5 (Suppl 6):S17–S22.
1994.PubMed/NCBI
|
|
9
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilkinson SP: The kidney in cirrhosis.
Tijdschr Gastroenterol. 19:155–169. 1976.PubMed/NCBI
|
|
11
|
Ozols RF: Carboplatin and Taxol
(paclitaxel) in advanced ovarian carcinoma. Ann Oncol. 5 (Suppl
6):S39–S43. 1994.PubMed/NCBI
|
|
12
|
Einzig AI: Review of phase II trials of
Taxol (paclitaxel) in patients with advanced ovarian cancer. Ann
Oncol. 5 (Suppl 6):S29–S32. 1994.PubMed/NCBI
|
|
13
|
Johnson DH, Chang AY and Ettinger DS:
Taxol (paclitaxel) in the treatment of lung cancer: The eastern
cooperative oncology group experience. Ann Oncol. 5 (Suppl
6):S45–S50. 1994.PubMed/NCBI
|
|
14
|
Fanale D, Bronte G, Passiglia F, Calò V,
Castiglia M, Di Piazza F, Barraco N, Cangemi A, Catarella MT,
Insalaco L, et al: Stabilizing versus destabilizing the
microtubules: A double-edge sword for an effective cancer treatment
option? Anal Cell Pathol (Amst). 2015:6909162015.PubMed/NCBI
|
|
15
|
Mukhtar E, Adhami VM and Mukhtar H:
Targeting microtubules by natural agents for cancer therapy. Mol
Cancer Ther. 13:275–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amaya C, Smith ER and Xu XX: Low intensity
ultrasound as an antidote to taxane/paclitaxel-induced
cytotoxicity. J Cancer. 13:2362–2373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walker FE: Paclitaxel (TAXOL): Side
effects and patient education issues. Semin Oncol Nurs. 9 (Suppl
2):S6–S10. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagumo Y, Villareal MO, Isoda H and Usui
T: RSK4 confers paclitaxel resistance to ovarian cancer cells,
which is resensitized by its inhibitor BI-D1870. Biochem Biophys
Res Commun. 679:23–30. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Januškevičienė I and Petrikaitė V:
Interaction of phenotypic sublines isolated from triple-negative
breast cancer cell line MDA-MB-231 modulates their sensitivity to
paclitaxel and doxorubicin in 2D and 3D assays. Am J Cancer Res.
13:3368–3383. 2023.PubMed/NCBI
|
|
20
|
Li Y, Zeng Y, Mooney SM, Yin B, Mizokami
A, Namiki M and Getzenberg RH: Resistance to paclitaxel increases
the sensitivity to other microenvironmental stresses in prostate
cancer cells. J Cell Biochem. 112:2125–2137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel A, Kalachand R, Busschots S, Doherty
B, Kapros E, Lawlor D, Hall N and Stordal BK: Taxane monotherapy
regimens for the treatment of recurrent epithelial ovarian cancer.
Cochrane Database Syst Rev. 7:CD0087662022.PubMed/NCBI
|
|
22
|
Wang L, Chen H, Wang F and Zhang X: The
development of peptide-drug conjugates (PDCs) strategies for
paclitaxel. Expert Opin Drug Deliv. 19:147–161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wender PA, Galliher WC, Bhat NM, Pillow
TH, Bieber MM and Teng NNH: Taxol-oligoarginine conjugates overcome
drug resistance in-vitro in human ovarian carcinoma. Gynecol Oncol.
126:118–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura J, Nakajima N, Matsumura K and
Hyon SH: Water-soluble taxol conjugates with dextran and targets
tumor cells by folic acid immobilization. Anticancer Res.
30:903–909. 2010.PubMed/NCBI
|
|
25
|
Falah M, Rayan M and Rayan A: A novel
paclitaxel conjugate with higher efficiency and lower toxicity: A
new drug candidate for cancer treatment. Int J Mol Sci.
20:49652019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shelanski ML, Gaskin F and Cantor CR:
Microtubule assembly in the absence of added nucleotides. Proc Natl
Acad Sci USA. 70:765–768. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JC and Timasheff SN: In vitro
reconstitution of calf brain microtubules: Effects of solution
variables. Biochemistry. 16:1754–1764. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Sun J, Chen X, Wang S, Scott H,
Zhang X and Zhang Q: Pharmacokinetics, tissue distribution and
anti-tumour efficacy of paclitaxel delivered by
polyvinylpyrrolidone solid dispersion. J Pharm Pharmacol.
64:775–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin HC, Cho H, Lai TC, Kozak KR, Kolesar
JM and Kwon GS: Pharmacokinetic study of 3-in-1 poly(ethylene
glycol)-block-poly(D, L-lactic acid) micelles carrying paclitaxel,
17-allylamino-17-demethoxygeldanamycin, and rapamycin. J Control
Release. 163:93–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersson BS and Way C: Parenternal
Paclitaxel in a stable non-toxic formulation. Patent US5877205A.
Filed June 28 1996; issued March 2, 1999.
|