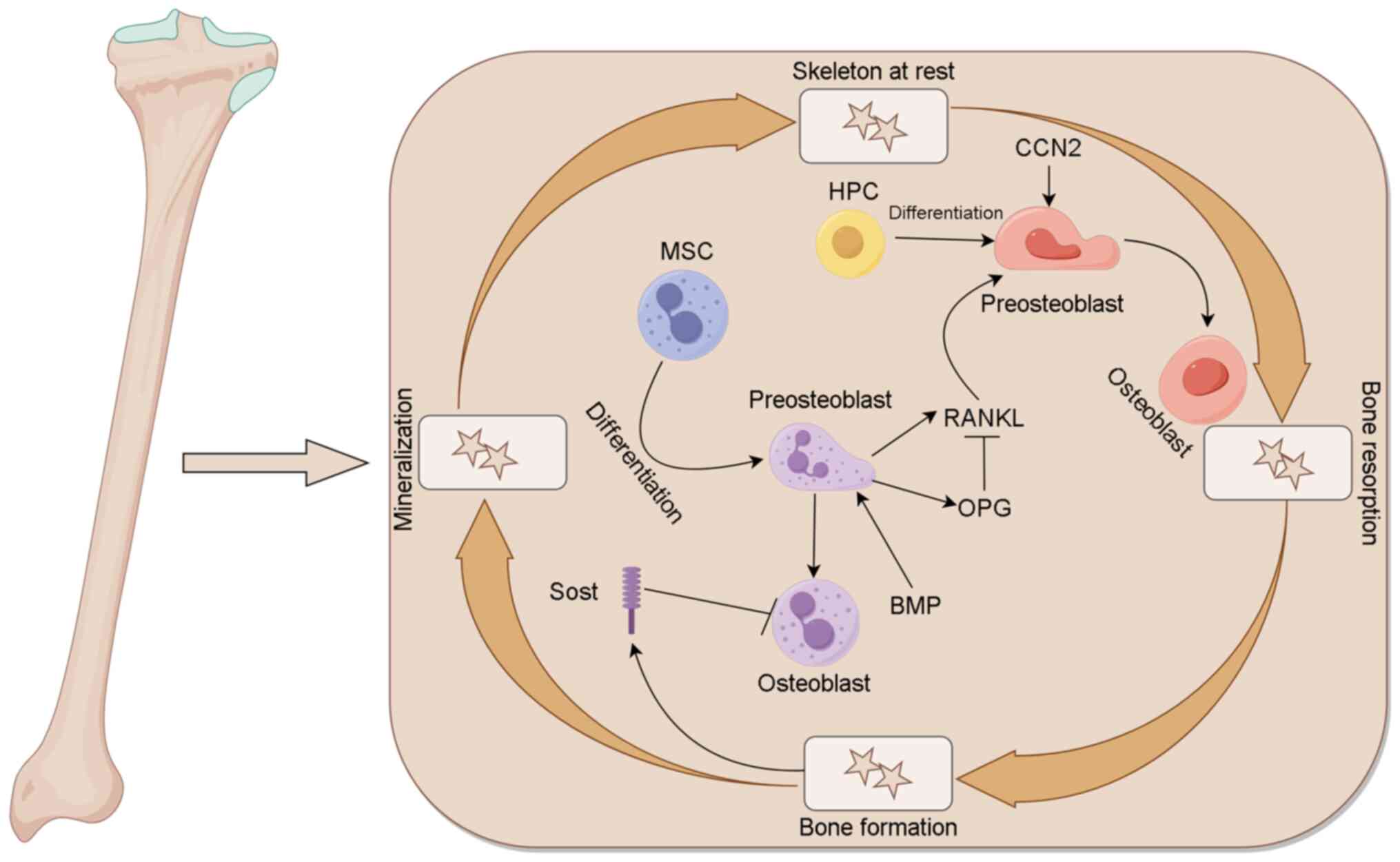
The dynamic bone remodeling cycle begins when
osteoclast precursors are attracted to sites of bone damage or
aging in response to chemokine signaling (9). These precursors then differentiate and
fuse to form multinucleated osteoclasts, which play an osteolytic
role. The binding of receptor activator of nuclear factor-κB (RANK)
with RANK ligand (RANKL) promotes the fusion, differentiation and
maturation of osteoclast precursors (10). RANK is expressed on the cell surface
of osteoclasts and their precursors, while RANKL is produced
primarily by osteocytes, osteoblasts, bone marrow stromal cells and
activated T cells (11).
Osteoprotegerin (OPG) also binds to RANKL, which prevents RANKL
from binding to RANK, thereby blocking RANKL signaling and
regulating osteoclastogenesis and osteolysis (12). In addition, Nozawa et al
(13) demonstrated that cellular
communication network factor 2 (CCN2), also known as a connective
tissue growth factor, induces osteoclast formation through
interaction with integrin αvβ3. CCN2 also regulates dynamic bone
remodeling by participation in the RANK-RANKL-OPG system (14). It has been suggested that bone
morphogenetic protein 9 (BMP9) can inhibit bone metastasis in
patients with breast cancer by downregulating CCN2 (15). Following osteoclast differentiation
and maturation, cytoskeletal changes lead to the formation of a
ruffled border. The osteoclasts then secrete proteolytic enzymes
and hydrochloric acid through the ruffled border to facilitate
osteolysis (15).
Following osteolysis, osteoclasts leave the bone
surface and undergo programmed cell death, specifically apoptosis,
which signals the beginning of bone formation (16). Osteoblast precursors are attracted
to the site of osteolysis. This process is regulated by the
osteoblast precursor transcription factors, including core-binding
factor subunit a-1, also known as Runt-related transcription factor
2 (RUNX2), and osterix, which bind to osteoblast-specific gene
enhancers to promote the development of an osteoblast-like
phenotype (17). In addition, BMPs
promote the proliferation and differentiation of osteoblast
precursors (18), and Wnt family
proteins promote bone formation via the activation of low-density
lipoprotein receptor-related protein 5 (LRP5), RUNX2 and osterix
(19). However, sclerostin (Sost)
produced by osteocytes inhibits bone formation by antagonizing the
effects of Wnt proteins (20).
Mature osteoblasts secrete noncalcified bone matrix onto the bone
surface, which subsequently mineralizes to form mature bone. During
this process, some osteoblasts are trapped by mineralized bone and
differentiate into osteocytes. Osteocytes are linked together by
elongated cytoplasmic extensions, which allows them to transmit
signals, such as mechanical loading signals, via nitric oxide and
prostaglandin signaling molecules (21).
Dynamic bone remodeling continues when bone
formation is complete. Various factors influence this dynamic
process, which can be classified into two categories: i) Chemical
factors or hormones and ii) physical factors (22). Inflammatory factors such as IL-1 and
tumor necrosis factor accelerate the remodeling cycle. Hormones
that regulate calcium levels, such as 1,25-dihydroxyvitamin D and
parathyroid hormone, promote bone remodeling and mobilize bone
calcium to maintain blood calcium levels (23). While thyroid and growth hormones
promote bone remodeling (24),
estrogens and androgens inhibit bone resorption and promote bone
formation, particularly in the trabecular and endocortical skeletal
compartments (25). These factors
regulate bone remodeling primarily by modulating the RANK-RANKL-OPG
signaling pathway (26). Notably,
mechanical loading affects bone remodeling; for example, increased
mechanical loading increases bone formation and reduces
osteolysis.
Bone metastasis has two main phenotypes: Osteolytic
and osteogenic. Most bone metastases exhibit both osteolytic and
osteogenic characteristics, with one phenotype being dominant. For
example, breast and lung cancers are frequently osteolytic, while
prostate cancer is frequently osteoblastic. Osteolytic lesions are
distinguished primarily by bone destruction, which generally
appears as cortical cavitation when analyzed using radiographic
imaging. By contrast, osteoblastic lesions are distinguished by the
excessive formation of new bone, which results in increased bone
density on imaging, frequently described as osteosclerosis on the
bone surface (2).
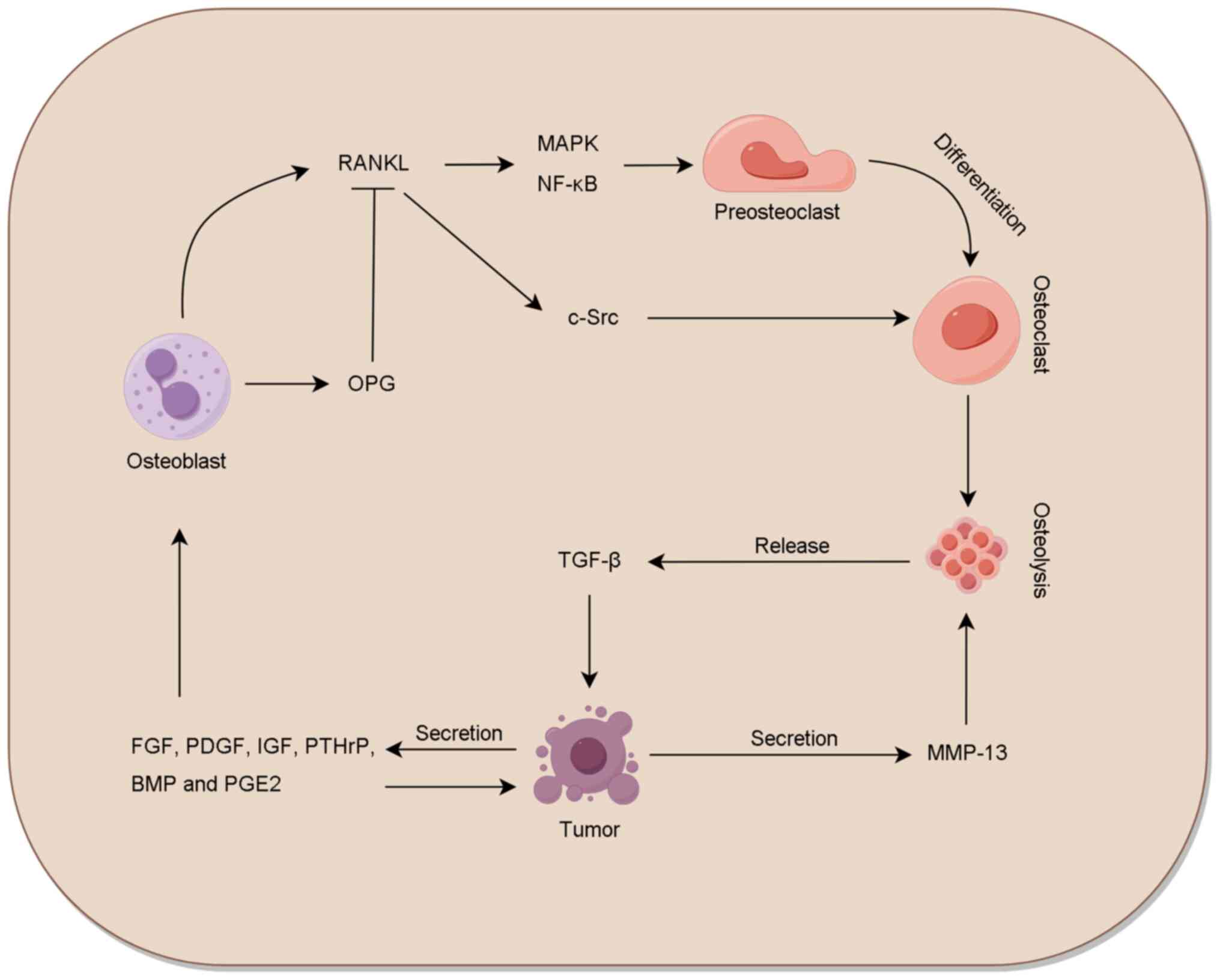
Bone remodeling involves a variety of cytokines,
growth factors and cell adhesion molecules, which makes bone an
attractive location for metastatic tumor cells (Fig. 2). The epiphysis, with its rich blood
supply and trabecular bone structure, provides an ideal environment
for the survival of bone metastatic cells (27). The slow blood flow in sinusoid
vessels further facilitates the colonization of the bone marrow by
hematopoietic stem cells and invasive tumor cells. Additionally,
endothelial cells in sinusoidal vessels express a variety of
adhesion molecules, including E-selectin, P-selectin, intracellular
adhesion molecules and vascular cell adhesion molecules (VCAMs),
which promote the homing of tumor cells to the bone marrow
(28–30). Following tumor colonization of the
bone, the bone microenvironment facilitates tumor growth and
invasion. Various resident and transient cells, including stromal
cells, osteoblasts and immune cells, affect tumor survival. Stromal
cells, which originate from mesenchymal cells within the bone
marrow and include adipocytes, fibroblasts and osteoblasts, promote
tumor cell proliferation and differentiation via the secretion of
VCAMs, syndecan and matrix metalloproteinase 2 (MMP-2) (31). Osteoclast-mediated osteolysis
further promotes tumor growth by releasing growth factors from the
bone, which increase tumor cell proliferation and osteolysis.
Transient cells, including red blood cells, platelets, and T cells,
also promote tumor growth and metastasis via a variety of pathways
and molecular interactions (28).
The RANK-RANKL-OPG system plays a crucial role in
the promotion of cancer cell proliferation, epithelial-mesenchymal
transition (EMT) and bone metastasis (32,33).
Tumor cells secrete various cytokines within the bone
microenvironment that affect the RANK-RANKL-OPG system (34). Tumor-derived parathyroid
hormone-related protein (PTHrP), insulin-like growth factor 1
(IGF-1), fibroblast growth factor (FGF) and platelet-derived growth
factor not only increase tumor cell growth in an autocrine manner
but also promote the production and release of RANKL by osteoblasts
and stromal cells (35). In
addition, tumor-derived PTHrP, IL-1, prostaglandin E2, BMP and
epithelial growth factor downregulate OPG expression in the stroma
and osteoblasts (36). Accordingly,
RANKL levels increase and OPG levels decrease in the tumor bone
microenvironment, which disrupts the dynamic balance of bone
remodeling. The binding of RANKL to RANK promotes the fusion,
differentiation and maturation of osteoclast precursors through
mitogen-activated protein kinase (MAPK) and nuclear factor-κB
signaling pathways, and enhances osteolysis through c-Src signaling
(37,38). In addition, PTHrP stimulates the
secretion of MMP-13 via the protein kinase C (PKC)-ERK signaling
pathway, and MMP-13 contributes to bone degradation and bone
fractures (39).
TGF-β acts as a suppressor in the early stages of
tumorigenesis but promotes tumor progression in later stages
(40). Bone metastasis occurs at an
advanced stage of cancer, and TGF-β plays a key role in bone
metastasis and the promotion of tumor development. TGF-β is
primarily released into the bone microenvironment by osteolysis,
and mediates the EMT, invasion, angiogenesis and immunosuppression
of tumor cells via TGF-β/Smad signaling (41,42).
Integrin αvβ3 has been demonstrated to be required for the
TGF-β/Smad signaling that triggers breast cancer metastasis
(43), but the underlying mechanism
remains to be further elucidated. Tumor cells undergoing EMT
experience cytoskeletal rearrangement and loss of intercellular
adhesion, ultimately increasing their invasion, motility and
metastatic potential. Preclinical experiments have shown that TGF-β
inhibitors have the ability to inhibit bone metastases in animal
models of breast cancer, but their effectiveness is limited in lung
cancer (44). These findings
suggest that the mechanism of TGF-β in bone metastases varies
according to the primary tumor type, highlighting that further
studies are necessary to classify primary tumors based on their
mechanism of metastasis.
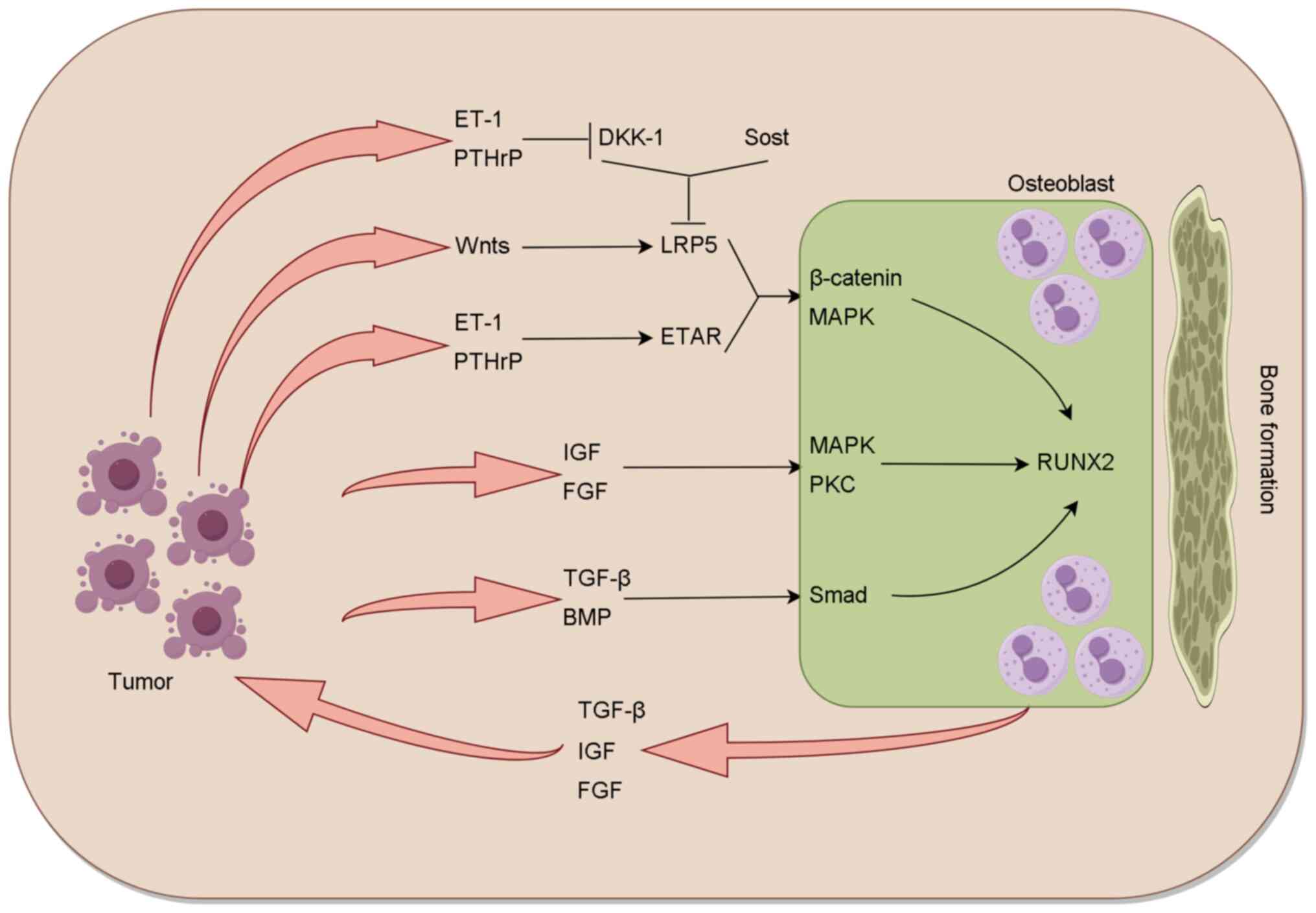
Tumor cells interact with osteoblasts, leading to
the production of TGF-β, BMP, IGF and FGF. This interaction also
promotes Wnt signaling, which induces osteogenic activity (Fig. 3). TGF-β and BMP activate Smad
signaling in osteoblasts, while growth factors activate MAPK and
PKC signaling in osteoclasts. In addition, Wnt activates the
β-catenin regulatory signaling pathway. These pathways converge and
interact with the RUNX2 transcriptional network to induce
osteoblast differentiation and proliferation. Research has shown
that the Wnt/β-catenin pathway is crucial for osteoblast function
and bone formation, with activation of this pathway stimulating
osteoblast development and promoting bone formation (45). Osteocytes secrete Sost, which binds
to LRP5, thereby blocking the Wnt-LRP5-β-catenin pathway and
inhibiting bone formation. The serum level of Sost is upregulated
in patients with multiple myeloma, as myeloma cells secrete Sost;
therefore, patients with multiple myeloma frequently present with
osteolytic lesions. In vitro data suggest that breast cancer
cells induce Sost expression to inhibit bone metastasis and
osteogenesis; however, in prostate cancer, Sost expression is
downregulated while BMP-6 expression is upregulated (46).
Downregulation of the RANKL-to-OPG ratio in the
RANK-RANKL-OPG system can inhibit osteolysis and lead to an
osteogenic phenotype. In prostate cancer, PSA has been shown to
inhibit osteolytic activity by inhibiting the expression of RANKL
in osteoblasts and promoting the function of osteoblasts (54). In addition, the expression levels of
RANK and RANKL in tumor tissue from patients with metastatic
prostate cancer are significantly higher compared with those in
patients with local disease (55).
Notably, RANKL-induced osteolysis appears to facilitate the
colonization of osteoblastic bone metastatic tumor cells (56). The ability of RANKL to promote the
intraosseous growth of prostate cancer cells has been found to be
associated with IGF signaling and hypoxia-inducible factors, which
create a bone microenvironment favorable for tumor growth (57).
In general, the mechanisms underlying the behavior
of cancer cells in bone metastasis are highly complex and largely
unknown, despite decades of research. Additional research, as well
as effective and suitable animal models, are required to elucidate
the specific mechanisms and identify novel therapeutic targets.
Cancer is the second leading cause of human death
worldwide, after cardiovascular diseases, with metastases typically
being the cause of mortality and bone being the most prevalent site
of metastasis (58). Bone
metastases are common among patients with breast, prostate or lung
cancer, and may also occur in patients with other tumors, including
myeloma, renal and thyroid cancers, Ewing's sarcoma and lymphoma
(59,60). The general pathogenesis of bone
metastasis involves several stages, including primary tumor
proliferation, local tissue invasion, intravascular invasion,
extravasation into the bone marrow, tumor cell dormancy,
intraosseous proliferation and changes in the intraosseous
microenvironment. In 1889, Stephen Paget proposed the ‘seed and
soil’ hypothesis (61). This
hypothesis proposes that the interaction between cancer cells and
the organ microenvironment influences cancer cell proliferation,
survival and expansion, and that the ability of the cancer cells to
recruit a blood supply determines whether the cancer cells will
metastasize. Understanding the molecular pathways involved in
cancer metastasis is crucial for preventing the formation and
growth of bone metastases. Animal models of bone metastasis are
essential for investigating these molecular pathways. An ideal
animal model should be clinically relevant, mimic human disease,
and reproducible. However, each model has both advantages and
disadvantages, and no single model is perfect. Researchers must
choose the most appropriate model based on the specific research
question.
The injection techniques that are commonly used to
study bone metastasis include tail vein, intracardiac,
intraosseous, orthotopic, subcutaneous and tail artery injections.
Each type of bone metastasis model offers unique benefits and
limitations. Therefore, it is critical to choose a model that is
suitable for the specific research direction and purpose.
Tail vein injection is a relatively simple method of
injection that is primarily used in studies of tumor blood
circulation and lung metastasis. This injection method rarely leads
to bone metastasis but more frequently leads to lung metastasis
(62).
Intracardiac inoculation typically involves
injecting tumor cells into the left ventricle of mice, allowing the
cells to circulate systemically and spread to the bone to establish
bone metastases. This type of model has a high tumorigenicity and a
short modeling time, allowing the distribution of tumor cells to be
monitored in real-time using bioluminescence imaging when
fluorescently labeled tumor cells are injected. It is the most
frequently used animal model of bone metastasis. However, the
successful construction of this model is challenging. In addition,
as tumor cells spread throughout the body via the systemic
circulation, multiple metastases can develop, sometimes leading to
fatal non-bone metastases before bone metastasis occurs, which may
complicate the specific study of bone metastasis (63).
Intraosseous injection involves directly injecting
tumor cells into the femur or tibia, leading to tumor formation in
the bone. Compared with left ventricular injection, intraosseous
injection results in a higher incidence of bone metastasis and a
shorter experimental period. However, it also disrupts the
integrity of the bone, which makes it less suitable for studying
the molecular mechanism of bone metastasis (64). In addition, as it does not involve
metastasis, with the movement of cells from a primary site to the
bone, this model is not strictly a model of metastasis. However, it
can be used for the investigation of tumor-bone interactions.
Orthotopic inoculation is a surgical method in which
tumor cells or tumor masses are directly inoculated into the organs
of mice, such as the prostate and mammary glands. Compared with the
spontaneous canine bone metastasis model, this method has greater
tumorigenicity and shorter latency while retaining the biological
characteristics of tumor cells. It can effectively simulate the
whole process of tumor metastasis from the primary site to the
bone. However, in numerous cases, mice in this model develop lung
and lymph node metastases before bone metastasis, and succumb to
other causes before bone metastases can develop.
Subcutaneous inoculation is a method of injecting
tumor cells or tumor fragments into the skin of mice to form
tumors. This method is simple and easy to manage; however, it
rarely causes bone metastasis, even when using cells derived from
the bone metastases of patients (65). Currently, subcutaneous tumor models
are primarily derived from patient samples and are used to evaluate
the inhibitory effects of antitumor drugs against human tumors
(66).
To date, dogs are the only nonrodent animals known
to develop spontaneous prostate cancer. However, their use in
research is challenging due to difficulties in experimental
control, the low incidence of spontaneous tumor formation and bone
metastasis, and the high cost (72). Lobund-Wistar rats, ACI/segHapBR
rats, C57BL/6 mice, BALB/c nude mice, severe combined
immunodeficiency mice and various transgenic mouse models have been
used to investigate bone metastasis in prostate cancer (73–75).
However, bone metastasis in rat prostate cancer models is very
rare. Due to their short experimental period, low economic cost and
research convenience, mice have become the most commonly used
animals for studying prostate cancer bone metastasis.
One of the most commonly used prostate cancer cell
lines is PC3, which was isolated in 1979 from a bone metastatic
lesion in a 62-year-old White male patient with grade IV prostate
cancer (76). This cell line has
high metastatic potential and exhibits characteristics more typical
of neuroendocrine carcinoma than adenocarcinoma (77). Numerous PC3 cell sublines now exist,
and PC3 cells can be reimplanted in vivo to screen for
highly metastatic variants. In 1984, Kozlowski et al
(78) injected PC3 cells into nude
mice and subsequently harvested metastatic cells from the liver,
which were designated PC3M cells. Later, in 1996, Pettaway et
al (79) orthotopically
injected PC3M cells into the prostate of mice, and subsequently
isolated metastatic cells from the lymph nodes and used them to
establish a PC3M-LN4 cell subline.
DU145 cells were originally derived from the brain
of a 69-year-old White male patient with metastatic prostate cancer
(80). In immunodeficient mice, the
intratibial or intracardiac injection of DU145 cells leads to the
formation of lytic bone lesions (77). DU145 cells offer advantages in the
study of prostate cancer, as their resemblance to adenocarcinoma is
closer than that of PC3 cells, and they are capable of producing
bone metastases in vivo. DU145 cell-derived bone metastases,
like those of PC3, are osteolytic (77).
Bone is one of the most prevalent sites for breast
cancer metastasis in females. Bone metastases in breast cancer are
often osteolytic and associated with high morbidity and mortality
rates; ~80% of patients with advanced breast cancer develop bone
metastases (84). The median
survival time of patients with breast cancer bone metastasis is
only 36 months (85). However,
animal models have contributed to improvements in the treatment of
bone metastasis in patients with breast cancer (86). Mice, rats, cats and dogs frequently
develop spontaneous benign and malignant mammary tumors. However,
these spontaneous breast cancers are usually unsuitable for
studying bone metastasis as most spontaneous breast tumors in mice
and rats do not metastasize, instead causing only local invasion
(87). Furthermore, the majority of
breast adenocarcinomas in rodents rapidly lose estrogen
responsiveness (88), making them
unsuitable models for the study of estrogen-responsive female
breast cancer.
MDA-MB-231 cells were originally isolated from the
breast of a 40-year-old White female patient with breast cancer,
and are commonly used in bone metastasis research. MDA-MB-231 and
its bone metastatic subline have been used in intracardiac, in
situ, intraosseous and caudal vein injection studies. These
cells metastasize almost entirely to the bone and cause osteolytic
metastasis 3–4 weeks after injection. Bisphosphonates have been
demonstrated to reduce the formation of new bone metastases from
MDA-MB-231 in animal models, and to hinder the growth of existing
bone metastases (89).
MCF7 cells are breast adenocarcinoma cells isolated
from the pleural effusion of a 69-year-old White female patient.
These cells express estrogen receptors and can generate mixed
osteolytic or osteogenic bone metastases following intraosseous
injection. Bone metastasis develops gradually, potentially taking
up to 6 months after intracardial injection (90).
ZR-75-1 cells were isolated from the breast tissue
of a 63-year-old White female patient with ductal breast cancer.
These cells have been used to establish an osteoblastic breast
cancer bone metastasis model in nude mice via intracardiac
injection. This model was used to demonstrate the critical
involvement of ET-1 in the pathophysiology of osteoblastic
metastasis (91).
4T1 is a mammary tumor cell line derived from
spontaneous mammary tumors in BALB/c mice. These cells are highly
invasive and tumorigenic, with growth and metastatic spread
patterns very similar to those of human breast cancer (92). 4T1 cells can easily be transplanted
into the mammary gland, allowing the primary tumor to grow in the
anatomically correct site, closely mimicking the metastatic pattern
observed in human breast cancer. In addition, the course of 4T1
metastasis to lymph nodes and other organs is very similar to that
of human breast cancer (93).
Tumors often form on days 7–10 following the orthotopic injection
of 4T1 cells into the mammary fat pads of female BALB/c mice, with
metastasis to the bone and internal organs occurring 3–4 weeks
later. Although the incidence of bone metastasis following the
orthotopic injection of 4T1 cells can reach 100%, the reliability
of this model is poor (94).
Lung cancer is classified into two subtypes: Small
cell lung cancer and non-small cell lung cancer, each with distinct
biological behaviors, clinical courses and treatment responses.
Bone metastases occur in 30–40% of patients with lung cancer
(95). These metastases are
primarily osteolytic lesions, osteoblastic lesions and mixed bone
lesions (96,97). Several cell lines, including A549,
ACC-LC319, H460, H727, H2030, HARA, LLC, SBC-3, NCI-H292, PC9,
PC14, SBC-5 and SPC-A-1, have been used in lung cancer bone
metastasis animal models (98).
Orthotopic, tail vein, intraosseous and intracardiac injections
have all been used in models of bone metastasis in lung cancer;
however, orthotopic injection more closely replicates the
biological behavior of lung cancer metastasis compared with other
methods. Intraosseous injections can be administered to the tibia
or spinal canal, and the direct injection of PC14 cells into the
spinal canal of mice was found to result in spinal metastases of
lung cancer (99). The osteoporosis
and spinal compression caused by bone metastases in this model
closely resemble those observed with human spinal metastases.
Certain cell lines can be selectively cultured in vivo to
generate sublines that more readily metastasize to the bone. For
example, although the bone metastasis of PC14 cells in mice is
rare, multiple in vivo selective cultures enabled a highly
metastatic PC14HM subline to be established (100). Similarly, following eight cycles
of in vivo selection of the SPC-A-1 cell line, the
SPC-A-1-BM cell subline achieved a bone metastasis success rate of
100% in mice following intracardiac injection (101). Table
II summarizes the specific conditions of the cell lines and
modeling methods commonly used for studying bone metastases
(77,85,86,102–120).
Direct cell injection is the most frequently used
approach for the development of bone metastasis models, with
intracardiac and intraosseous injection methods being the most
successful in bone metastasis research. Tail vein injection is
simpler than cardiac injection but frequently leads to lung
metastasis, which complicates the study of bone metastasis.
Although cell injection models are straightforward and easy to
maintain, they provide limited information about the process by
which tumors metastasize to bone. Animal models are very important
in the study of the pathogenesis of bone metastasis. The
development of different animal models for different research
purposes and research directions is critical. The identification of
key targets for the treatment of bone metastasis may improve
therapy efficacy and patient quality of life, and ultimately extend
patient survival.
Not applicable.
This study was supported by Special funding for Key R&D
Program Project of Guangxi (grant code Guike AB24010083) and the
National Natural Science Foundation of China (grant nos. 82160590,
82460541 and 82260602).
Not applicable.
MD, JG and GQ were responsible for conception and
design. JG and GQ supervised the study. MD, YZ and HD wrote the
original manuscript. JG, GQ and MD reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papalia GF, Brigato P, Sisca L, Maltese G,
Faiella E, Santucci D, Pantano F, Vincenzi B, Tonini G, Papalia R
and Denaro V: Artificial intelligence in detection, management, and
prognosis of bone metastasis: A systematic review. Cancers (Basel).
16:27002024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sousa S and Clézardin P: Bone-targeted
therapies in Cancer-induced bone disease. Calcif Tissue Int.
102:227–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Bado IL, Hu J, Wan YW, Wu L, Wang
H, Gao Y, Jeong HH, Xu Z, Hao X, et al: The bone microenvironment
invigorates metastatic seeds for further dissemination. Cell.
184:2471–86.e20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coleman R, Hadji P, Body JJ, Santini D,
Chow E, Terpos E, Oudard S, Bruland Ø, Flamen P, Kurth A, et al:
Bone health in cancer: ESMO Clinical Practice Guidelines. Ann
Oncol. 31:1650–1663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trompet D, Melis S, Chagin AS and Maes C:
Skeletal stem and progenitor cells in bone development and repair.
J Bone Miner Res. 39:633–654. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boyce BF: Advances in the regulation of
osteoclasts and osteoclast functions. J Dent Res. 92:860–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikebuchi Y, Aoki S, Honma M, Hayashi M,
Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et
al: Coupling of bone resorption and formation by RANKL reverse
signalling. Nature. 561:195–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamagishi T, Kawashima H, Ogose A,
Ariizumi T, Oike N, Sasaki T, Hatano H, Ohashi R, Umezu H, Ajioka Y
and Endo N: Expression profiling of Receptor-activator of nuclear
Factor-Kappa B ligand in soft tissue tumors. Tohoku J Exp Med.
248:87–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gostage J, Kostenuik P, Goljanek-Whysall
K, Bellantuono I, McCloskey E and Bonnet N: Extra-osseous roles of
the RANK-RANKL-OPG axis with a focus on skeletal muscle. Curr
Osteoporos Rep. 22:632–650. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nozawa K, Fujishiro M, Kawasaki M, Kaneko
H, Iwabuchi K, Yanagida M, Suzuki F, Miyazawa K, Takasaki Y, Ogawa
H, et al: Connective tissue growth factor promotes articular damage
by increased osteoclastogenesis in patients with rheumatoid
arthritis. Arthritis Res Ther. 11:R1742009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoyama E, Kubota S, Khattab HM, Nishida T
and Takigawa M: CCN2 enhances RANKL-induced osteoclast
differentiation via direct binding to RANK and OPG. Bone.
73:242–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren W, Sun X, Wang K, Feng H, Liu Y, Fei
C, Wan S, Wang W, Luo J, Shi Q, et al: BMP9 inhibits the bone
metastasis of breast cancer cells by downregulating CCN2
(connective tissue growth factor, CTGF) expression. Mol Biol Rep.
41:1373–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sims NA and Martin TJ: Coupling the
activities of bone formation and resorption: A multitude of signals
within the basic multicellular unit. Bonekey Rep. 3:4812014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia J and Delany AM: MicroRNAs
regulating TGFβ and BMP signaling in the osteoblast lineage. Bone.
143:1157912021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caetano-Lopes J, Canhão H and Fonseca JE:
Osteoblasts and bone formation. Acta Reumatol Port. 32:103–110.
2007.PubMed/NCBI
|
|
20
|
Abhishek Shah A, Chand D, Ahamad S, Porwal
K, Chourasia MK, Mohanan K, Srivastava KR and Chattopadhyay N:
Therapeutic targeting of Wnt antagonists by small molecules for
treatment of osteoporosis. Biochem Pharmacol. 230:1165872024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van't Hof RJ and Ralston SH: Nitric oxide
and bone. Immunology. 103:255–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danilchenko S, Kalinkevich A, Zhovner M,
Kuznetsov V, Li H and Wang J: Anisotropic aspects of solubility
behavior in the demineralization of cortical bone revealed by XRD
analysis. J Biol Phys. 45:77–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitcharanant N, Chattipakorn N and
Chattipakorn SC: The effect of intermittent parathyroid hormone on
bone lengthening: Current evidence to inform future effective
interventions. Osteoporos Int. 34:1657–1675. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niwczyk O, Grymowicz M, Szczęsnowicz A,
Hajbos M, Kostrzak A, Budzik M, Maciejewska-Jeske M, Bala G,
Smolarczyk R and Męczekalski B: Bones and hormones: Interaction
between hormones of the hypothalamus, pituitary, adipose tissue and
bone. Int J Mol Sci. 24:68402023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grigoryan S and Clines GA: Hormonal
control of bone architecture throughout the lifespan: Implications
for fracture prediction and prevention. Endocr Pract. 30:687–694.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagy V and Penninger JM: The RANKL-RANK
Story. Gerontology. 61:534–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mastro AM, Gay CV and Welch DR: The
skeleton as a unique environment for breast cancer cells. Clin Exp
Metastasis. 20:275–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bussard KM, Gay CV and Mastro AM: The bone
microenvironment in metastasis; what is special about bone? Cancer
Metastasis Rev. 27:41–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma R, Sharma R, Khaket TP, Dutta C,
Chakraborty B and Mukherjee TK: Breast cancer metastasis: Putative
therapeutic role of vascular cell adhesion molecule-1. Cell Oncol
(Dordr). 40:199–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Q and Massagué J: Molecular pathways:
VCAM-1 as a potential therapeutic target in metastasis. Clin Cancer
Res. 18:5520–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lipton A: Implications of bone metastases
and the benefits of bone-targeted therapy. Semin Oncol. 37 (Suppl
2):S15–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Leon-Oliva D, Barrena-Blázquez S,
Jiménez-Álvarez L, Fraile-Martinez O, García-Montero C,
López-González L, Torres-Carranza D, García-Puente LM, Carranza ST,
Álvarez-Mon MÁ, et al: The RANK-RANKL-OPG System: A multifaceted
regulator of homeostasis, immunity, and cancer. Medicina (Kaunas).
59:17522023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Liu Y, Huang Z, Chen X and Zhang
B: The roles of osteoprotegerin in cancer, far beyond a bone
player. Cell Death Discov. 8:2522022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clézardin P: The role of
RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone
diseases: Physiopathology and clinical implications. Bull Cancer.
98:837–846. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Susperregui AR, Viñals F, Ho PW, Gillespie
MT, Martin TJ and Ventura F: BMP-2 regulation of PTHrP and
osteoclastogenic factors during osteoblast differentiation of C2C12
cells. J Cell Physiol. 216:144–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Izawa T, Zou W, Chappel JC, Ashley JW,
Feng X and Teitelbaum SL: c-Src links a RANK/αvβ3 integrin complex
to the osteoclast cytoskeleton. Mol Cell Biol. 32:2943–2953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou S, Li J, Ying T, Wang Y, Wang Q, Li X
and Zhao F: StemRegenin 1 attenuates the RANKL-induced
osteoclastogenesis via inhibiting AhR-c-src-NF-κB/p-ERK MAPK-NFATc1
signaling pathway. iScience. 27:1096822024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ibaragi S, Shimo T, Iwamoto M, Hassan NM,
Kodama S, Isowa S and Sasaki A: Parathyroid hormone-related peptide
regulates matrix metalloproteinase-13 gene expression in bone
metastatic breast cancer cells. Anticancer Res. 30:5029–5036.
2010.PubMed/NCBI
|
|
40
|
Giarratana AO, Prendergast CM, Salvatore
MM and Capaccione KM: TGF-β signaling: Critical nexus of
fibrogenesis and cancer. J Transl Med. 22:5942024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Juárez P and Guise TA: TGF-β in cancer and
bone: Implications for treatment of bone metastases. Bone.
48:23–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan C, Wang Q, Kuipers TB, Cats D, Iyengar
PV, Hagenaars SC, Mesker WE, Devilee P, Tollenaar RAEM, Mei H and
Ten Dijke P: LncRNA LITATS1 suppresses TGF-β-induced EMT and cancer
cell plasticity by potentiating TβRI degradation. EMBO J.
42:e1128062023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Drabsch Y, Pujuguet P, Ren J, van
Laar T, Zhang L, van Dam H, Clément-Lacroix P and Ten Dijke P:
Genetic depletion and pharmacological targeting of αv integrin in
breast cancer cells impairs metastasis in zebrafish and mouse
xenograft models. Breast Cancer Res. 17:282015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luis-Ravelo D, Antón I, Vicent S, Zandueta
C, Martínez S, Valencia K, Ormazábal C and Lecanda F: Divergent
effects of TGF-β inhibition in bone metastases in breast and lung
cancer. Rev Osteoporos Metab Miner. 5:79–84. 2013. View Article : Google Scholar
|
|
45
|
Zhu S, Chen W, Masson A and Li YP: Cell
signaling and transcriptional regulation of osteoblast lineage
commitment, differentiation, bone formation, and homeostasis. Cell
Discov. 10:712024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gkotzamanidou M, Dimopoulos MA, Kastritis
E, Christoulas D, Moulopoulos LA and Terpos E: Sclerostin: A
possible target for the management of cancer-induced bone disease.
Expert Opin Ther Targets. 16:761–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pinzone JJ, Hall BM, Thudi NK, Vonau M,
Qiang YW, Rosol TJ and Shaughnessy JD: The role of Dickkopf-1 in
bone development, homeostasis, and disease. Blood. 113:517–525.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Irani S, Salajegheh A, Smith RA and Lam
AK: A review of the profile of endothelin axis in cancer and its
management. Crit Rev Oncol Hematol. 89:314–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bagnato A, Loizidou M, Pflug BR, Curwen J
and Growcott J: Role of the endothelin axis and its antagonists in
the treatment of cancer. Br J Pharmacol. 163:220–233. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xin Z, Qin L, Tang Y, Guo S, Li F, Fang Y,
Li G, Yao Y, Zheng B, Zhang B, et al: Immune mediated support of
metastasis: Implication for bone invasion. Cancer Commun (Lond).
44:967–991. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma X and Yu J: Role of the bone
microenvironment in bone metastasis of malignant tumors-therapeutic
implications. Cell Oncol (Dordr). 43:751–761. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Baldessari C, Pipitone S, Molinaro E,
Cerma K, Fanelli M, Nasso C, Oltrecolli M, Pirola M, D'Agostino E,
Pugliese G, et al: Bone metastases and health in prostate cancer:
From pathophysiology to clinical implications. Cancers (Basel).
15:15182023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Jiang P and Wang C: The role of
prostate-specific antigen in the osteoblastic bone metastasis of
prostate cancer: A literature review. Front Oncol. 13:11276372023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yonou H, Horiguchi Y, Ohno Y, Namiki K,
Yoshioka K, Ohori M, Hatano T and Tachibana M: Prostate-specific
antigen stimulates osteoprotegerin production and inhibits receptor
activator of nuclear factor-kappaB ligand expression by human
osteoblasts. Prostate. 67:840–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Christoph F, König F, Lebentrau S, Jandrig
B, Krause H, Strenziok R and Schostak M: RANKL/RANK/OPG cytokine
receptor system: mRNA expression pattern in BPH, primary and
metastatic prostate cancer disease. World J Urol. 36:187–192. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kuchimaru T, Hoshino T, Aikawa T, Yasuda
H, Kobayashi T, Kadonosono T and Kizaka-Kondoh S: Bone resorption
facilitates osteoblastic bone metastatic colonization by
cooperation of insulin-like growth factor and hypoxia. Cancer Sci.
105:553–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jin R, Sterling JA, Edwards JR, DeGraff
DJ, Lee C, Park SI and Matusik RJ: Activation of NF-kappa B
signaling promotes growth of prostate cancer cells in bone. PLoS
One. 8:e609832013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Choi SW, Sun AK, Cheung JP and Ho JC:
Circulating tumour cells in the prediction of bone metastasis.
Cancers (Basel). 16:2522024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Roth ES, Fetzer DT, Barron BJ, Joseph UA,
Gayed IW and Wan DQ: Does colon cancer ever metastasize to bone
first? a temporal analysis of colorectal cancer progression. BMC
Cancer. 9:2742009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Uccella S, Morris JM, Bakkum-Gamez JN,
Keeney GL, Podratz KC and Mariani A: Bone metastases in endometrial
cancer: Report on 19 patients and review of the medical literature.
Gynecol Oncol. 130:474–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
62
|
Elkin M and Vlodavsky I: Tail vein assay
of cancer metastasis. Curr Protoc Cell Biol. Chapter
19:19.2.1-19.2.7.2001.doi: 10.1002/0471143030.cb1902s12. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kuchimaru T, Kataoka N, Nakagawa K,
Isozaki T, Miyabara H, Minegishi M, Kadonosono T and Kizaka-Kondoh
S: A reliable murine model of bone metastasis by injecting cancer
cells through caudal arteries. Nat Commun. 9:29812018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Neudert M, Fischer C, Krempien B, Bauss F
and Seibel MJ: Site-specific human breast cancer (MDA-MB-231)
metastases in nude rats: Model characterisation and in vivo effects
of ibandronate on tumour growth. Int J Cancer. 107:468–477. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hoffman RM: Patient-derived orthotopic
xenografts: Better mimic of metastasis than subcutaneous
xenografts. Nat Rev Cancer. 15:451–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stribbling SM and Ryan AJ: The
cell-line-derived subcutaneous tumor model in preclinical cancer
research. Nat Protoc. 17:2108–2128. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Farhoodi HP, Segaliny AI, Wagoner ZW,
Cheng JL, Liu L and Zhao W: Optimization of a syngeneic murine
model of bone metastasis. J Bone Oncol. 23:1002982020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Winnard PT Jr, Vesuna F, Bol GM,
Gabrielson KL, Chenevix-Trench G, Ter Hoeve ND, van Diest PJ and
Raman V: Targeting RNA helicase DDX3X with a small molecule
inhibitor for breast cancer bone metastasis treatment. Cancer Lett.
604:2172602024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han Y, Azuma K, Watanabe S, Semba K and
Nakayama J: Metastatic profiling of HER2-positive breast cancer
cell lines in xenograft models. Clin Exp Metastasis. 39:467–477.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ye X, Huang X, Fu X, Zhang X, Lin R, Zhang
W, Zhang J and Lu Y: Myeloid-like tumor hybrid cells in bone marrow
promote progression of prostate cancer bone metastasis. J Hematol
Oncol. 16:462023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhong L, Miller HD, Zhang Y, Jin B, Ge D
and You Z: Intra-arterial injection to create bone metastasis of
prostate cancer in mice. Am J Clin Exp Urol. 8:93–100.
2020.PubMed/NCBI
|
|
72
|
Simmons JK, Dirksen WP, Hildreth BE III,
Dorr C, Williams C, Thomas R, Breen M, Toribio RE and Rosol TJ:
Canine prostate cancer cell line (Probasco) produces osteoblastic
metastases in vivo. Prostate. 74:1251–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Abou DS, Ulmert D, Doucet M, Hobbs RF,
Riddle RC and Thorek DL: Whole-Body and microenvironmental
localization of Radium-223 in naïve and mouse models of prostate
cancer metastasis. J Natl Cancer Inst. 108:djv3802025. View Article : Google Scholar
|
|
74
|
Pollard HB, Levine MA, Eidelman O and
Pollard M: Pharmacological ascorbic acid suppresses syngeneic tumor
growth and metastases in hormone-refractory prostate cancer. In
Vivo. 24:249–255. 2010.PubMed/NCBI
|
|
75
|
Wang N, Reeves KJ, Brown HK, Fowles AC,
Docherty FE, Ottewell PD, Croucher PI, Holen I and Eaton CL: The
frequency of osteolytic bone metastasis is determined by conditions
of the soil, not the number of seeds; evidence from in vivo models
of breast and prostate cancer. J Exp Clin Cancer Res. 34:1242015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
77
|
Dai J, Hensel J, Wang N, Kruithof-de Julio
M and Shiozawa Y: Mouse models for studying prostate cancer bone
metastasis. Bonekey Rep. 5:7772016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kozlowski JM, Fidler IJ, Campbell D, Xu
ZL, Kaighn ME and Hart IR: Metastatic behavior of human tumor cell
lines grown in the nude mouse. Cancer Res. 44:3522–3529.
1984.PubMed/NCBI
|
|
79
|
Pettaway CA, Pathak S, Greene G, Ramirez
E, Wilson MR, Killion JJ and Fidler IJ: Selection of highly
metastatic variants of different human prostatic carcinomas using
orthotopic implantation in nude mice. Clin Cancer Res. 2:1627–1636.
1996.PubMed/NCBI
|
|
80
|
Stone KR, Mickey DD, Wunderli H, Mickey GH
and Paulson DF: Isolation of a human prostate carcinoma cell line
(DU 145). Int J Cancer. 21:274–281. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Horoszewicz JS, Leong SS, Chu TM, Wajsman
ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK and
Sandberg AA: The LNCaP cell line-a new model for studies on human
prostatic carcinoma. Prog Clin Biol Res. 37:115–132.
1980.PubMed/NCBI
|
|
82
|
Thalmann GN, Anezinis PE, Chang SM, Zhau
HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC and Chung LW:
Androgen-independent cancer progression and bone metastasis in the
LNCaP model of human prostate cancer. Cancer Res. 54:2577–2581.
1994.PubMed/NCBI
|
|
83
|
Sobel RE and Sadar MD: Cell lines used in
prostate cancer research: A compendium of old and new lines-part 1.
J Urol. 173:342–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Guo Q, Jin Y, Lin M, Zeng C and Zhang J:
NF-κB signaling in therapy resistance of breast cancer: Mechanisms,
approaches, and challenges. Life Sci. 348:1226842024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou J and Ottewell PD: The role of IL-1B
in breast cancer bone metastasis. J Bone Oncol. 46:1006082024.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shu ST, Nadella MV, Dirksen WP, Fernandez
SA, Thudi NK, Werbeck JL, Lairmore MD and Rosol TJ: A novel
bioluminescent mouse model and effective therapy for adult T-cell
leukemia/lymphoma. Cancer Res. 67:11859–11866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Isaacs JT, Heston WD, Weissman RM and
Coffey DS: Animal models of the hormone-sensitive and -insensitive
prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and
R-3327-AT. Cancer Res. 38:4353–4359. 1978.PubMed/NCBI
|
|
89
|
Padalecki SS and Guise TA: Actions of
bisphosphonates in animal models of breast cancer. Breast Cancer
Res. 4:35–41. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ooi LL, Zheng Y, Zhou H, Trivedi T,
Conigrave AD, Seibel MJ and Dunstan CR: Vitamin D deficiency
promotes growth of MCF-7 human breast cancer in a rodent model of
osteosclerotic bone metastasis. Bone. 47:795–803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yin JJ, Mohammad KS, Käkönen SM, Harris S,
Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM and
Guise TA: A causal role for endothelin-1 in the pathogenesis of
osteoblastic bone metastases. Proc Natl Acad Sci USA.
100:10954–10959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pulaski BA and Ostrand-Rosenberg S: Mouse
4T1 breast tumor model. Curr Protoc Immunol. Chapter 20: Unit 20.2.
2001.doi: 10.1002/0471142735.im2002s39. PubMed/NCBI
|
|
93
|
Pulaski BA and Ostrand-Rosenberg S:
Reduction of established spontaneous mammary carcinoma metastases
following immunotherapy with major histocompatibility complex class
II and B7.1 cell-based tumor vaccines. Cancer Res. 58:1486–1493.
1998.PubMed/NCBI
|
|
94
|
Lelekakis M, Moseley JM, Martin TJ, Hards
D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J and
Anderson RL: A novel orthotopic model of breast cancer metastasis
to bone. Clin Exp Metastasis. 17:163–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Feeley BT, Liu NQ, Conduah AH, Krenek L,
Roth K, Dougall WC, Huard J, Dubinett S and Lieberman JR: Mixed
metastatic lung cancer lesions in bone are inhibited by noggin
overexpression and Rank:Fc administration. Bone Miner Res.
21:1571–1580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Miki T, Yano S, Hanibuchi M and Sone S:
Bone metastasis model with multiorgan dissemination of human
small-cell lung cancer (SBC-5) cells in natural killer
cell-depleted SCID mice. Oncol Res. 12:209–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tannehill-Gregg SH, Levine AL, Nadella MV,
Iguchi H and Rosol TJ: The effect of zoledronic acid and
osteoprotegerin on growth of human lung cancer in the tibias of
nude mice. Clin Exp Metastasis. 23:19–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Taube T, Beneton MN, McCloskey EV, Rogers
S, Greaves M and Kanis JA: Abnormal bone remodelling in patients
with myelomatosis and normal biochemical indices of bone
resorption. Eur J Haematol. 49:192–198. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nakano T, Shimizu K, Kawashima O,
Kamiyoshihara M, Kakegawa S, Sugano M, Ibe T, Nagashima T, Kaira K,
Sunaga N, et al: Establishment of a human lung cancer cell line
with high metastatic potential to multiple organs: Gene expression
associated with metastatic potential in human lung cancer. Oncol
Rep. 28:1727–1735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang S, Dong Q, Yao M, Shi M, Ye J, Zhao
L, Su J, Gu W, Xie W, Wang K, et al: Establishment of an
experimental human lung adenocarcinoma cell line SPC-A-1BM with
high bone metastases potency by (99m)Tc-MDP bone scintigraphy. Nucl
Med Biol. 36:313–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gravina GL, Mancini A, Muzi P, Ventura L,
Biordi L, Ricevuto E, Pompili S, Mattei C, Di Cesare E, Jannini EA
and Festuccia C: CXCR4 pharmacogical inhibition reduces bone and
soft tissue metastatic burden by affecting tumor growth and
tumorigenic potential in prostate cancer preclinical models.
Prostate. 75:1227–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao
C, Murphy CF, Yang H, Zhau HE, Balian G and Chung LW: Establishing
human prostate cancer cell xenografts in bone: Induction of
osteoblastic reaction by prostate-specific antigen-producing tumors
in athymic and SCID/bg mice using LNCaP and lineage-derived
metastatic sublines. Int J Cancer. 77:887–894. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shevrin DH, Kukreja SC, Ghosh L and Lad
TE: Development of skeletal metastasis by human prostate cancer in
athymic nude mice. Clin Exp Metastasis. 6:401–409. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Havens AM, Pedersen EA, Shiozawa Y, Ying
C, Jung Y, Sun Y, Neeley C, Wang J, Mehra R, Keller ET, et al: An
in vivo mouse model for human prostate cancer metastasis.
Neoplasia. 10:371–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang M, Jiang P, Sun FX, Hasegawa S,
Baranov E, Chishima T, Shimada H, Moossa AR and Hoffman RM: A
fluorescent orthotopic bone metastasis model of human prostate
cancer. Cancer Res. 59:781–786. 1999.PubMed/NCBI
|
|
107
|
Fisher JL, Schmitt JF, Howard ML, Mackie
PS, Choong PF and Risbridger GP: An in vivo model of prostate
carcinoma growth and invasion in bone. Cell Tissue Res.
307:337–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bonfil RD, Dong Z, Trindade Filho JC,
Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni SR, Zhao H,
Mobashery S, et al: Prostate cancer-associated membrane type
1-matrix metalloproteinase: A pivotal role in bone response and
intraosseous tumor growth. Am J Pathol. 170:2100–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zou M, Jiao J, Zou Q, Xu Y, Cheng M, Xu J
and Zhang Y: Multiple metastases in a novel LNCaP model of human
prostate cancer. Oncol Rep. 30:615–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Corey E, Quinn JE, Bladou F, Brown LG,
Roudier MP, Brown JM, Buhler KR and Vessella RL: Establishment and
characterization of osseous prostate cancer models: Intra-tibial
injection of human prostate cancer cells. Prostate. 52:20–33. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jantscheff P, Ziroli V, Esser N, Graeser
R, Kluth J, Sukolinskaya A, Taylor LA, Unger C and Massing U:
Anti-metastatic effects of liposomal gemcitabine in a human
orthotopic LNCaP prostate cancer xenograft model. Clin Exp
Metastasis. 26:981–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wetterwald A, van der Pluijm G, Que I,
Sijmons B, Buijs J, Karperien M, Löwik CW, Gautschi E, Thalmann GN
and Cecchini MG: Optical imaging of cancer metastasis to bone
marrow: A mouse model of minimal residual disease. Am J Pathol.
160:1143–1153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sasaki SI, Zhang D, Iwabuchi S, Tanabe Y,
Hashimoto S, Yamauchi A, Hayashi K, Tsuchiya H, Hayakawa Y, Baba T
and Mukaida N: Crucial contribution of GPR56/ADGRG1, expressed by
breast cancer cells, to bone metastasis formation. Cancer Sci.
112:4883–4893. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim B, Kim H, Jung S, Moon A, Noh DY, Lee
ZH, Kim HJ and Kim HH: A CTGF-RUNX2-RANKL axis in breast and
prostate cancer cells promotes tumor progression in bone. J Bone
Miner Res. 35:155–166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yoneda T, Michigami T, Yi B, Williams PJ,
Niewolna M and Hiraga T: Actions of bisphosphonate on bone
metastasis in animal models of breast carcinoma. Cancer.
88:2979–2988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yi B, Williams PJ, Niewolna M, Wang Y and
Yoneda T: Tumor-derived platelet-derived growth factor-BB plays a
critical role in osteosclerotic bone metastasis in an animal model
of human breast cancer. Cancer Res. 62:917–923. 2002.PubMed/NCBI
|
|
117
|
Sun J, Huang J, Lan J, Zhou K, Gao Y, Song
Z, Deng Y, Liu L, Dong Y and Liu X: Overexpression of CENPF
correlates with poor prognosis and tumor bone metastasis in breast
cancer. Cancer Cell Int. 19:2642019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hung JY, Horn D, Woodruff K, Prihoda T,
LeSaux C, Peters J, Tio F and Abboud-Werner SL: Colony-stimulating
factor 1 potentiates lung cancer bone metastasis. Lab Invest.
94:371–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu H, Cheng Q, Xu DS, Wang W, Fang Z, Xue
DD, Zheng Y, Chang AH and Lei YJ: Overexpression of CXCR7
accelerates tumor growth and metastasis of lung cancer cells.
Respir Res. 21:2872020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang Q, Zhao B, Li J, Zhao J, Wang C, Li
Q, Yang W, Xu L and Gong Y: Qilian formula inhibits tumor cell
growth in a bone metastasis model of lung cancer. Integr Cancer
Ther. 22:153473542312172742023. View Article : Google Scholar : PubMed/NCBI
|