Introduction
Cervical cancer is the fourth most prevalent
malignancy among women globally (1). Adenocarcinoma, which accounts for
10–25% of cervical cancer cases, typically exhibits distinct
cytological features on cytology smears. These include uniform
polygonal cells arranged in flat sheets and glandular structures,
granular cytoplasm, indistinct cell boundaries and round to oval
nuclei with prominent nucleoli (2).
Moreover, due to its higher propensity for metastasis and poorer
prognosis, adenocarcinoma also presents significant challenges in
patient management (3,4). The current treatment strategy for
cervical adenocarcinoma primarily involves surgery for early stage
disease and chemoradiotherapy for advanced-stage disease. The
overall 5-year survival rate for early stage cases is 70–90%, while
the survival rate for advanced-stage cases is typically <20%
(5). Despite advancements in
immunotherapy that have provided therapeutic breakthroughs for
patients with cervical cancer, the prognosis of cervical
adenocarcinoma remains poor (6).
There is an urgent need to explore new targeted therapies to
improve patient survival. Therefore, it is necessary to study new
biomarkers to improve prognostic accuracy and promote the further
development of targeted therapies.
Human epidermal growth factor receptor 2 (HER2), a
member of the EGFR family, serves a pivotal role in tumor growth
and progression by inhibiting apoptosis and promoting angiogenesis
and metastasis (7). HER2
upregulation occurs in various types of cancer (8), and numerous studies (9–12) have
indicated that HER2 protein expression levels are a poor prognostic
factor in malignant tumors and are associated with tumor
sensitivity to chemotherapy and biological therapy. Particularly in
breast and gastric cancer, HER2 has been extensively studied and
successfully applied as a therapeutic target in clinical practice
(13–15). However, in cervical cancer,
especially in the subtypes of cervical adenocarcinoma and squamous
cell carcinoma, the role and clinical significance of HER2 remains
controversial. Studies (16,17)
have shown that HER2 expression levels are higher in cervical
adenocarcinoma compared with those in squamous cell carcinoma,
particularly in advanced and high-risk patients, suggesting that
HER2 may be involved in the pathological progression of
adenocarcinoma. Other studies (17–19)
have suggested that HER2 could be a valuable therapeutic target in
cervical adenocarcinoma, especially for patients with advanced
disease or disease progression. Although some studies (20–22)
have found that HER2 expression levels are associated with a poor
prognosis, significant variations in HER2 expression levels and
prognostic significance across studies could be attributed to
factors such as sample size, and detection methods such as
immunohistochemistry (IHC)/fluorescence in situ
hybridization (FISH). Moreover, in previous studies (17,23),
the expression of HER2 in cervical adenocarcinoma was low according
to the traditional assessment method of combined IHC/FISH, and its
association with prognosis in cervical adenocarcinoma remains
unclear.
In recent years, anti-HER2-drug conjugates (ADCs)
have garnered attention for their ability to target HER2-expressing
cancer cells and improve therapeutic outcomes (24). The prevalence of IHC 1+ and
2+/FISH-HER2 expression in gynecological tumors has become an
important focus for clinicians. Research has demonstrated that in
the C018 cervical cancer cohort study, the range of patients
benefiting from RC48 anti-HER2 therapy expanded to include IHC 1+,
2+ and 3+ groups. Notably, an objective response rate (ORR) of up
to 50% was observed in IHC 1+ patients (25). This underscores the importance of
more precise classification of HER2 expression levels, which is
essential for guiding personalized treatment strategies for
cervical cancer.
At present, traditional HER2 detection methods have
the disadvantage of low detection rates, which limits treatment
options for HER2 patients with low expression and thus affects
prognosis (26). Standalone IHC
could provide the sensitivity of HER2 status detection, with the
advantages of being fast, simple, cost-effective and highly
specific, making it more effective in assessing clinical prognosis,
and will help more accurately evaluate the therapeutic effects of
HER2-targeted drugs (27). However,
there is a lack of research investigating the association between
standalone IHC and clinical prognosis. The present study aimed to
investigate HER2 expression levels in patients with cervical
adenocarcinoma, with a particular focus on IHC 1+ and
2+/FISH-cases, and to evaluate its prognostic significance, as well
as its association with programmed death-ligand 1 (PD-L1)
expression levels.
Patients and methods
Patients
The present study retrospectively analyzed HER2
status via IHC in 179 female patients with cervical adenocarcinoma
undergoing radical surgery (including 10 patients who received
neoadjuvant chemotherapy before surgery) between January 2018 and
November 2020 at Zhejiang Cancer Hospital (Hangzhou, China). The
present study was conducted in accordance with the Declaration of
Helsinki with the approval of the Medical Ethics Committee of
Zhejiang Cancer Hospital (approval no. IRB-2023-656), and all
patients provided written informed consent for publication of their
data.
Patients included in the present study were required
to meet the following criteria: Aged between 18 and 70 years, with
pathologically confirmed cervical adenocarcinoma, who received
radical surgery, with complete clinical and pathological data, and
who signed an informed consent form at admission (which included
consent for their samples to be used for research). Exclusion
criteria included patients lost to follow-up or those with
insufficient tissue samples for HER2 testing.
Data collection and follow up
Patients' age, clinical stage, pathological
diagnosis, tumor size, lymph vascular space invasion (LVSI), extent
of invasion, lymph node metastasis status, whether neoadjuvant
therapy was administered before surgery, HER2 expression status,
pre-treatment carbohydrate antigen 19-9 (CA19-9), pre-treatment
cancer antigen 125 (CA-125), recurrence status, recurrence time,
survival status, last follow-up time and time of death were
recorded. Progression-free survival (PFS) and overall survival (OS)
were followed up through outpatient visits and telephone
interviews. The data cut-off date was June 2024.
Clinical characteristics [including age,
International Federation of Gynecology and Obstetrics (FIGO) stage,
tumor size, depth of invasion, vascular invasion or perineural
invasion, pre-treatment CA19-9 values and pre-treatment CA-125
values] and prognostic factors (metastasis, recurrence and survival
data) were analyzed in the IHC alone group. Additionally, the
expression ratio between PD-L1 and HER2 in patients with cervical
adenocarcinoma who died was analyzed. OS and PFS were measured from
pathological diagnosis to death from any cause and to the first
disease progression, respectively. The clinical data were obtained
from the medical records of Zhejiang Cancer Hospital and tissue
samples were obtained from the pathology archives of the
hospital.
HER2 status analysis
According to the guidelines (23) for HER2 testing in gastric cancer and
other malignancies, HER2 1+ and HER2 2+/FISH- are defined as
negative, while HER2 2+/FISH+ and HER2 3+ are defined as positive.
Targeted therapy is recommended for patients with HER2-positive
status (28). To broaden the scope
of treatment and improve patient prognosis, the present study
population was divided into groups. Patient tumor samples were
tested using IHC and FISH methods. The detection results were used
to classify patients by HER2 status into the following groups based
on IHC alone and the traditional combined IHC/FISH method: i) IHC
alone, patients were divided into the HER2 zero expression (IHC 0)
group and the HER2 expression (IHC 1+, 2+ and 3+) group; and ii)
traditional combined IHC/FISH classification, patients were
categorized as HER2-negative (IHC 0, IHC 1+ or IHC 2+/FISH-) or
HER2-positive (IHC 3+ or IHC 2+/FISH+).
IHC
Paraffin-embedded samples were fixed with 10%
neutral formalin for 24 h at room temperature. IHC was performed on
3-µm thick sections obtained from formalin-fixed, paraffin-embedded
tissue blocks. The immunohistochemistry images were captured using
a light microscope. PD-L1 and HER2 were targeted using specific
primary antibodies. The gold standard for detecting PD-L1
expression is IHC (29), which
utilizes the high specificity of antibody-antigen binding to
display the location and intensity of antigen-antibody binding
through histochemical techniques, thereby detecting the expression
levels of PD-L1 on the surface of tumor cells or immune cells. In
the present study, PD-L1 expression levels were assessed using
slides stained for PD-L1 22C3 (catalog number M3666; Dako, Agilent
Technologies, Inc.) and the combined positive score (CPS) method
was applied for evaluation. The procedure was performed according
to the manufacturer's instructions. CPS was calculated as: (Number
of PD-L1-positive tumor cells + number of PD-L1-positive immune
cells)/total number of viable tumor cells ×100. A CPS threshold of
≥1 was used to determine PD-L1 positivity (30). CPS=0 indicated that PD-L1 expression
was negative (Fig. S1) with no
brown staining observed in tumor cells or immune cells. By
contrast, when PD-L1 was positively expressed in tumor cells or
immune cells, partial brown staining was observed (Fig. S2). This indicates a certain level
of PD-L1 expression in the tumor microenvironment, providing a
reference for the evaluation of immunotherapy. In addition, the
present study adhered to American Society of Clinical
Oncology/College of American Pathologists (ASCO/CAP) guidelines
(31) for gastric cancer to
establish HER2 IHC scoring criteria. These criteria were defined as
follows: 0, no staining or <10% of tumor cell membranes stained;
1+, ≥10% of tumor cells with faint or barely perceptible membrane
staining, with only part of the membrane stained; 2+, ≥10% of tumor
cells with weak to moderate complete, basolateral or lateral
membrane staining; and 3+, ≥10% of tumor cells with strong
complete, basolateral or lateral membrane staining. Based on HER2
expression scoring standards in gastric and gastroesophageal
adenocarcinoma, HER2 staining was categorized as: 0, negative; 1+,
negative; 2+, equivocal; and 3+, positive (32). Cases with equivocal HER2
upregulation (2+) results were further analyzed using FISH
(33).
FISH
FISH tests were conducted using the Vysis PathVysion
HER2 DNA Probe Kit (Abbott Pharmaceutical Co. Ltd.), according to
the manufacturer's protocol. The dual-color FISH method, involving
two labeled DNA probes, was performed on sections cut from the same
tissue microarray block. The LSI HER2 probe, which spans the entire
HER2 gene, was labeled in Spectrum Orange, while the CEP17 probe
(chromosome-17 centromere probe for chromosome 17 enumeration) was
labeled in Spectrum Green. These probes hybridized to the a
satellite DNA located at the centromere of chromosome 17
(17p11.1-q11.1). For analysis, two separate fields comprising ≥20
cells each were counted. The HER2 signal ratio was calculated by
tallying red (HER2 gene) and green (chromosome 17) signals in
preselected tumor areas. Tumor cells from the same sites analyzed
by IHC were typically assessed for signal counts. Images were
captured using an ECLIPSE 80i (Nikon Corporation) fluorescence
microscope with a PlanFluor (Nikon Corporation) 100X oil objective
and a double band-pass filter enabling simultaneous visualization
of green and red colors.
IHC and FISH interpretation and
quality control
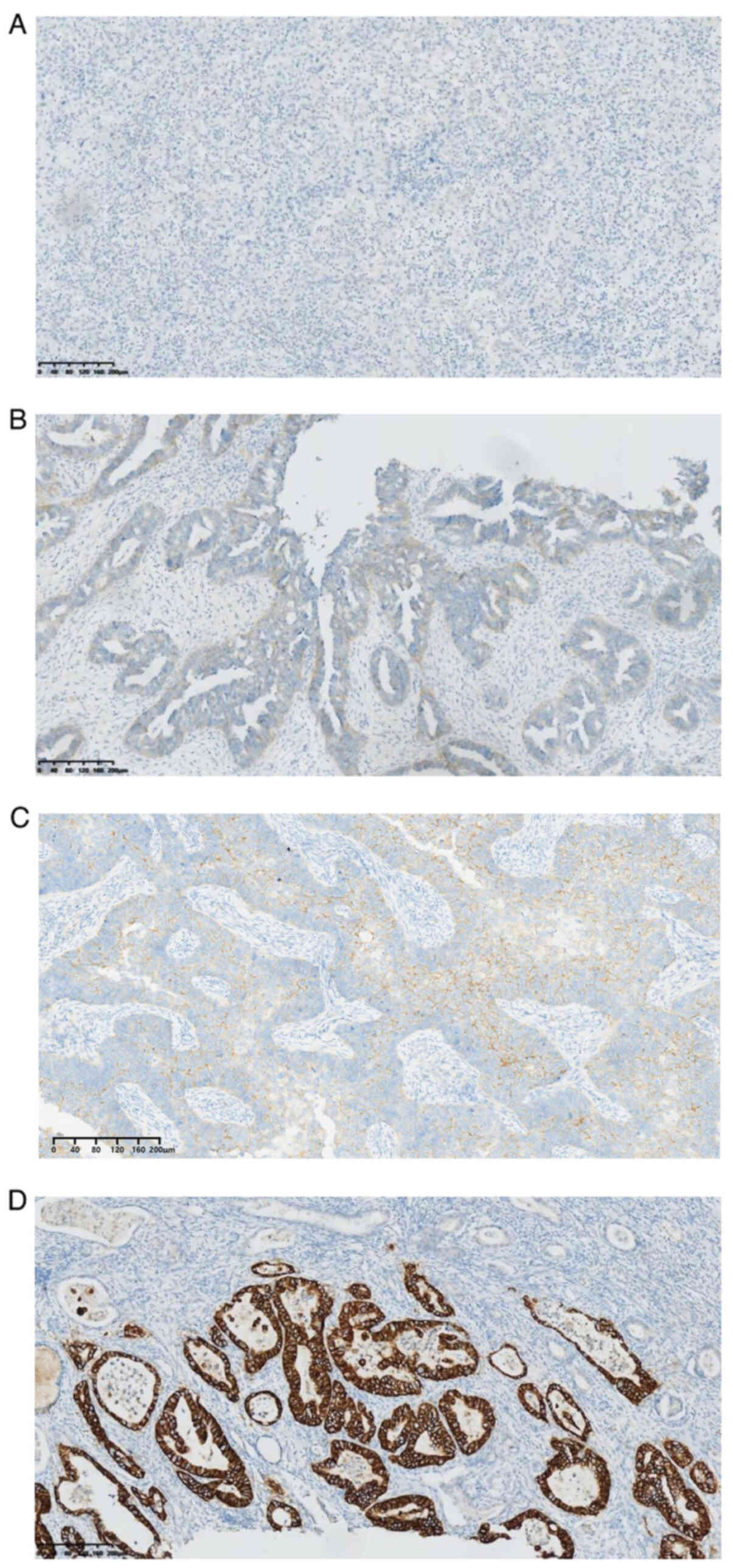
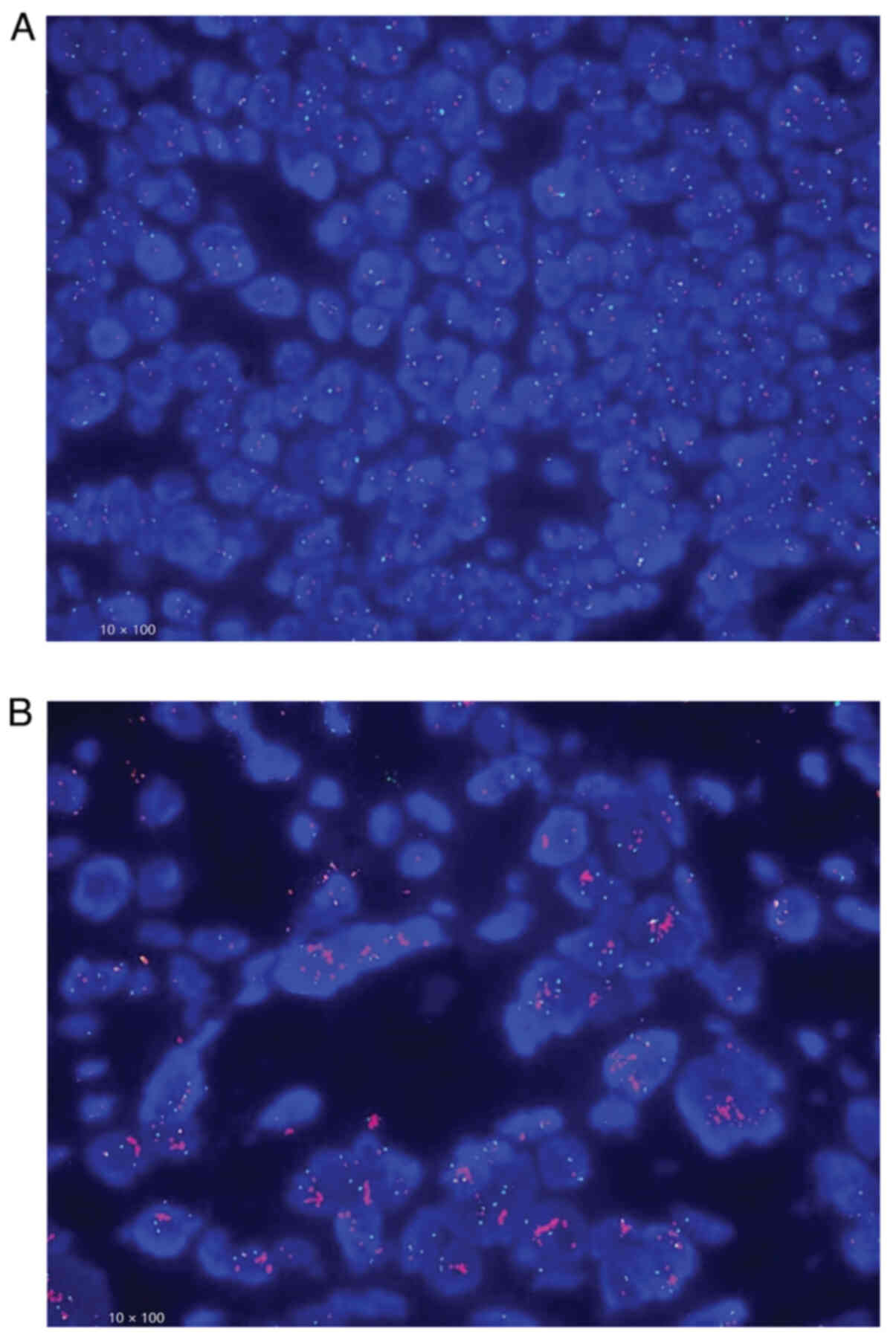
Representative images of different levels of HER2
expression using IHC and FISH are shown in Figs. 1 and 2, respectively, and data presented in
Tables I and II. For quality control of IHC/FISH
analysis, two pathologists with >5 years of experience
independently interpreted the results. In cases of disagreement and
the inability to reach a consensus, a third senior reviewer made
the final judgment.
 | Table I.Description of representative images
of HER2 expression using IHC. |
Table I.
Description of representative images
of HER2 expression using IHC.
| IHC status,
score | Characteristics of
IHC | IHC results |
|---|
| 0 | The cells appear
blue, with no significant membrane staining observed in the
glandular cells. | Fig. 1A |
| 1+ | The cells in the
glandular structures show slight staining. | Fig. 1B |
| 2+ | Some glandular
structures exhibit an incomplete membranous staining pattern
(basolateral or ‘U-shaped’). | Fig. 1C |
| 3+ | The membranous
staining of glandular cells is relatively complete, displaying a
pattern that encircles the glands. | Fig. 1D |
 | Table II.Description of representative images
of HER2 2+ using FISH. |
Table II.
Description of representative images
of HER2 2+ using FISH.
| Category | HER2/CEP17 signal
ratio | FISH results |
|---|
|
Non-amplification | <2.0 | Fig. 2A |
| Amplification | ≥2.0 | Fig. 2B |
Statistical analysis
Statistical analyses were conducted using SPSS
(version 25; IBM Corp.). Clinical and pathological characteristics
were compared using the χ2 test or Fisher's exact test
as appropriate. Survival analysis was performed using the
Kaplan-Meier method and comparisons were made using log-rank tests.
Multivariate analysis was performed using the Cox proportional
hazards regression model to identify independent prognostic
factors. All reported P-values were two-sided. P<0.05 was
considered to indicate a statistically significant difference.
Results
HER2 expression levels in cervical
adenocarcinoma
The present study included 179 patients with
cervical adenocarcinoma who underwent radical surgery, and HER2
expression was evaluated through IHC and FISH results (Figs. 1 and 2). A novel classification method
specifically designed to evaluate HER2 expression using IHC alone
was utilized (Fig. 3). In the IHC
alone group, the expression rates of IHC 1+, 2+ and 3+ were 27.4%
(49/179), 12.8% (23/179) and 3.9% (7/179), respectively, which
indicated that 44.1% of samples exhibited HER2 expression. In the
traditional combined IHC/FISH method group, there were 7 HER2 3+
cases and 23 HER2 2+ cases (data not shown). After FISH testing,
only 2 of the 23 cases were FISH-positive. Therefore, the total
number of HER2-positive patients was 9, accounting for only 5% of
included patients.
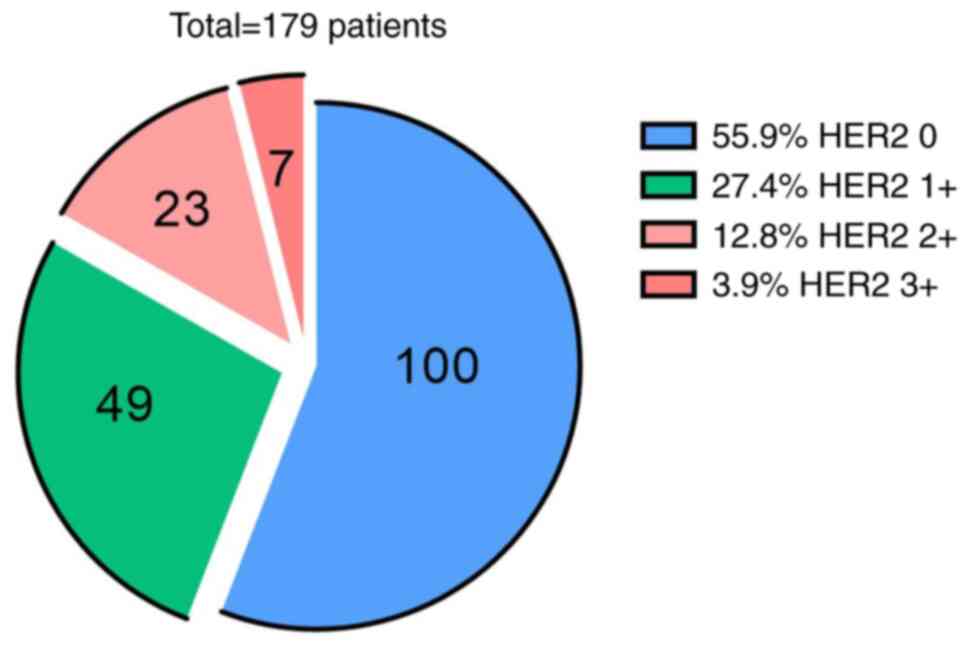 | Figure 3.Distribution of patient numbers and
detection rates across different HER2 expression levels (IHC 0, 1+,
2+ and 3+) assessed using IHC alone. HER2 zero expression (IHC 0)
was the most common (100 cases, 55.9%), followed by HER2 1+ (49
cases, 27.4%), HER2 2+ (23 cases, 12.8%) and HER2 3+ (7 cases,
3.9%), indicating a relatively high proportion of positive HER2
expression in the present study cohort. HER2, human epidermal
growth factor receptor 2; IHC, immunohistochemistry. |
Association of HER2 expression and
clinical factors in the IHC group
There were 100 patients with HER2 zero expression
and 79 patients with HER2 expression. As shown in Table III, the age distribution between
the HER2 zero expression group and the HER2 expression group was
generally similar and showed no statistically significant
difference among patients with cervical adenocarcinoma who
underwent radical surgery. However, the mean age at onset was
49.75±11.40 years for patients in the HER2 zero expression group
and 47.97±10.72 years for those in the HER2 expression group among
patients with cervical adenocarcinoma who underwent radical surgery
(Table III). HER2 expression was
significantly associated with advanced FIGO stages (P=0.001) and
higher recurrence rates (P=0.032). Among the tumor markers,
abnormal pre-treatment CA-125 levels were significantly associated
with HER2 expression (P=0.023), whereas pre-treatment CA19-9 levels
did not reach statistical significance (P=0.15).
 | Table III.Association between HER2 status and
characteristics in 179 cases in the HER2 zero expression (n=100)
and HER2 expression (n=79) groups. |
Table III.
Association between HER2 status and
characteristics in 179 cases in the HER2 zero expression (n=100)
and HER2 expression (n=79) groups.
| A, Total patient
cohort |
|---|
|
|---|
| Variables | HER2 zero
expression | HER2
expression | χ2 | P-value |
|---|
| No. of
patients | 100 | 79 |
|
|
| Age, n (%) |
|
| 1.735 | 0.188 |
| ≤60
years | 83 (53.9) | 71 (46.1) |
|
|
| >60
years | 17 (68.0) | 8 (32.0) |
|
|
| FIGO stage, n
(%) |
|
| 10.116 | 0.001 |
|
I–IIB | 78 (63.9) | 44 (36.1) |
|
|
|
III–IV | 22 (38.6) | 35 (61.4) |
|
|
| Recurrence status,
n (%) |
|
| 4.581 | 0.032 |
| No
progression | 80 (60.6) | 52 (39.4) |
|
|
|
Progression | 20 (42.6) | 27 (57.4) |
|
|
| Survival status, n
(%) |
|
| 1.237 | 0.266 |
|
Survival | 86 (57.7) | 63 (42.3) |
|
|
|
Death | 14 (46.7) | 16 (53.3) |
|
|
| Pre-treatment
CA19-9 levels, n (%) |
|
| 2.072 | 0.15 |
|
Normal | 69 (59.0) | 48 (41.0) |
|
|
|
Abnormal | 26 (47.3) | 29 (52.7) |
|
|
| Not
available | 5 (71.4) | 2 (28.6) |
|
|
| Pre-treatment
CA-125 levels, n (%) |
|
| 5.141 | 0.023 |
|
Normal | 76 (60.8) | 49 (39.2) |
|
|
|
Abnormal | 20 (41.7) | 28 (58.3) |
|
|
| Not
Available | 4 (66.7) | 2 (33.3) |
|
|
|
| B, Excluding 10
patients who received neoadjuvant chemotherapy |
|
|
Variables | HER2 zero
expression | HER2
expression |
χ2 | P-value |
|
| No. of
patients | 94 | 75 |
|
|
| Vascular or
perineural invasion, n (%) |
|
| 4.751 | 0.029 |
|
Negative | 62 (62.6) | 37 (37.4) |
|
|
|
Positive | 32 (45.7) | 38 (54.3) |
|
|
| Lymph node
metastasis, n (%) |
|
| 9.979 | 0.002 |
|
Negative | 77 (63.1) | 45 (36.9) |
|
|
|
Positive | 17 (36.2) | 30 (63.8) |
|
|
| Depth of invasion,
n (%) |
|
| 1.180 | 0.277 |
| Not
infiltrated into deep muscle layer | 48 (60.0) | 32 (40.0) |
|
|
|
Infiltration into the deep
muscle layer | 46 (51.7) | 43 (48.3) |
|
|
| Tumor size, n
(%) |
|
| 0.629 | 0.428 |
| ≤4
cm | 69 (53.9) | 59 (46.1) |
|
|
| >4
cm | 25 (61.0) | 16 (39.0) |
|
|
Patients in the HER2 zero expression group exhibited
a survival rate of 57.7% and a mortality rate of 46.7%, compared
with a survival rate of 42.3% and a mortality rate of 53.3% for the
HER2 expression group, suggesting a trend towards improved
prognosis for patients without HER2 expression, although this was
not statistically significant (P=0.266). When excluding the 10
patients who had received neoadjuvant chemotherapy, postoperative
pathological findings indicated that HER2 expression was
significantly associated with vascular or perineural invasion
(P=0.029) and lymph node metastasis (P=0.002), with no significant
differences observed in tumor size or invasion depth.
Association between HER2 expression
and prognosis
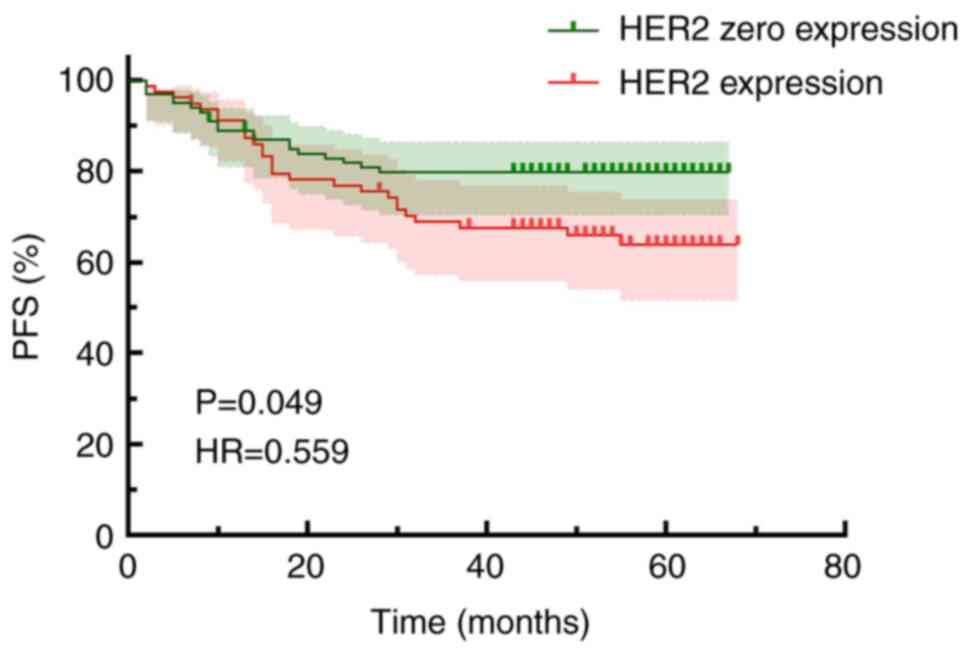
IHC alone and combined IHC/FISH both indicated that
patients with HER2 expression experienced significantly poorer PFS
times compared with those without HER2 expression. In the IHC alone
group, the PFS time was 56.01±2.22 months for the HER2 IHC 0 group
and 51.02±2.75 months for the HER2 expression group, with a
statistically significant difference when compared [hazard ratio
(HR), 0.559; 95% confidence interval (CI), 0.313–0.998; P=0.049;
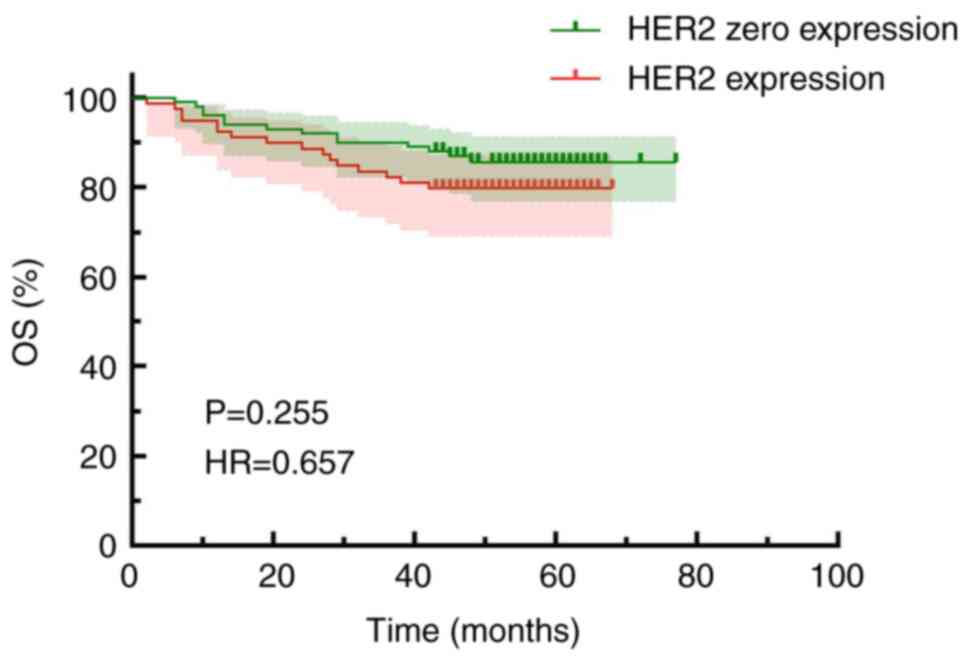
Fig. 4]. Kaplan-Meier survival
curves were used to evaluate OS based on HER2 status, which
demonstrated that the OS time of the patients with HER2 expression
was slightly lower compared with that of the patients without HER2
(Fig. 5). Specifically, the OS was
69.47±1.93 months for the HER2 zero expression group and 58.47±2.22
months for the HER2 expression group, although this difference did
not reach statistical significance (HR, 0.657; 95% CI, 0.318–1.356;
P=0.255).
In the traditional method group, which employed
combined IHC/FISH, the PFS time was 55.17±1.78 months for the
HER2-negative group, compared with 36.44±7.85 months for the
HER2-positive group, showing a statistically significant difference
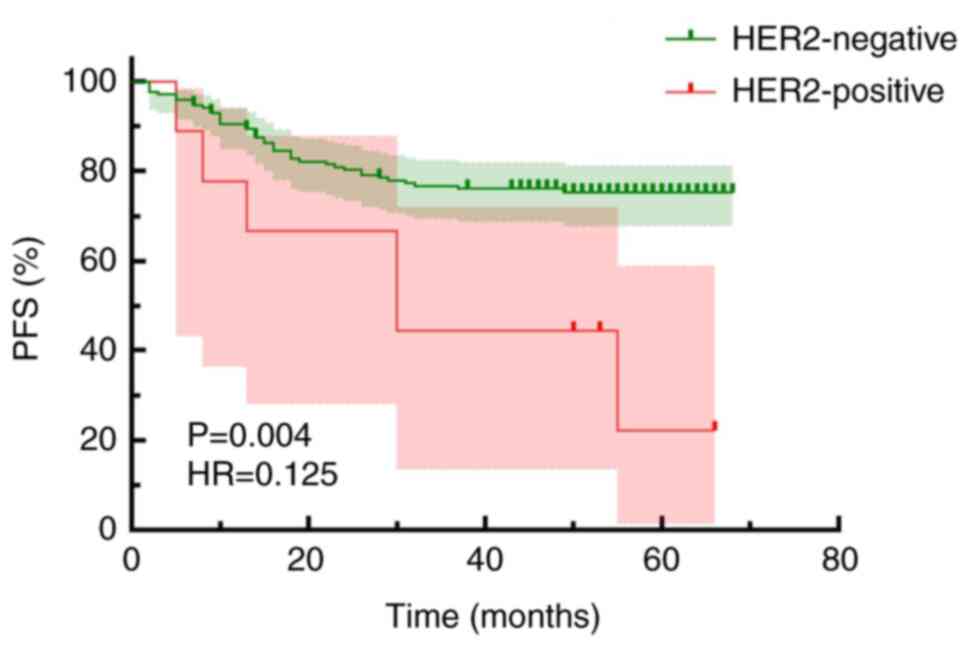
(HR, 0.125; 95% CI, 0.03033–0.5156; P=0.004; Fig. 6). The present study showed that the
risk of poor survival in HER2-negative patients was significantly
lower compared with that of HER2-positive patients, with an HR of
0.1250, which indicated that the survival risk in the HER2-negative
group was only 12.5% of that in the HER2-positive group. Therefore,
HER2 negativity was associated with an improved prognosis.
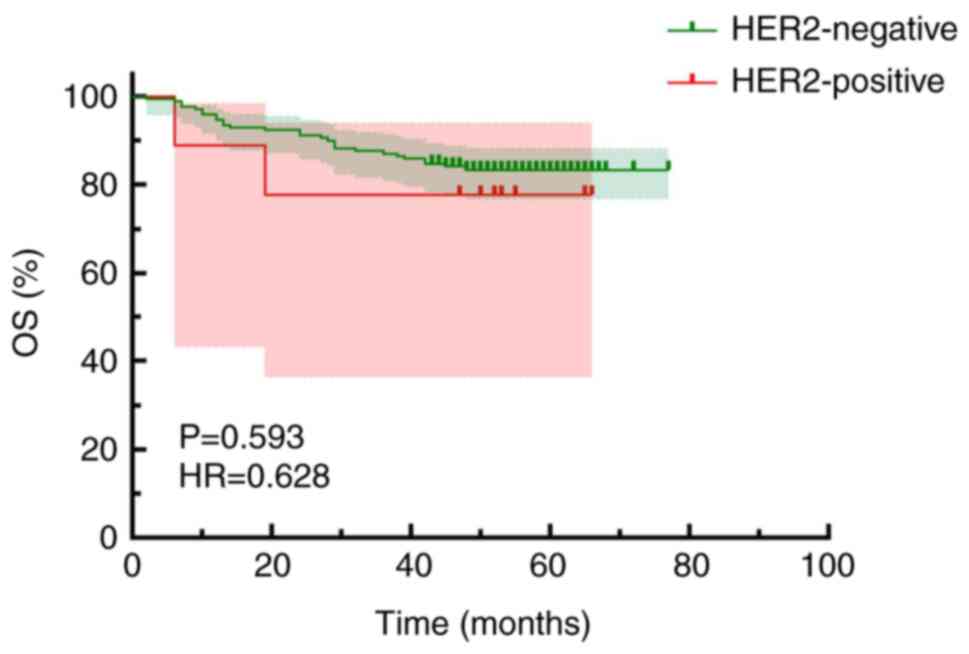
Kaplan-Meier survival curves comparing HER2 positivity and OS
indicated an OS time of 68.06±1.60 months for the HER2-negative
group and 54.11±7.48 months for the HER2-positive group, with no
statistically significant difference observed (OS; HR, 0.628; 95%
CI, 0.114–3.465; P=0.593; Fig.
7).
Association between HER2 and PD-L1
expression in cervical adenocarcinoma
The association between HER2 expression and PD-L1
expression in 37 cases of cervical adenocarcinoma with fatal
outcomes was analyzed (Table IV).
Among these cases, 21 (56.8%) exhibited PD-L1 expression. The data
suggested a statistically significant association between HER2
expression and increased levels of PD-L1 expression (χ2,
4.259; P=0.039). Furthermore, the overlap in HER2 and PD-L1
expression was found to be 40.5%. Specifically, among the 16
PD-L1-negative patients, 6 cases (37.5%) showed HER2 expression. By
contrast, among the 21 PD-L1-positive patients, 15 cases (71.4%)
showed HER2 expression. The proportion of HER2-positive patients in
the PD-L1-positive group was higher compared with that in the
PD-L1-negative group.
 | Table IV.Expression rates of PD-L1 and
HER2. |
Table IV.
Expression rates of PD-L1 and
HER2.
| PD-L1
expression | HER2 expression, n
(%) | HER2 zero
expression, n (%) | χ2 | P-value |
|---|
| Negative | 6 (37.5) | 10 (62.5) | 4.259 | 0.039 |
| Positive | 15 (71.4) | 6 (28.6) |
|
|
Multivariate analysis of factors
associated with disease progression in patients
In the multivariate Cox regression analysis of
factors influencing disease progression in 179 patients with
cervical adenocarcinoma after radical surgery, factors such as
LVSI, depth of invasion, tumor size, pre-treatment levels of CA19-9
and CA-125, HER2 expression, age, staging and lymph node metastasis
were included. These factors were selected as they are commonly
considered relevant in cancer prognosis and disease progression
(34). Patient prognosis was
significantly associated with LVSI (P=0.003), but not HER2
expression (P=0.401), age (P=0.520), depth of invasion (P=0.284),
tumor size (P=0.261) or the tumor markers CA19-9 (P=0.654) and
CA-125 (P=0.630) (Table V). The
confidence intervals for staging and lymph node metastasis were
excessively wide.
 | Table V.Multivariate analysis of factors
associated with disease progression in 179 patients after radical
surgery for cervical adenocarcinoma. |
Table V.
Multivariate analysis of factors
associated with disease progression in 179 patients after radical
surgery for cervical adenocarcinoma.
| Subgroup | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
≤60 | 1.000 |
|
|
>60 | 0.751
(0.313–1.799) | 0.520 |
| FIGO stage |
|
|
|
I–IIB | 1.000 |
|
|
III–IV | 1407.350
(0–1.006×1055) | 0.905 |
| Tumor size, cm |
|
|
| ≤4 | 1.000 |
|
|
>4 | 1.505
(0.738–3.068) | 0.261 |
| Vascular or
perineural invasion |
|
|
|
Negative | 1.000 |
|
|
Positive | 0.279
(0.12–0.651) | 0.003 |
| Depth of
invasion |
|
|
| Not
infiltrated into deep muscle layer | 1.000 |
|
|
Infiltration into the deep
muscle layer | 0.592
(0.227–1.544) | 0.284 |
| Pre-treatment
CA19-9 levels |
|
|
|
Normal | 1.000 |
|
|
Abnormal | 1.161
(0.604–2.231) | 0.654 |
| Pre-treatment
CA-125 levels |
|
|
|
Normal | 1.000 |
|
|
Abnormal | 1.187
(0.59–2.389) | 0.630 |
| HER2 expression
status |
|
|
|
Negative | 1.000 |
|
|
Positive | 0.752
(0.386–1.462) | 0.401 |
| Lymph node
metastasis |
|
|
|
Negative | 1.000 |
|
|
Positive | 0.000
(0–1.937×1048) | 0.893 |
Discussion
Although numerous studies have reported that HER2
expression may impact the prognosis of patients with various types
of cancer (35,36), data specific to cervical cancer
remains limited. According to the latest National Comprehensive
Cancer Network guidelines (version 1, 2024), HER2 IHC testing is
recommended for patients with advanced recurrent or metastatic
cervical cancer (37). However, a
standardized protocol for HER2 testing and interpretation in
cervical cancer has yet to be established. In gynecological tumors,
HER2 testing often references guidelines for breast cancer, which
report very low rates of high HER2 expression and gene
amplification in cervical cancer, with rates of 5.7 and 1.2%,
respectively (38). In the present
study, 179 patients with cervical adenocarcinoma were evaluated
using breast cancer guidelines, which identified only 9
HER2-positive cases. Based on the traditional combined IHC/FISH
classification, the HER2 positivity rate was 5%. Meanwhile, using
the ASCO/CAP gastric cancer guidelines, IHC alone has shown higher
rates of high HER2 expression in cervical cancer, at 27.0%
(38). In the present study, when
assessed by gastric cancer guidelines, IHC alone (1+ to 3+)
identified 72 cases of low HER2 expression (40.2%), with an overall
HER2 expression rate of 44.1%. In a study by Shi et al
(17), involving 209 patients with
cervical adenocarcinoma, 123 were identified as HER2-positive,
resulting in a positivity rate of 58.9%. Among these, 100 patients
(47.8%) exhibited low HER2 expression. The study also observed
higher HER2 expression in gastric-type adenocarcinoma compared with
ordinary adenocarcinoma, which was potentially associated with poor
prognosis. These findings were consistent with the present results.
Additionally, a previous study indicated that HER2 staining in
cervical adenocarcinoma frequently exhibits incomplete membrane
patterns (such as basolateral or U-shaped) in 12.2% of cases
(15/123) and intratumoral staining heterogeneity in 17.1% of cases
(21/123), resembling patterns observed in gastric and
gastroesophageal adenocarcinomas (17). Given these similarities, the HER2
assessment criteria that aligned with those used for gastric cancer
were adopted in the present study.
HER2, a receptor tyrosine kinase, exerts its effects
through several key biological pathways. First, the PI3K/AKT/mTOR
pathway serves a pivotal role in cell survival, proliferation and
metabolic regulation, with its aberrant activation frequently
observed in various types of cancer (39). The MAPK signaling pathway
contributes to tumor development and progression by promoting cell
proliferation, differentiation and survival (40). Additionally, the JAK2/STAT3 pathway
facilitates uncontrolled cancer cell growth, resistance to
apoptosis and regulation of cell survival. These pathways are not
only integral to tumor biology but also serve as potential
therapeutic targets (41). With
increasing focus on HER2 expression in cervical cancer, the use of
ADCs in metastatic HER2-expressing cervical cancer has emerged as a
key area of interest. Current clinical trial data on ADCs in
cervical cancer treatment shows promising outcomes for patients.
The open-label phase II study, DESTINY-PanTumor02 (42), aimed to evaluate the efficacy and
safety of trastuzumab deruxtecan (T-DXd; 5.4 mg/kg) as a
second-line treatment for HER2-expressing solid tumors. In the
cervical cancer cohort, 40 patients were enrolled and T-DXd
demonstrated significant antitumor activity, with an ORR of 50%.
Additionally, the RC48-C018 study included a cervical cancer cohort
of patients with HER2-expressing recurrent or metastatic disease
who had undergone at least one prior line of treatment. As of
October 2023, 25 patients with cervical cancer were enrolled, with
22 being evaluable for efficacy. The ORR was 36.4% (including 1
complete response), the disease control rate was 86.4%, the median
duration of response was 5.52 months and the median PFS time was
4.37 months (25). The advent of
ADCs represents a novel treatment strategy for cervical cancer,
making the detection of HER2 (especially IHC 1+ and 2+/FISH-)
particularly important.
In recent years, the emergence of immunotherapy has
brought new hope to some patients with advanced cervical cancer.
PD-L1 is a critical co-stimulatory molecule in immune responses and
exhibits a high expression rate in cervical cancer (43). This characteristic suggests that
PD-L1 may serve an important role in the immune regulation of
cervical cancer. Additionally, numerous studies have shown a
potential synergy between the expression levels of HER2 and PD-L1.
Oki et al (44) found that
in gastric cancer cells, HER2 expression levels were positively
correlated with PD-L1 expression levels. Among patients with HER2
3+ status, 72.4% demonstrated high levels of PD-L1 expression.
Furthermore, when HER2 expression was downregulated using siRNA,
PD-L1 protein levels were significantly reduced, indicating that
HER2 signaling pathways might directly or indirectly regulate PD-L1
expression. Similarly, Chaganty et al (45) proposed that a combination of
anti-HER2 therapy and anti-programmed cell death protein 1/PD-L1
therapy could be a potential strategy to improve therapeutic
outcomes in breast cancer. In the present study, data from 37
patients who died were analyzed. The results showed that among the
21 PD-L1-positive patients, 15 (71.4%) also demonstrated HER2
expression, indicating a high level of co-expression between the
two markers. Furthermore, the overlap in HER2 and PD-L1 expression
was found to be 40.5%, and the present results demonstrated a
statistically significant association between HER2 expression and
PD-L1 expression levels (χ2, 4.259; P=0.039), suggesting
a potential association between these two markers in the tumor
microenvironment. These findings provide an important basis for
further investigation into the synergistic effects of HER2 and
PD-L1 and their potential application in dual-targeted therapy.
This observation was consistent with previous studies in gastric,
esophageal and esophago-gastric cancer, which supported dual HER2
and PD-L1 targeting, showing clinical benefits (46,47).
Such evidence offers a foundation for more individualized treatment
strategies for patients with cervical cancer with dual HER2 and
PD-L1 expression.
Univariate analysis in the present study
demonstrated a significant association between HER2 expression in
patients with cervical adenocarcinoma and adverse
clinicopathological characteristics, such as advanced FIGO stage,
lymph node metastasis, presence of vascular cancer thrombus, nerve
invasion and abnormal CA-125 levels. Patients with HER2 expression
typically exhibited multiple poor prognostic factors, contributing
to an overall worse prognosis. The present study indicated HER2 to
be a potentially key biomarker for prognostic evaluation in
patients with cervical adenocarcinoma. However, the multivariate
analysis did not show consistent results. The absence of
statistical significance between HER2 expression and disease
progression may be attributed to insufficient sample size or
collinearity among variables, potentially masking its effect.
Although HER2 failed to demonstrate independent prognostic
significance, this does not exclude its potential role, and further
investigations with larger sample sizes are essential to validate
these findings. Numerous studies have demonstrated that adverse
clinicopathological factors significantly impact the prognosis of
patients with cervical cancer. These high-risk factors include
lymph node metastasis, vascular or perineural invasion and FIGO
staging (48,49). The present study suggests that HER2
expression may be associated with a poorer prognosis. Martinho
et al conducted a pathological study using IHC techniques to
analyze HER2 expression in cancer tissues from 229 patients with
cervical cancer, including 194 adenocarcinomas and 35 squamous
carcinomas (20). Univariate
analysis suggested a notable but non-significant trend toward an
association between HER2 positivity and poor prognosis (P=0.07).
Kaplan-Meier curve analysis further associated HER2 upregulation
with adverse prognosis (P=0.014). Multivariate analysis confirmed
metastasis and HER2 upregulation as independent prognostic factors
for patients with cervical cancer (20). In the present analysis, PFS time for
patients in the HER2 zero expression group was increased compared
with that of patients with HER2 expression, with an HR of 0.559,
indicating a ~44.1% reduction in the risk of disease progression or
death. The 95% CI (0.313–0.998) and a P-value of 0.049 further
supported the statistical significance of this observation.
In summary, patients with HER2 expression exhibited
worse PFS time compared with those in the HER2 zero expression
group. Moreover, OS time for patients in the HER2 zero expression
group appeared improved compared with that for patients with HER2
expression, with an HR of 0.656, indicating a potential 34.4%
reduction in mortality risk. However, the 95% CI (0.318–1.356) and
a non-significant P-value of 0.255 suggested uncertainty in these
results. Although the OS in HER2 zero expression shows a better
trend compared to HER2 expression, it did not reach statistical
significance, and longer-term studies may elucidate significant
differences. Therefore, HER2 status holds significant implications
for assessing disease progression and could potentially serve as a
pivotal biomarker for treatment and prognosis monitoring in
cervical adenocarcinoma.
The present study had several limitations that
warrant consideration. First, the analysis of HER2 expression in
the samples was conducted retrospectively. Due to the extended time
between surgery and sample collection, samples that originally
expressed HER2 may have lost the protein over time, leading to
insufficient staining. Therefore, the actual HER2 expression rate
may be higher. Second, due to the lack of established HER2
assessment guidelines specifically for cervical adenocarcinoma, the
present study, similar to others (42), referenced guidelines originally
developed for breast or gastric cancer. HER2 assessment standards
can differ significantly across such different cancer types
(50). Third, the present study was
limited by its retrospective design, and insufficient available
data and sample size, which made conducting sensitivity analyses
challenging and resulted in the absence of sensitivity analysis.
Larger-scale studies or clinical trials are needed to validate and
generalize these findings.
In summary, the present study evaluated HER2 IHC
expression in patients with cervical adenocarcinoma using gastric
cancer assessment criteria. HER2 expression was analyzed based on
both traditional and newly established standalone IHC
classification methods. The findings underscore the association
between HER2 expression and adverse prognostic factors in patients
undergoing radical surgery for cervical adenocarcinoma.
HER2-positive patients experienced poorer PFS times, emphasizing
the critical role of early HER2 status assessment in guiding
treatment decisions for cervical adenocarcinoma. Tumor patients
expressing HER2 who have died may also exhibit higher PD-L1
expression levels, and the combined expression of these markers
could help physicians develop personalized treatment strategies.
This approach holds promise for predicting patient outcomes and
optimizing therapeutic strategies to improve treatment
efficacy.
Future research will explore the pathological
characteristics of cervical cancer in greater depth, particularly
focusing on the association between HER2 expression and cervical
squamous cell carcinoma. This effort aims to develop tailored
treatment approaches that could ultimately enhance survival rates
and improve the quality of life for patients with cervical
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Project of Medicine and
Health Major Science and Technology Plan of Zhejiang Province
(grant no. WKJ-ZJ-2020).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QX, ZY, YL, XZ and JN were responsible for data
collection, processing, and interpretation. QX was responsible for
methodology, software, formal analysis and manuscript writing. ZY
partook in formal analysis and investigation. YL performed formal
analysis. XZ contributed to the study design and data analysis, as
well as to the processing of data images. JN contributed to study
conception and design, and reviewed and edited the manuscript. HL
undertook study conceptualization, resources, project
administration, funding acquisition and supervision. All authors
read and approved the final manuscript. QX and JN confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki with the approval of the Medical Ethics
Committee of Zhejiang Cancer Hospital (Hangzhou, China; approval
no. IRB-2023-656). Patients signed an informed consent form at
admission, which included consent for their samples to be used for
research.
Patient consent for publication
Patients provided written informed consent for
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
IHC
|
immunohistochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
|
PD-L1
|
programmed death-ligand 1
|
|
ADC
|
antibody-drug conjugate
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albadri ST and Salomão D: Metastatic
prostate adenocarcinoma to cervical lymph nodes: An unusual
diagnosis on fine-needle aspiration biopsy. J Am Soc Cytopathol.
10:231–238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saida T, Sakata A, Tanaka YO, Ochi H,
Ishiguro T, Sakai M, Takahashi H, Satoh T and Minami M: Clinical
and MRI characteristics of uterine cervical adenocarcinoma: Its
variants and mimics. Korean J Radiol. 20:364–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadducci A, Guerrieri ME and Cosio S:
Adenocarcinoma of the uterine cervix: Pathologic features,
treatment options, clinical outcome and prognostic variables. Crit
Rev Oncol Hematol. 135:103–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn MA, Benedet JL, Odicino F,
Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY and
Pecorelli S: Carcinoma of the cervix uteri. FIGO 26th Annual Report
on the results of treatment in gynecological cancer. Int J Gynaecol
Obstet. 95 (Suppl 1):S43–S103. 2006.
|
|
6
|
Xie Y, Kong W, Zhao X, Zhang H, Luo D and
Chen S: Immune checkpoint inhibitors in cervical cancer: Current
status and research progress. Front Oncol. 12:9848962022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu J, Fang T, Yun C, Liu X and Cai X:
Antibody-drug conjugates targeting the human epidermal growth
factor receptor family in cancers. Front Mol Biosci. 9:8478352022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhan N, Dong WG, Tang YF, Wang ZS and
Xiong CL: Analysis of HER2 gene amplification and protein
expression in esophageal squamous cell carcinoma. Med Oncol.
29:933–940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seshadri R, Firgaira FA, Horsfall DJ,
McCaul K, Setlur V and Kitchen P: Clinical significance of
HER-2/neu oncogene amplification in primary breast cancer. The
South Australian Breast Cancer Study Group. J Clin Oncol.
11:1936–1942. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jørgensen JT and Hersom M: HER2 as a
prognostic marker in gastric cancer-a systematic analysis of data
from the literature. J Cancer. 3:137–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ,
Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, et al: HER-2/neu
amplification is an independent prognostic factor in gastric
cancer. Dig Dis Sci. 51:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimozaki K, Fukuoka S, Ooki A and
Yamaguchi K: HER2-low gastric cancer: Is the subgroup targetable?
ESMO Open. 9:1036792024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harbeck N: Advances in targeting
HER2-positive breast cancer. Curr Opin Obstet Gynecol. 30:55–59.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kunos CA, Fabian D, Piecoro DW, Napier D,
Miller RW and Ueland FR: Human epidermal growth factor receptor 2
expression in women with uterine cervix adenocarcinoma from
Appalachian Kentucky. Front Oncol. 13:9483482023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Shao Y, Lu W and Lu B: An analysis
of HER2 amplification in cervical adenocarcinoma: Correlation with
clinical outcomes and the International Endocervical Adenocarcinoma
Criteria and Classification. J Pathol Clin Res. 7:86–95. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedman CF, D'Souza A, Bello Roufai D,
Tinker AV, de Miguel M, Gambardella V, Goldman J, Loi S, Melisko
ME, Oaknin A, et al: Targeting HER2-mutant metastatic cervical
cancer with neratinib: Final results from the phase 2 SUMMIT basket
trial. Gynecol Oncol. 181:162–169. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zhuo Y, Wang F, Li Z, Lin Y, Li L,
Pan J, Song Y, Du H, Li C and Xu Q: A metastatic cervical
adenocarcinoma patient carrying HER2 G292R achieved complete
response upon pyrotinib treatment. Onco Targets Ther. 14:4833–4836.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinho O, Silva-Oliveira R, Cury FP,
Barbosa AM, Granja S, Evangelista AF, Marques F, Miranda-Gonçalves
V, Cardoso-Carneiro D, de Paula FE, et al: HER family receptors are
important theranostic biomarkers for cervical cancer: Blocking
glucose metabolism enhances the therapeutic effect of HER
inhibitors. Theranostics. 7:717–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dang HZ, Yu Y and Jiao SC: Prognosis of
HER2 over-expressing gastric cancer patients with liver metastasis.
World J Gastroenterol. 18:2402–2407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, Su Y and Zhou L: Expression of HER2
and BRCA1 correlates with prognosis in patients with breast cancer
after radiotherapy: A Case-control study. Cancer Biother
Radiopharm. 37:603–611. 2022.PubMed/NCBI
|
|
23
|
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from ToGA: Targeting HER2 in gastric and
gastroesophageal junction cancer. Gastric Cancer. 18:476–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Gong J, Wang A, Wei J, Peng Z,
Wang X, Zhou J, Qi C, Liu D, Li J, et al: Disitamab vedotin (RC48)
plus toripalimab for HER2-expressing advanced gastric or
gastroesophageal junction and other solid tumours: A multicentre,
open label, dose escalation and expansion phase 1 trial.
EClinicalMedicine. 68:1024152024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lingying W, Li G, Zhang Y, An R, Sun L,
Zhang K, Huang Y, Guo R, Li Q, Miao J, et al: Evaluation of the
effectiveness and safety of disitamab vedotin For HER2-expressing
recurrent cervical cancer after progression on platinum-based
treatment-a single-arm, multicenter, open-label, phase II clinical
study. Int J Gynecological Cancer. 34 (Suppl 1):A6.1–A6. 2024.
|
|
26
|
Nicolò E, Boscolo Bielo L, Curigliano G
and Tarantino P: The HER2-low revolution in breast oncology: Steps
forward and emerging challenges. Ther Adv Med Oncol.
15:175883592311528422023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schildhaus HU: Immunohistochemistry-based
predictive biomarkers for lung cancer. Pathologe. 41:21–31.
2020.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swain SM, Miles D, Kim SB, Im YH, Im SA,
Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al:
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic
breast cancer (CLEOPATRA): End-of-study results from a
double-blind, randomised, placebo-controlled, phase 3 study. Lancet
Oncol. 21:519–530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim H and Chung JH: PD-L1 Testing in
Non-small cell lung cancer: Past, present, and future. J Pathol
Transl Med. 53:199–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang X, Ding Q, Guo H, Gong Y, Zhao J,
Zhao M, Sui D, Wu Y, Chen H, Liu H, et al: Comparison of three
FDA-approved diagnostic immunohistochemistry assays of PD-L1 in
triple-negative breast carcinoma. Hum Pathol. 108:42–50. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartley AN, Washington MK, Colasacco C,
Ventura CB, Ismaila N, Benson AB III, Carrato A, Gulley ML, Jain D,
Kakar S, et al: HER2 testing and clinical decision making in
gastroesophageal adenocarcinoma: Guideline from the college of
american pathologists, american society for clinical pathology, and
the american society of clinical oncology. J Clin Oncol.
35:446–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vakiani E: HER2 testing in gastric and
gastroesophageal adenocarcinomas. Adv Anat Pathol. 22:194–201.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoang MP, Sahin AA, Ordòñez NG and Sneige
N: HER-2/neu gene amplification compared with HER-2/neu protein
overexpression and interobserver reproducibility in invasive breast
carcinoma. Am J Clin Pathol. 113:852–859. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou J, Chen Y, Xu X, Yan D and Lou H:
Postoperative clinicopathological factors affecting cervical
adenocarcinoma: Stages I–IIB. Medicine (Baltimore). 97:e93232018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dieci MV, Miglietta F, Griguolo G and
Guarneri V: Biomarkers for HER2-positive metastatic breast cancer:
Beyond hormone receptors. Cancer Treat Rev. 88:1020642020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sawaki A, Ohashi Y, Omuro Y, Satoh T,
Hamamoto Y, Boku N, Miyata Y, Takiuchi H, Yamaguchi K, Sasaki Y, et
al: Efficacy of trastuzumab in Japanese patients with HER2-positive
advanced gastric or gastroesophageal junction cancer: A subgroup
analysis of the Trastuzumab for Gastric Cancer (ToGA) study.
Gastric Cancer. 15:313–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN Guidelines® Insights: Cervical cancer,
version 1.2024. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Itkin B, Garcia A, Straminsky S,
Adelchanow ED, Pereyra M, Haab GA and Bardach A: Prevalence of HER2
overexpression and amplification in cervical cancer: A systematic
review and meta-analysis. PLoS One. 16:e02579762021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang HP, Jiang RY, Zhu JY, Sun KN, Huang
Y, Zhou HH, Zheng YB and Wang XJ: PI3K/AKT/mTOR signaling pathway:
An important driver and therapeutic target in triple-negative
breast cancer. Breast Cancer. 31:539–551. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coussy F, El Botty R, Lavigne M, Gu C,
Fuhrmann L, Briaux A, de Koning L, Dahmani A, Montaudon E, Morisset
L, et al: Combination of PI3K and MEK inhibitors yields durable
remission in PDX models of PIK3CA-mutated metaplastic breast
cancers. J Hematol Oncol. 13:132020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maniaci A, Giurdanella G, Chiesa Estomba
C, Mauramati S, Bertolin A, Lionello M, Mayo-Yanez M, Rizzo PB,
Lechien JR and Lentini M: Personalized treatment strategies via
integration of gene expression biomarkers in molecular profiling of
laryngeal cancer. J Pers Med. 14:10482024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meric-Bernstam F, Makker V, Oaknin A, Oh
DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L,
Manzano A, et al: Efficacy and safety of trastuzumab deruxtecan in
patients With HER2-expressing solid tumors: Primary results from
the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 42:47–58.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Enwere EK, Kornaga EN, Dean M, Koulis TA,
Phan T, Kalantarian M, Köbel M, Ghatage P, Magliocco AM,
Lees-Miller SP and Doll CM: Expression of PD-L1 and presence of
CD8-positive T cells in pre-treatment specimens of locally advanced
cervical cancer. Mod Pathol. 30:577–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oki E, Okano S, Saeki H, Umemoto Y,
Teraishi K, Nakaji Y, Ando K, Zaitsu Y, Yamashita N, Sugiyama M, et
al: Protein expression of programmed Death 1 Ligand 1 and HER2 in
gastric carcinoma. Oncology. 93:387–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C,
Calin GA, Weiner LM and Fan Z: Trastuzumab upregulates PD-L1 as a
potential mechanism of trastuzumab resistance through engagement of
immune effector cells and stimulation of IFNγ secretion. Cancer
Lett. 430:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alsina M, Arrazubi V, Diez M and Tabernero
J: Current developments in gastric cancer: From molecular profiling
to treatment strategy. Nat Rev Gastroenterol Hepatol. 20:155–170.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Valkema MJ, Mostert B, Lagarde SM,
Wijnhoven BPL and van Lanschot JJB: The effectivity of targeted
therapy and immunotherapy in patients with advanced metastatic and
non-metastatic cancer of the esophagus and esophago-gastric
junction. Updates Surg. 75:313–323. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pinto PJJ, Chen MJ, Santos Neto E, Faloppa
CC, De Brot L, Guimaraes APG and Baiocchi G: Prognostic factors in
locally advanced cervical cancer with pelvic lymph node metastasis.
Int J Gynecol Cancer. 32:239–245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gai J, Wang X, Meng Y, Xu Z, Kou M and Liu
Y: Clinicopathological factors influencing the prognosis of
cervical cancer. J BUON. 24:291–295. 2019.PubMed/NCBI
|
|
50
|
Abrahao-Machado LF and Scapulatempo-Neto
C: HER2 testing in gastric cancer: An update. World J
Gastroenterol. 22:4619–4625. 2016. View Article : Google Scholar : PubMed/NCBI
|