Introduction
Papillary thyroid carcinoma (PTC) is the most common
subtype of thyroid cancer, accounting for ~90% of the major
histological types (1). The
widespread use of imaging techniques has led to a year-on-year
increase in the incidence of PTC among the general population of
the world. Although thyroid carcinoma has a relatively low
mortality rate compared with its incidence, due to its slow
progression and effective treatment options, PTC has the highest
relative survival rate among thyroid cancer types (1). Globally, the age-standardized
incidence of thyroid cancer in 2020 was 10.1 per 100,000 women and
3.1 per 100,000 men, with age-standardized mortality rates of 0.5
per 100,000 women and 0.3 per 100,000 men (2).
Castleman disease (CD), first described in 1954
(3), is a heterogeneous
lymphoproliferative disorder that commonly occurs in the neck,
mediastinum and abdomen (4,5). Based on lymph node involvement, CD can
be classified into unicentric CD (UCD) and multicentric CD (MCD).
UCD involves the enlargement of a single lymph node or multiple
lymph nodes within a single lymph node station, and most patients
are asymptomatic (5). By contrast,
MCD is a systemic, progressive and often fatal disease
characterized by widespread lymphadenopathy (6). Histologically, CD can be divided into
three types: Hyaline vascular (HV), plasma cell (PC) and mixed
variant (MV), with the latter encompassing features of both HV and
PC (7). Most UCD cases are
histologically classified as the HV type (5).
PTC frequently metastasizes to the cervical lymph
nodes. In previous studies, it was shown that lateral cervical
lymph node metastasis was identified in 3,915 (20.9%) of 18,741
patients with papillary thyroid carcinoma (PTC) (8). Therefore, when PTC presents with
lateral cervical masses, the possibility of tumor metastasis should
be considered. However, mediastinal masses, which can be benign or
malignant, require differential diagnosis. Primary mediastinal
tumors, such as congenital, inflammatory, neurogenic tumors,
thymomas and benign cysts account for ~60% of surgically resected
lesions. Lymphomas, teratomas and granulomatous diseases comprise
for another 30%, while vascular lesions, typically aortic
aneurysms, account for 10% in non-surgical series (9). Rare diseases such as CD and
schwannomas should also be considered. The case presented in the
current study initially suggested PTC with metastasis to different
lymph nodes, but the patient was ultimately diagnosed as PTC
combined with contralateral UCD, a rare occurrence. The present
report aims to improve understanding, reduce the missed or
incorrect diagnosis of CD, and enhance patient survival and
treatment outcomes.
Case report
In May 2023, a 28-year-old Asian woman underwent a
medical examination at Weixin County People's Hospital (Zhaotong,
China). Her primary care physician's initial B-ultrasound of the
thyroid revealed a solid hypoechoic nodule in the right thyroid
lobe (class 4a) measuring 1.1×0.8 cm2. The patient was
initially monitored with follow-up observation and no further
treatment. In October 2023, the patient came to the Affiliated
Hospital of Southwest Medical University (Luzhou, China) for
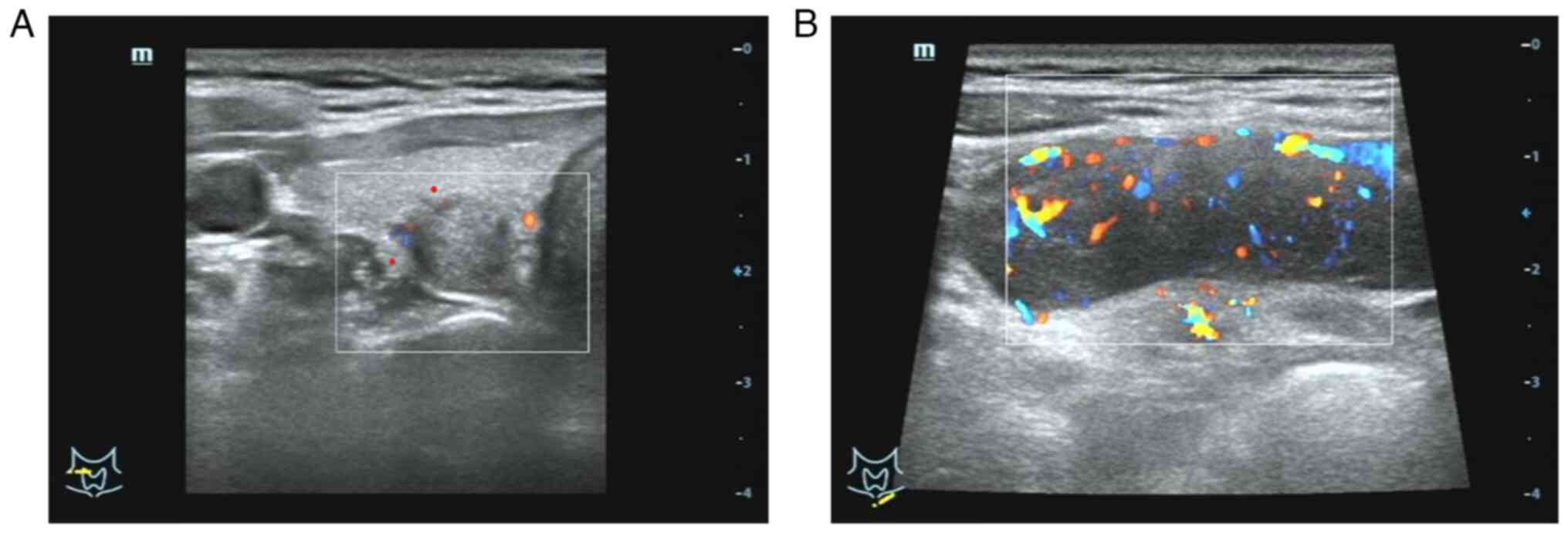
further evaluation and treatment. A thyroid B-ultrasound performed
in the thyroid surgery clinic showed a slightly hypoechoic mass in
the middle and upper part of the right thyroid lobe, measuring
~1.17×0.95 cm2. The mass was located close to the
capsule, with an aspect ratio >1, irregular shape, ill-defined
boundaries, a solid internal structure and strong punctate echo
(Fig. 1A). The left thyroid lobe
and isthmus showed no apparent lesions. Multiple low-echo areas
were found in the right neck region IV, with a regular shape and
clear boundaries, the largest measuring 0.5×0.3 cm2, and
in region VI, the largest measuring 0.8×0.6 cm2. A
4.6×1.9 cm2 hypoechoic mass was detected in the left
supraclavicular fossa (Fig. 1B),
showing a regular shape, clear boundaries and poorly defined
cortico-medullary boundaries. Clinical examination revealed an
asymptomatic, enlarged, painless and fixed mass in the left
supraclavicular fossa. Fine-needle aspiration under ultrasound
guidance showed the following: A right thyroid nodule; Bethesda
System for Reporting Thyroid Cytopathology (10) grade VI; papillary thyroid carcinoma;
and a left supraclavicular mass. Microscopic evaluation showed
numerous lymphocytes. A chest computed tomography (CT) scan showed
tracheal diverticulum with no significant findings. Nasal
fibrolaryngoscopy showed chronic pharyngitis and vocal nodules. The
patient had no significant medical, surgical or social history, no
known exposure to infection, and routine blood tests showed no
significant abnormalities in liver and kidney function.
Based on preoperative findings, the initial
diagnosis was papillary thyroid cancer with left supraclavicular
lymph node metastasis. A preliminary plan was made for right
lobectomy and isthmus thyroidectomy with central lymph node
dissection and bilateral cervical lymph node exploration.
Intraoperative frozen section results showed papillary carcinoma
metastasis in the right cervical lymph nodes (2/18). No papillary
carcinoma metastasis was detected in the left cervical lymph nodes.
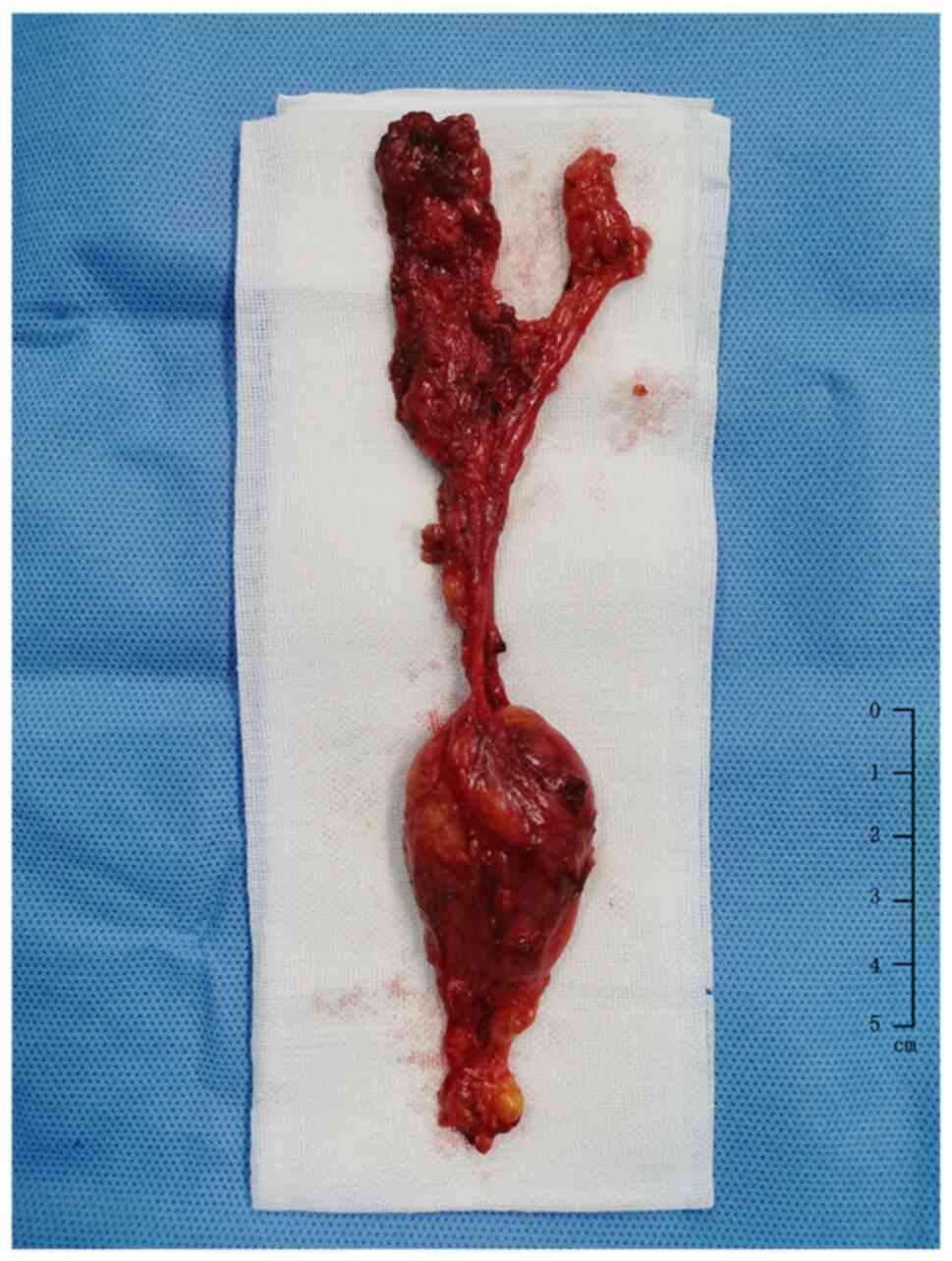
A complete capsular mass (Fig. 2)
was found in the left supraclavicular fossa, suggestive of CD.
Following standard thyroid cancer treatment
guidelines (11), the procedure was
expanded to a total thyroidectomy and right cervical lymph node
dissection. The patient experienced no significant complications
postoperatively and was discharged on postoperative day 5.
Postoperative pathology revealed papillary carcinoma in the right
lobe of the thyroid. The tumor cells were arranged in a papillary,
follicular structure. The tumor nuclei were enlarged and
overlapped, round or oval, ground glass like, the nuclear membrane
thickened, the karyotype was irregular and nuclear furrows were
visible. Obvious interstitial hyperplasia and sclerosis were
detected (Fig. 3A), measuring
0.6×0.4×0.4 cm3, with capsular invasion but no vascular
or nerve invasion. No malignancy was found in the left thyroid
lobe. Cancer metastasis was detected in the central lymph nodes: A
total of 6 lymph nodes were examined, with 2 showing cancer
metastasis (2/6). No cancer metastasis was found in the left
lateral cervical lymph nodes: A total of 15 lymph nodes were
examined, with 0 showing cancer metastasis (0/15). Cancer
metastasis was detected in the right lateral cervical lymph nodes:
A total of 30 lymph nodes were examined, with 2 showing cancer
metastasis (2/30). The left supraclavicular fossa showed a complete
capsular tubercle that is characteristic of lymphoproliferative
disease, and postoperative pathological examination showed that the
lymph node follicles were increased, dispersed, the sheath area was
widened, the germinal center was atrophic, and a sheath area
surrounded multiple germinal centers. The small lymphocytes in the
mantle area were widened, showing ‘onion skin’-like changes, the
interfollicular area and the germinal center showed hyalinous
vascular hyperplasia, and the endothelial cells in the germinal
center proliferated into a glassy shape. In addition, the small
blood vessels were vertically inserted into the atrophied germinal
center, resembling ‘lollipop’-like changes (Fig. 3B and C).
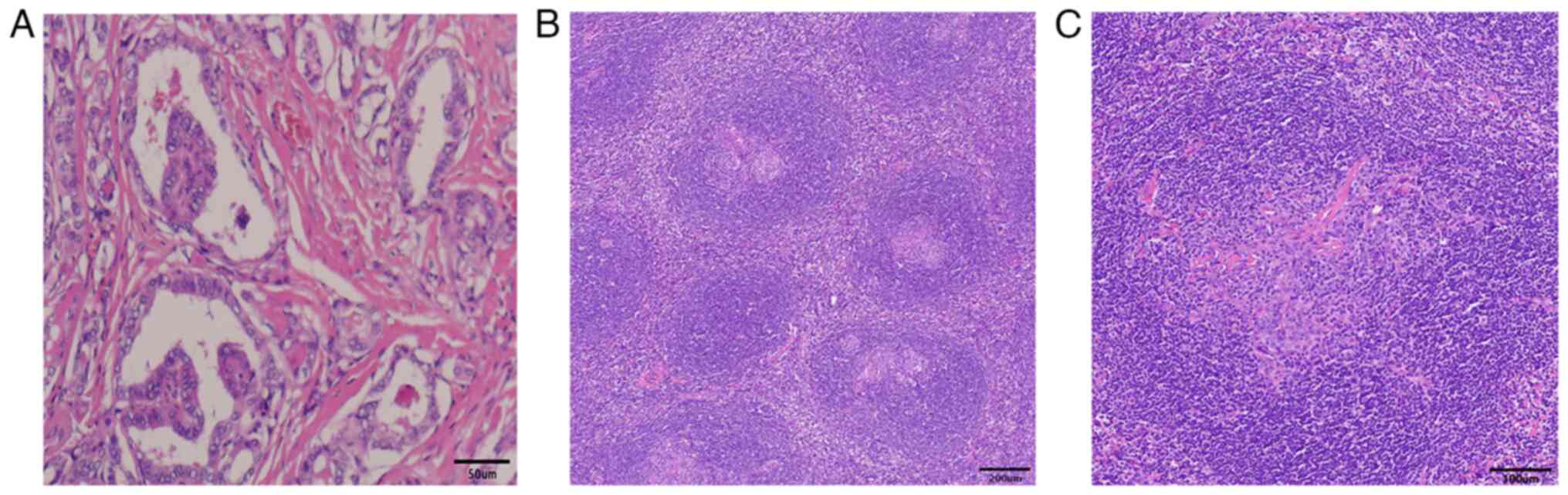 | Figure 3.Histopathological examination. (A) The
tumor cells are arranged in a papillary, follicular structure. The
tumor nuclei were enlarged and overlapped, round or oval, ground
glass-like, the nuclear membrane thickened, the karyotype was
irregular and nuclear furrows were visible. Obvious interstitial
hyperplasia and sclerosis (magnification, ×200; scale bar, 50 µm).
(B) The follicles of lymph nodes were increased, had a scattered
distribution, the sheath area was widened, the germinal center
atrophied and it was observed that one mantle area surrounded
multiple germinal centers. The small lymphocytes in the mantle area
were widened and showed ‘onion-skin’-like changes, and the
hyperplastic hyalinoid vessels were observed in the interfollicular
area and germinal center (magnification, ×40; scale bar, 200 µm).
(C) Vascular endothelial cells in the germinal center proliferated
and became glassy, and small blood vessels can be observed
inserting vertically into the atrophied germinal center, a
‘lollipop’-like change (magnification, ×100; scale bar, 100
µm). |
Positive immunohistochemical results of right
cervical lymph node supported metastatic papillary thyroid
carcinoma with thyroglobulin (Tg)(+) (Fig. 4A) and thyroid transcription factor 1
(TTF-1)(+) (Fig. 4B).
Immunophenotyping of the left supraclavicular mass suggested
mixed-type CD with cluster of differentiation 3 (T region +),
cluster of differentiation 20 (B region +), cluster of
differentiation 79a (B region +), cluster of differentiation 5 (T
region +), cluster of differentiation 10 (germinal center +), Bcl-2
(low expression in the germinal center, high expression outside)
(Fig. 4C), Bcl-6 (germinal center
+) (Fig. 4D), cluster of
differentiation 21 (follicular dendritic network), CyclinD1(−)
(Fig. 4E), cluster of
differentiation 38 (focally+) (Fig.
4F), Ki-67 (high expression in the germinal center, low
expression outside); in situ hybridization for Epstein-Barr
(EB) virus-encoded small RNA (EBER) was negative.
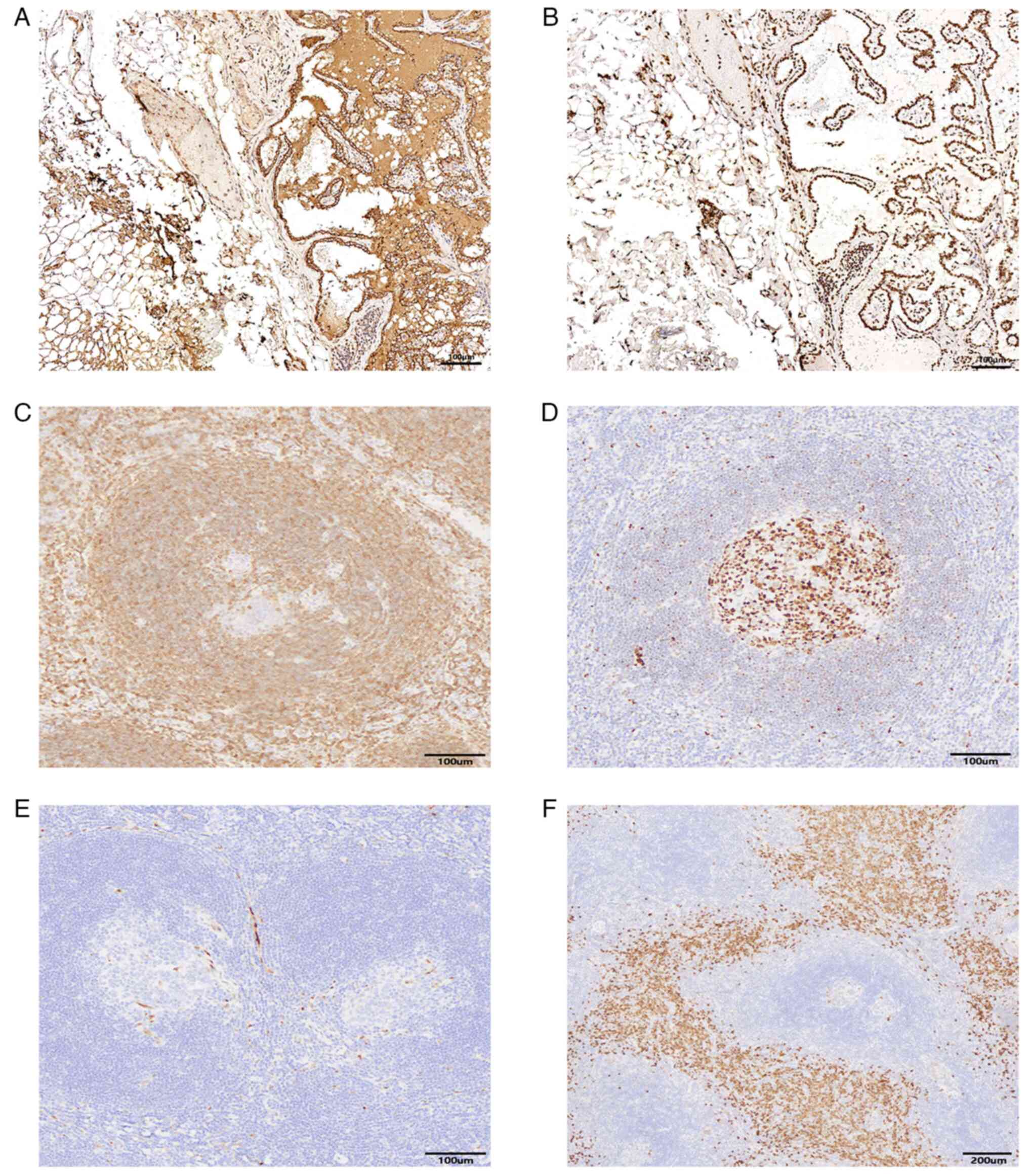 | Figure 4.Immunohistochemical results of right
cervical lymph node and Immunohistochemical results of left
supraclavicular fossa mass. Right cervical lymph node: (A)
Thyroglobulin immunohistochemical staining was positive
(magnification, ×100; scale bar, 100 µm); (B) Thyroid transcription
factor-1 immunohistochemical staining was positive (magnification,
×100; scale bar, 100 µm). Left supraclavicular fossa mass: (C)
BCL-2 immunohistochemical staining showed that the sheath area
widened significantly and atrophy of the germinal center
(magnification, ×100; scale bar, 100 µm); (D) BCL-6
immunohistochemical staining showed positive germinal center
staining (magnification, ×100; scale bar, 100 µm); (E) Cyclin D1
immunohistochemical staining was negative (magnification, ×100;
scale bar, 100 µm); (F) cluster of differentiation 38
immunohistochemical staining showed a large number of plasma cells
in the interfollicular region (magnification, ×40; scale bar, 200
µm). |
At 21 days postoperatively, the patient returned for
Iodine-131 radiotherapy. To rule out MCD, a comprehensive CT
examination of the abdomen, chest and pelvis was repeated, showing
no enlarged lymph nodes elsewhere, no abnormal symptoms and there
were no laboratory abnormalities.
Discussion
CD was first described in 1954 (3), and its prevalence remains low, with an
estimated annual incidence of 4,300 to 5,200 in the United States
(12). CD that coexists with PTC is
even rarer, and in cases of PTC with a neck mass, CD is often
overlooked. Initial assessments in the present case presumed PTC
metastasis; however, the contralateral mass did not support this,
ultimately leading to a diagnosis of PTC with contralateral UCD
based on pathology.
UCD can occur at any age, and opinions on sex
predilection differ. Some studies suggest no sex preference for UCD
(5,13), while others suggest a slight female
predominance (4,14). The average age of onset for MCD is
higher than that for UCD, with a higher male proportion (12). Among 65 patients diagnosed with CD
in the pathological database of Henan Provincial People's Hospital
(Zhengzhou, China), 60% (n=20) of patients with UCD were female,
while 65% (n=21) of patients with MCD were male, with mean onset
ages of 36.1±18.0 years (UCD) and 40.9±19.9 years (MCD) (14). From a review of 775 articles from
PubMed in 2017, 1,133 cases were extracted, and it was found that
58% of patients with UCD were female and 63% of patients with MCD
were male, with mean ages of 34±17 years (UCD) and 48±18 years
(MCD) (15). Both PTC and UCD are
more common in female patients, and cases of PTC combined with UCD
are rare. When PTC and UCD appear in female patients at the same
time, evaluating whether there are potential pathogenic factors is
worthy of further study.
UCD most commonly affects single lymph nodes or
lymph node regions in the mediastinum, neck, abdomen and
retroperitoneum (13). UCD can also
occur in rare locations, such as the orbit, lungs, kidneys,
nasopharynx and small intestine (16–20).
UCD is typically asymptomatic, and produce normal laboratory tests.
Severe complications such as paraneoplastic pemphigus (PNP),
polyneuropathy, pulmonary complications and autoimmune hemolytic
anemia may occur (21). By
contrast, MCD presents a broader range of clinical and laboratory
abnormalities (22). In a
retrospective study, the most common symptoms and findings in MCD
were shown as follows: i) Fever (78.8%); ii) inguinal
lymphadenopathy (83.3%); iii) hepatomegaly (74.1%); iv)
splenomegaly (90.3%); v) arthralgia (83.3%); vi) abdominal pain
(40%); vii) fatigue (33.3%); viii) diarrhea (100%); and elevated
serum C-reactive protein levels (63.4%) (22). The present case involved unicentric
CD in the left supraclavicular region, with no symptoms and normal
laboratory tests, and no complications observed post-surgery.
Preoperative diagnosis of CD is difficult.
Currently, international consensus guidelines for diagnosis and
treatment have been developed for idiopathic Multicentric CD (iMCD)
and UCD (23,24). CD can only be definitively diagnosed
by biopsy and microscopic examination (5). In addition, the guidelines recommend a
series of laboratory and radiological tests such as CT or PET-CT,
blood tests, bone marrow tests, inflammatory markers and organ
function determination tests, EBER and latency associated nuclear
antig-1 staining to exclude diseases with similar histopathological
features to UCD, such as MCD, lymphoma, infection, autoimmune
disease and primary or acquired immune deficiency (23). Preoperative imaging and fine-needle
aspiration cytology often lead to misdiagnosis as lymphoma or
autoimmune disease. However, imaging can assist in identifying
lesion location and number to differentiate UCD from MCD and rule
out other conditions. CD often shows well-defined, mildly hypodense
or isodense, homogeneous lymph nodules on nonenhanced CT/MRI, with
intermediate and marked enhancement on contrast-enhanced CT/MRI.
Calcification and hypertrophied vessels may be valuable diagnostic
features (25).
PC is the most common histological type in MCD, and
HV is common in UCD, and the MV is rare for both (4,5).
Analysis of 1,086 cases indicated that 77% of UCD cases were of the
HV type, 16% of the PC type and 7% mixed type, while 62% of MCD
cases were PC type, 20% HV type and 18% were of the mixed type
(15). The results of
immunohistochemistry also have certain significance for the
diagnosis of CD. For mixed-type CD, Bcl-2 staining shows low
expression in the germinal center and high expression outside the
germinal center; Bcl-6 staining shows positive staining in the
germinal center; and cluster of differentiation 38 staining shows
positive staining of interfollicular plasma cells (26). In the present case, the
immunohistochemical staining results of the left supraclavicular
mass were: i) Bcl-2 (low expression in the germinal center, high
expression outside); ii) Bcl-6 (germinal center +); and iii)
cluster of differentiation 38 (focally+), which were consistent
with the immunohistochemical results of mixed-type CD reported in
the literature (26,27).
In addition, the diagnosis of CD needs to be
differentiated, such as mantle cell lymphoma. Some articles have
shown that the Cyclin D1 immunohistochemical staining of mantle
cell lymphoma is positive (28).
However, the Cyclin D1 staining result in the present case was
negative, which can be used as evidence to exclude mantle cell
lymphoma.
Compared with CD, the diagnosis of PTC lymph node
metastasis is much easier. Among the immunohistochemical
indicators, TG and TTF-1 are more important. TG is a protein
secreted by thyroid follicular epithelial cells, and TTF-1 is a
transcription factor expressed in the thyroid follicular epithelium
and alveolar epithelium. When TG and TTF-1 are both positive, it
strongly suggests that the tumor originates from thyroid tissue. In
the present case, the immunohistochemical staining of TG and TTF-1
in the right cervical lymph node was positive, suggesting that the
tumor originated from the right papillary thyroid carcinoma. This
is consistent with the results reported in the previous literature
(29,30). At 21 days after surgery, abdominal,
chest and pelvic CT ruled out MCD, thereby confirming a diagnosis
of PTC with mixed UCD based on international guidelines. To date,
to the best of our knowledge, no similar case of PTC with mixed CD
involving heterogenous regions has been reported.
Complete surgical resection is typically curative
for UCD, making it the preferred first-line treatment (4,13,23).
For unresectable UCD, patients with an inflammatory syndrome, the
anti-interleukin-6 monoclonal antibody Siltuximab is recommended
(23). For unresectable UCD with
symptoms due to compression of nearby critical structures,
medication treatment (such as rituximab or steroids) can be
employed. After conservative treatment, if surgery becomes
feasible, surgical resection is preferred (23). For patients who remain unresectable
after conservative treatment, asymptomatic cases may be monitored,
while radiotherapy may be considered for symptomatic cases
(23). UCD treatment prognosis is
favorable, with most surgically resected patients experiencing good
survival and quality of life. In a retrospective study, it was
shown that 43 of 47 surgical patients achieved complete remission
post-surgery, while 11 out of 13 patients in the watchful waiting
group remained stable for up to 17 years, with an estimated 5-year
overall survival rate of 98.4% (21). However, patients with UCD have an
increased risk of developing PNP, a rare and fatal autoimmune
disease, which is considered an independent adverse prognostic
factor (31).
MCD treatment typically involves systemic therapy,
and surgery is generally not recommended. Patients MCD often have a
poorer prognosis than patients with UCD. A Japanese study of 342 CD
patients reported a median disease duration of 3.7 years, with
59.0, 40.6 and 20.1% surviving for more than 3, 5 and 10 years,
respectively (32).
The present case study involved PTC associated with
UCD. The patient underwent total thyroidectomy and resection of the
Castleman mass. The patient was followed up for >21 days
postoperatively without any significant discomfort or abnormal
findings. With no complications or HIV infection, a diagnosis of
UCD was favored, and further adjuvant therapy was deemed
unnecessary, with follow-up observation considered sufficient.
Based on this case, it is suggested that when imaging shows local
enlarged lymph nodes, clinicians should not only consider thyroid
cancer metastasis but also other possible causes of cervical lymph
node enlargement, including CD.
In conclusion, in future clinical practice, when
encountering PTC with mediastinal masses, clinicians should
consider not only common lymph node metastases but also the
possibility of CD. For the diagnosis of CD, postoperative
histopathology is a necessary condition for the diagnosis of CD,
but laboratory and imaging tests and clinical symptoms are still
important.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Southwest Medical University
University-Level Project (grant no. 2020ZRQNB052).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FW and HL obtained and analyzed the patient's
information and wrote the manuscript. RH, KC, JY, SL and XZ
obtained and analyzed the patient's information and reviewed the
discussion part of the clinical diagnosis and treatment. YL
provided the pathological images and made pathological diagnosis.
SL and XZ partially revised the article and generated the figures.
FW and XZ confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Southwest Medical University (LuZhou,
China; ethics approval no. KY2024265).
Patient consent for publication
Written informed consent to publish this case
information and accompanying images was obtained from the patient
and their family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitahara CM and Schneider AB: Epidemiology
of thyroid cancer. Cancer Epidemiol Biomarkers Prev. 31:1284–1297.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pizzato M, Li M, Vignat J, Laversanne M,
Singh D, La Vecchia C and Vaccarella S: The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN estimates for
incidence and mortality rates in 2020. Lancet Diabetes Endocrinol.
10:264–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castleman B and Towne VW: Case records of
the massachusetts general hospital: Case No. 40231. N Engl J Med.
250:1001–1005. 1954.PubMed/NCBI
|
|
4
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in Castleman's disease: A systematic review of 404
published cases. Ann Surg. 255:677–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone A, Borok M, Damania B, Gloghini A,
Polizzotto MN, Jayanthan RK, Fajgenbaum DC and Bower M: Castleman
disease. Nat Rev Dis Primers. 7:842021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oksenhendler E, Boutboul D, Fajgenbaum D,
Mirouse A, Fieschi C, Malphettes M, Vercellino L, Meignin V, Gérard
L and Galicier L: The full spectrum of Castleman disease: 273
Patients studied over 20 years. Br J Haematol. 180:206–216. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keller AR, Hochholzer L and Castleman B:
Hyaline-vascular and plasma-cell types of giant lymph node
hyperplasia of the mediastinum and other locations. Cancer.
29:670–683. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
So YK, Kim MJ, Kim S and Son YI: Lateral
lymph node metastasis in papillary thyroid carcinoma: A systematic
review and meta-analysis for prevalence, risk factors, and
location. Int J Surg. 50:94–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strollo DC, Rosado de Christenson ML and
Jett JR: Primary mediastinal tumors. Part 1: Tumors of the anterior
mediastinum. Chest. 112:511–522. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cibas ES and Ali SZ: The 2017 Bethesda
system for reporting thyroid cytopathology. Thyroid. 27:1341–1346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Filetti S, Durante C, Hartl D, Leboulleux
S, Locati LD, Newbold K, Papotti MG and Berruti A; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguideclinicalguidelines@esmo.org:
Thyroid cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up†. Ann Oncol. 30:1856–1883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpson D: Epidemiology of castleman
disease. Hematol Oncol Clin North Am. 32:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong RSM: Unicentric castleman disease.
Hematol Oncol Clin North Am. 32:65–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XQ, Zhong NN, Sun Q, Yan SC, Xu GC,
Wang YG, Peng LW, Liu B and Bu LL: Comprehensive analysis of 65
patients with Castleman disease in a single center in China. Sci
Rep. 12:86942022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haap M, Wiefels J, Horger M, Hoyer A and
Müssig K: Clinical, laboratory and imaging findings in Castleman's
disease-The subtype decides. Blood Rev. 32:225–234. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akram W, Degliuomini J, Wallack MK, Huang
S, Okechukwu E and Eric T: Unicentric Castleman's disease
masquerading as a carcinoid tumor of the small intestine. Am Surg.
82:e287–e289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang D, Lee J, Lee H and Baek S:
Unicentric Castleman's disease in the orbit: A case report. Indian
J Ophthalmol. 63:555–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai MH, Pai HH, Yen PT, Huang TS and Ho
YS: Nasopharyngeal Castleman's disease. J Formos Med Assoc.
95:877–880. 1996.PubMed/NCBI
|
|
19
|
Liu Y, Chen G, Qiu X, Xu S, Wu Y, Liu R,
Zhou Q and Chen J: Intrapulmonary unicentric Castleman disease
mimicking peripheral pulmonary malignancy. Thorac Cancer.
5:576–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang E, Li Y and Lang N: Case report:
Castleman's disease involving the renal sinus resembling renal cell
carcinoma. Front Surg. 9:10013502022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boutboul D, Fadlallah J, Chawki S, Fieschi
C, Malphettes M, Dossier A, Gérard L, Mordant P, Meignin V,
Oksenhendler E and Galicier L: Treatment and outcome of unicentric
Castleman disease: A retrospective analysis of 71 cases. Br J
Haematol. 186:269–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gündüz E, Kırkızlar HO, Ümit EG, Karaman
Gülsaran S, Özkocaman V, Özkalemkaş F, Candar Ö, Elverdi T,
Küçükyurt S, Paydaş S, et al: Castleman disease: A multicenter case
series from turkey. Turk J Haematol. 39:130–135. 2022.PubMed/NCBI
|
|
23
|
van Rhee F, Oksenhendler E, Srkalovic G,
Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S,
Streetly M, et al: International evidence-based consensus
diagnostic and treatment guidelines for unicentric Castleman
disease. Blood Adv. 4:6039–6050. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fajgenbaum DC, Uldrick TS, Bagg A, Frank
D, Wu D, Srkalovic G, Simpson D, Liu AY, Menke D, Chandrakasan S,
et al: International, evidence-based consensus diagnostic criteria
for HHV-8-negative/idiopathic multicentric Castleman disease.
Blood. 129:1646–1657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao S, Wan Y, Huang Z, Song B and Yu J:
Imaging and clinical features of Castleman Disease. Cancer Imaging.
19:532019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Cao F, Gong H, Liu P, Sun G and
Zhang W: Embolization of blood-supply artery followed by surgery
for treatment of mesorectal Castleman's disease: Case report and
literature review. Gastroenterol Rep (Oxf). 7:141–145. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Y, Shao S, Kang H, Xu Z, Wen G, Shan Y,
Gong Z, Al-Sharabi A, Qu B, Ren Y, et al: A unicentric center,
multicenter, and mixed-type Castleman disease: Three case reports
and a review of the literature. Medicine (Baltimore).
103:e377222024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jain P and Wang M: Mantle cell lymphoma:
2019 Update on the diagnosis, pathogenesis, prognostication, and
management. Am J Hematol. 94:710–725. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sathiyamoorthy S and Maleki Z:
Cytomorphologic overlap of differentiated thyroid carcinoma and
lung adenocarcinoma and diagnostic value of TTF-1 and TGB on
cytologic material. Diagn Cytopathol. 42:5–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kreft A, Hansen T and Kirkpatrick CJ:
Thyroid transcription factor 1 expression in cystic lesions of the
neck: An immunohistochemical investigation of thyroglossal duct
cysts, branchial cleft cysts and metastatic papillary thyroid
cancer. Virchows Arch. 447:9–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua Y, Liang C, Yang J, Wang L, Xu A, Xi
L, Wang S and Wang Z: Clinical characteristics and prognosis of
patients with Castleman disease in a Chinese hospital:
Paraneoplastic pemphigus is an independent risk factor. Am J Transl
Res. 14:1051–1059. 2022.PubMed/NCBI
|
|
32
|
Murakami M, Johkoh T, Hayashi S, Ohshima
S, Mizuki M, Nakatsuka SI, Tomobe M, Kuroyanagi K, Nakasone A and
Nishimoto N: Clinicopathologic characteristics of 342 patients with
multicentric Castleman disease in Japan. Mod Rheumatol. 30:843–851.
2020. View Article : Google Scholar : PubMed/NCBI
|