Introduction
Adrenal cortical carcinoma (ACC) is a rare endocrine
tumor with an annual incidence rate of 0.5 to 2 cases per million
individuals globally, accounting for 0.02–0.2% of all
cancer-related deaths (1). The
male-to-female ratio of patients with ACC is ~1.5:1, with ACC being
more common in middle-aged women aged 40–50 years. The prognosis of
patients with ACC is poor, with an average 5-year survival rate of
32–45% (2). Furthermore, >50% of
patients exhibit excessive adrenal cortex hormone levels, with
adrenocorticotropic hormone (ACTH) secretion by adrenal tumor
cells, leading to ACTH-dependent Cushing's syndrome (3,4).
Tumor-induced hypoglycemia may manifest in cases with late-stage
tumors or paraneoplastic syndrome; nonetheless, this presentation
is rare (5). Hypoglycemic symptoms
have been proposed to be related to insulin overdose, with patients
experiencing drowsiness, fatigue and even hypoglycemic coma.
Hypoglycemia is often sporadic in patients with cancer and caused
by physiological factors such as prolonged fasting. Pathological
hypoglycemia is generally reported in patients with
insulin-secreting tumors, non-islet cell tumors, myeloma, lymphoma,
leukemia and metastatic tumors (6,7).
While the most common manifestations of ACC are
hyperglycemia and Cushing's syndrome, tumor-associated hypoglycemia
is relatively rare, with non-islet cell tumor hypoglycemia (NICTH)
being a severe and rare malignant presentation of paraneoplastic
syndrome (8). The incidence rate of
NICTH is four times lower than that of insulinoma, mainly owing to
insulin-like growth factor II (IGF-II) secretion by the tumor
(9). NICTH may be associated with
paraneoplastic syndrome caused by tumor cell secretion of IGF-II
(10). ACC has been reported in
various neuroendocrine and adrenal tumors in patients with
recurrent hypoglycemia (11). Tumor
resection remains the definitive treatment for hypoglycemia and
related hormonal complications in patients with ACC and
hypoglycemia (8,12). The current report presents the case
of a middle-aged woman diagnosed with right-sided ACC based on
recurrent hypoglycemic episodes.
Case report
A 53-year-old woman was admitted to the Affiliated
Hospital of Ningxia Medical University (Yinchuan, China) in May
2024 with abdominal pain and recurrent hypoglycemic coma. The
patient continued to experience hypoglycemic coma episodes during
hospitalization, which improved with active symptomatic glucose
supplementation treatment. However, the cause of these episodes
remained unidentified. Even after discharge, the patient
experienced hypoglycemic coma episodes while resting at home. For
further diagnosis and treatment, the patient visited Hexi
University Affiliated Zhangye People's Hospital (Zhangye, China) in
July 2024. The patient's vital signs were evaluated upon admission,
and were all within normal ranges (blood pressure, 140/90 mmHg;
body temperature, 36.3°C; heart rate, 76 beats/min; and respiratory
rate, 16 breaths/min). The patient had right upper abdominal
tenderness, a palpable mass, facial acne and obesity, with a body
weight increasing from 54 to 69 kg in the past 2 months. The
patient experienced mental distress and general fatigue, the
patient exhibited weight gain and redistribution of body fat,
resulting in the characteristic appearance of a moon face and
buffalo hump. Laboratory examination revealed a normal white blood
cell count, creatinine level and coagulation, accompanied by
hypokalemia (potassium level, 2.64 mmol/l; normal reference range,
3.5-.5 mmol/l)). The cortisol level at 4:00 p.m. was 610.54 nmol/l
(normal reference range, <276 nmol/l) and did not normalize
after treatment with oral dexamethasone (2 mg, once every 6 h) for
2 days. Moreover, the patient's ACTH level was 4.09 pg/ml (normal
reference range, 7.26–62.73 pg/ml) and the aldosterone-to-renin
ratio was 2.01 (normal reference range, <5.7). The patient had a
history of multiple hypoglycemia episodes induced by an unknown
cause before hospitalization. Post-hospitalization, the patient
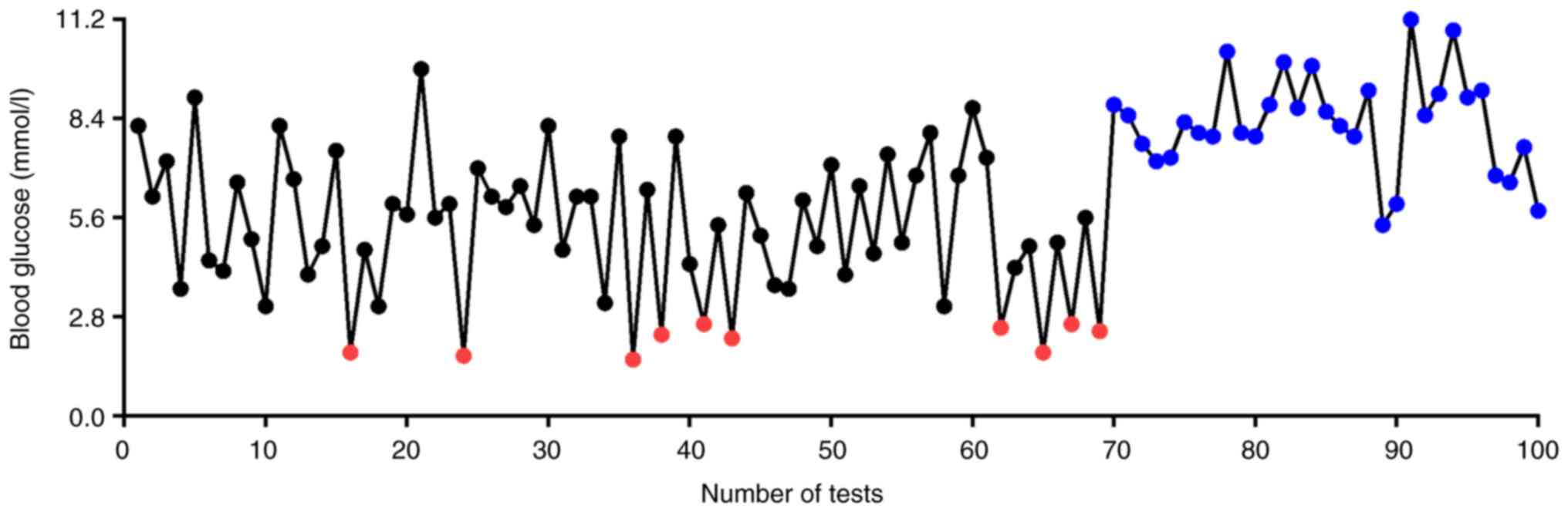
underwent an average of seven daily blood glucose monitoring tests;
however, 10 episodes of hypoglycemia occurred before surgical
treatment despite regular glucose monitoring (Fig. 1).
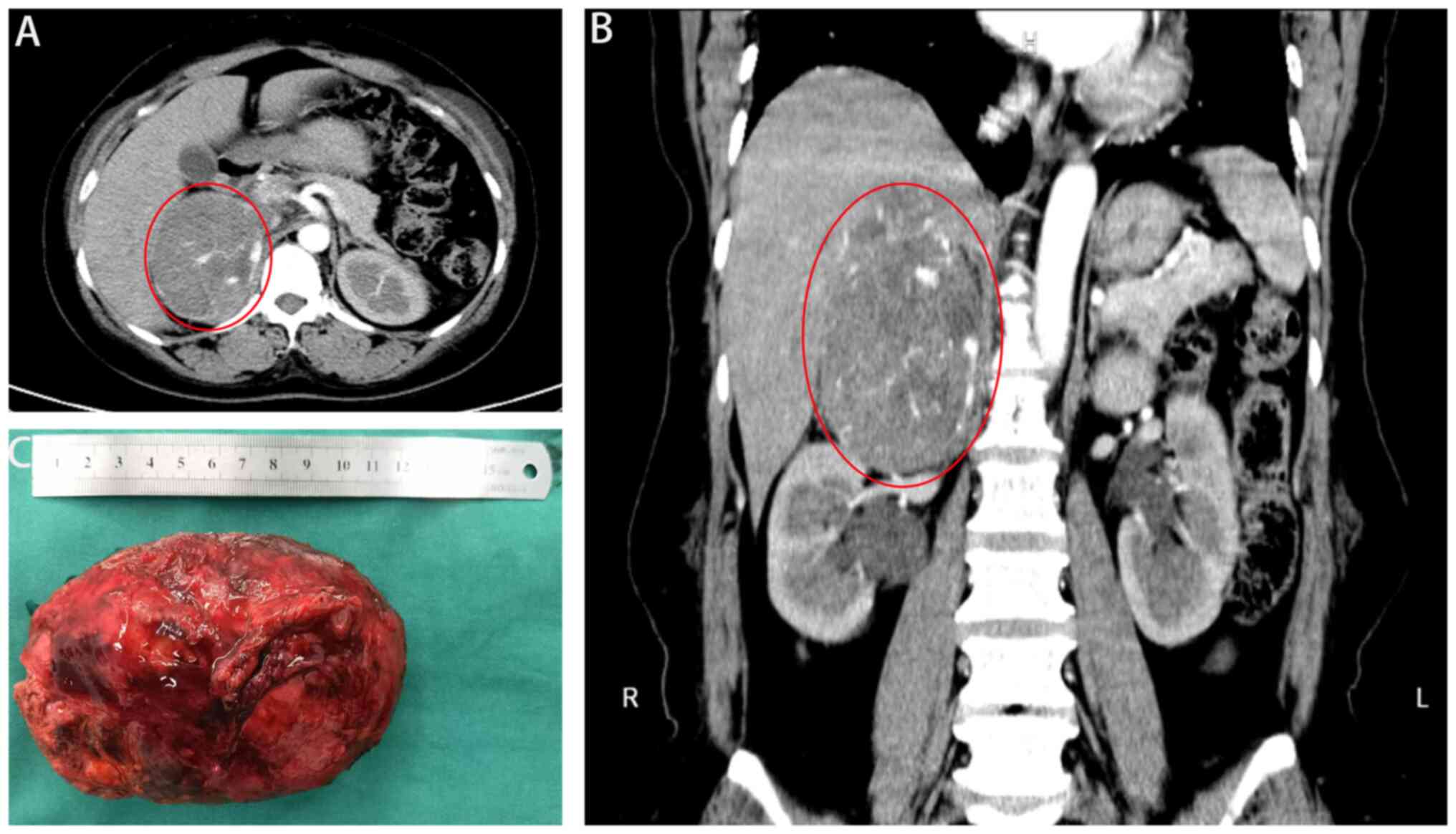
Abdominal computed tomography revealed a 12-cm
malignant tumor in the right adrenal gland, liver S7 invasion with
the right inferior phrenic artery as the blood supply, superior
vena cava invasion, and the right renal artery and vein closely
attached to the lower edge of the lesion (Fig. 2A and B). During hospitalization,
patients often experience symptomatic hypoglycemia, needing
continuous glucose infusion to prevent the recurrence of
hypoglycemia. The patient was administered an intravenous glucose
infusion (10% glucose 1,000 ml), oral glucose (20% glucose 100 ml),
starch supplemented with blood potassium (3 g potassium chloride)
and oral potassium chloride (potassium chloride sustained-release
tablets, 1 g three times a day) daily. After the blood potassium
and glucose levels stabilized, the patient underwent an open right
adrenal tumor resection (Fig. 2C).
Immunohistochemistry was performed on 4-µm paraffin-embedded tissue
sections during postoperative analysis. Slides were heated at 60°C
for 2 h, dewaxed in xylene and dehydrated by a gradient
concentration of alcohol. After retrieving and blocking the
endogenous peroxidase and non-specific staining with 3% (v/v)
hydrogen peroxide and normal goat serum, the sections were
incubated with primary antibody overnight at 4°C. The slides were
then incubated with HRP-conjugated goat anti-mouse/rabbit IgG
secondary antibody for 30 min at 37°C. Finally, they were
visualized by DAB solution (Dako; Agilent Technologies, Inc.) and
counterstained with haematoxylin. The quantity of the positively
stained cells was measured using a light microscope. Postoperative
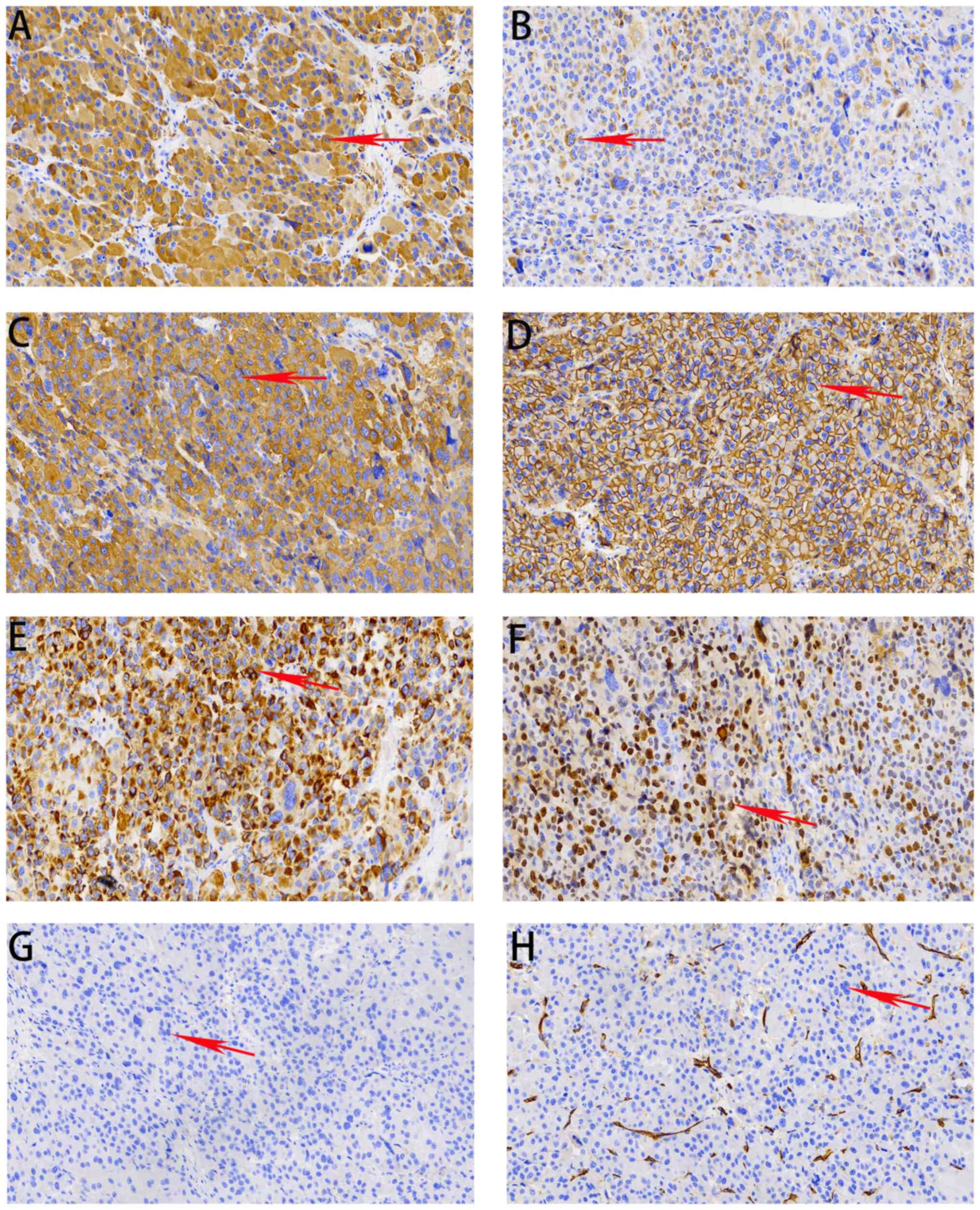
pathological analysis using immunohistochemistry revealed a
high-grade ACC according to the Weiss criteria (13), with the following results: Partially
positive for rabbit anti-human melan-A (cat. no. ab51061; 1:1,000;
Abcam); positive for mouse anti-human Inhibin-α (cat. no. ab273454;
1:1,000; Abcam), rabbit anti-human synaptophysin (cat. no. ab32127;
1:400; Abcam), rabbit anti-human CD56 (cat. no. ab220360; 1:1,000;
Abcam), rabbit anti-human pan-cytokeratin (cat. no. ab308262;
1:2,000; Abcam) and rabbit anti-human Ki-67 (cat. no. ab16667;
1:200; Abcam); and negative for rabbit anti-human chromogranin A
(cat. no. ab308262; 1:2,000; Abcam) and rabbit anti-human CD31
(cat. no. ab308262; 1:50; Abcam) (Fig.
3). Postoperative follow-up revealed that the patient recovered
well from the tumor resection. However, due to slow healing of the
open surgical wound, the patient was discharged half a month later
with significant weight loss but a better mental state than before.
No further episodes of hypoglycemia or hypokalemia were observed.
The possibility of recurrence is high, but the patient is satisfied
with the control of hypoglycemia and bloating symptoms, and is
followed up monthly.
Discussion
ACC is a rare, highly invasive endocrine tumor with
excessive adrenal cortex hormone secretion first manifesting as
increased cortisol levels in most patients (14). Hypercortisolism can cause
masculinization and Cushing's syndrome in these patients. Some
patients also have paraneoplastic syndromes, including
hyperreninemic aldosteronism (15).
In the present case, recurrent hypokalemia was closely associated
with aldosterone secretion; however, cases of concomitant
hypoglycemia are extremely rare with a poor prognosis (16,17).
Khadka et al (17) reported
the case of a 30-year-old man with ACC metastasis and hypoglycemia
symptoms. The patient died after 2 months of chemotherapy. Karimi
et al (16) reported the
case of a 26-year-old woman with hypoglycemia caused by an ACC
secreting cortisol and androgens. The patient was treated with
mitotane, but eventually succumbed to the disease a few months
later. The present patient experienced a potentially fatal
hypoglycemic coma repeatedly before hospitalization; however, the
specific cause of hypoglycemia was not identified, and the patient
was treated only with a high-glucose infusion. For patients with
frequent hypoglycemic episodes, the priority is to prevent a
hypoglycemic coma or even death. However, owing to the
unpredictability of the tumor-induced hypoglycemia, access to
glucose is important. If hypoglycemia symptoms are observed, timely
glucose supplementation, either orally or through intravenous
administration is necessary, as it is the only preventive approach
currently available. Thus, curing the tumors is the fundamental and
effective method to eliminate these tumor-induced hypoglycemic
episodes.
NICTH is a rare paraneoplastic syndrome, second only
to insulinoma in causing tumor-induced hypoglycemia (18). NICTH is less commonly associated
with ACC. The main pathophysiological mechanism of NICTH in ACC is
the secretion of the 10- to 20-kDa IGF-II resulting in the
insulin-like activity of the tumor (19). Abnormal transcription and expression
of the tumor IGF-II gene leads to increased secretion
abnormalities. Currently, hypoglycemia in ACC has been proposed to
be caused by excessive IGF-II secretion activating insulin
receptors and promoting glucose uptake (20,21).
Furthermore, inhibition of glycogen breakdown and gluconeogenesis
induces severe recurrent hypoglycemia. Moreover, 25–30% of the
patients are diagnosed with ACC only after tumor metastasis, making
them ineligible for surgery owing to the lack of specific clinical
manifestations and obvious symptoms during early ACC, making an
early diagnosis difficult (22,23).
Numerous primary hospitals in China do not perform blood tests for
ACC; therefore, patients with recurrent hypoglycemia are not
diagnosed or treated in a timely and effective manner.
Surgical resection is the gold-standard treatment
for ACC, and mitotane is the only drug approved by the US Food and
Drug Administration and European Medicines Agency for ACC treatment
(15,24). Mitotane combined with cytotoxic
chemotherapy, etoposide, doxorubicin and cisplatin has been
reported to be associated with good response rates in patients with
ACC (25). However, adjuvant
treatment is needed post-surgery for giant ACC. Patients with
metastases cannot undergo surgery and can only be administered
systemic drug therapy. However, a number of countries, including
China, have not yet approved these therapies, and patients do not
receive effective treatment. In the present case, after surgical
treatment, the patient's hypoglycemic symptoms were quickly
controlled and the hypoglycemia was no longer evident. The
hypokalemia gradually improved and the patient experienced marked
weight loss. Tumor-associated hypoglycemia can be treated with
glucose supplements and glucagon. Supplementation with
glucocorticoids can stimulate gluconeogenesis, inhibiting IGF-II
after surgery, preventing adrenal crises in patients, and providing
sustained benefits in hypoglycemia treatment (26). Therefore, glucocorticoids are
routinely administered in Hexi University Affiliated Zhangye
People's Hospital before and after surgery, and the patient stopped
using them after discharge without any adrenal-related
complications.
The present study has some limitations. Although the
patient had a huge tumor invading the liver and underwent surgical
treatment, standard postoperative adjuvant therapy with mitotane
was not administered, decreasing the treatment efficacy. The
patient received radiotherapy and chemotherapy in the Department of
Oncology, and new complications may still require close
follow-up.
In recent decades, the understanding of ACC
pathology has gradually led to its recognition and familiarity.
However, ACC has a poor prognosis with a low 5-year survival rate
and complex surgical and perioperative management (27). Therefore, treatment is challenging,
and the early symptoms of patients need to be closely monitored and
physical examinations need to be conducted to detect and treat ACC
at an early stage to improve the patient's prognosis.
Acknowledgements
Not applicable.
Funding
This study was funded by grants from the Hexi University 14th
Science and Technology Innovation Project (grant no. 164).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YPL, RY and JXY contributed to the drafting of the
manuscript and the design of the study. LZ, JW, FYY, ST and JQ
contributed to the conceptualization and design of the study. ST
and JXY assisted with the completion of the surgery. JQ and JXY
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the Ethics Committee
of Hexi University Affiliated Zhangye People's Hospital (approval
number B2024-018).
Patient consent for publication
The patient provided written informed consent for
the publication of this report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adrenal cortical carcinoma
|
|
IGF-II
|
insulin-like growth factor II
|
|
NICTH
|
non-islet cell tumor hypoglycemia
|
References
|
1
|
Torti JF and Correa R: Adrenal Cancer [M].
StatPearls; Treasure Island: 2025
|
|
2
|
Wang Z, Deng JH, Wang X, Liu Y, Chen JY
and Zhang YS: Changes in WHO classification of adrenal tumors and
new ideas for multi-dimensional diagnosis and treatment. Zhonghua
Wai Ke Za Zhi. 62:1001–1007. 2024.(In Chinese). PubMed/NCBI
|
|
3
|
Yip L, Duh QY, Wachtel H, Jimenez C,
Sturgeon C, Lee C, Velázquez-Fernández D, Berber E, Hammer GD,
Bancos I, et al: American association of endocrine surgeons
guidelines for adrenalectomy: Executive summary. JAMA Surg.
157:870–877. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiseljak-Vassiliades K, Bancos I,
Hamrahian A, Habra M, Vaidya A, Levine AC and Else T: American
association of clinical endocrinology disease state clinical review
on the evaluation and management of adrenocortical carcinoma in an
adult: A practical approach. Endocr Pract. 26:1366–1383. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iglesias P and Díez JJ: Management of
endocrine disease: A clinical update on tumor-induced hypoglycemia.
Eur J Endocrinol. 170:R147–R157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siddiqui M, Vora A, Ali S, Abramowitz J
and Mirfakhraee S: Pasireotide: A novel treatment for tumor-induced
hypoglycemia due to insulinoma and non-islet cell tumor
hypoglycemia. J Endocr Soc. 5:bvaa1712020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Axelrod L and Ron D: Insulin-like growth
factor II and the riddle of tumor-induced hypoglycemia. N Engl J
Med. 319:1477–1479. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SW, Lee SE, Oh YL, Kim S, Park SH and
Kim JH: Nonislet cell tumor hypoglycemia in a patient with adrenal
cortical carcinoma. Case Rep Endocrinol.
2016:57314172016.PubMed/NCBI
|
|
9
|
Marchetti KR, Pereira MA, Lichtenstein A
and Paiva EF: Refractory hypoglycemia in a patient with functional
adrenal cortical carcinoma. Endocrinol Diabetes Metab Case Rep.
201:16–0101. 2016.PubMed/NCBI
|
|
10
|
Fukuda I, Hizuka N, Takano K,
Asakawa-Yasumoto K, Shizume K and Demura H: Characterization of
insulin-like growth factor II (IGF-II) and IGF binding proteins in
patients with non-islet-cell tumor hypoglycemia. Endocr J.
40:111–119. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikura K, Takamura T, Takeshita Y,
Nakagawa A, Imaizumi N, Misu H, Taji K, Kasahara K, Oshinoya Y,
Suzuki S, et al: Cushing's syndrome and big IGF-II associated
hypoglycaemia in a patient with adrenocortical carcinoma. BMJ Case
Rep. 2010.bcr07.2009.2100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sinclair TJ, Gillis A, Alobuia WM, Wild H
and Kebebew E: Surgery for adrenocortical carcinoma: When and how?
Best Pract Res Clin Endocrinol Metab. 34:1014082020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamai T, Murakami S, Arai K, Ishida K and
Kijima T: Association of Nrf2 expression and mutation with Weiss
and Helsinki scores in adrenocortical carcinoma. Cancer Sci.
13:2368–2377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaujoux S, Weinandt M, Bonnet S, Reslinger
V, Bertherat J and Dousset B: Surgical treatment of adrenal
carcinoma. J Visc Surg. 154:335–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puglisi S, Calabrese A, Basile V, Pia A,
Reimondo G, Perotti P and Terzolo M: New perspectives for mitotane
treatment of adrenocortical carcinoma. Best Pract Res Clin
Endocrinol Metab. 34:1014152020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karimi F, Dehghanian A, Fallahi M and
Dalfardi B: Pure androgen-secreting adrenocortical carcinoma
presenting with hypoglycemia. Arch Iran Med. 22:527–530.
2019.PubMed/NCBI
|
|
17
|
Khadka S, Mandal S, Kasireddy V, Ghimire
S, Maganti T and Mols-Kowalczewski B: Hypoglycemia in a patient
with hypercortisolism and adrenocortical carcinoma: A paradoxical
entity. J Adolesc Young Adult Oncol. 11:122–125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Douillard C, Jannin A and Vantyghem MC:
Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris).
81:110–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scalia P, Marino IR, Asero S, Pandini G,
Grimberg A, El-Deiry WS and Williams SJ: Autocrine
IGF-II-associated cancers: From a rare paraneoplastic event to a
hallmark in malignancy. Biomedicines. 12:402023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller BS, Rogol AD and Rosenfeld RG: The
history of the insulin-like growth factor system. Horm Res
Paediatr. 95:619–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holly JMP, Biernacka K and Perks CM: The
neglected insulin: IGF-II, a metabolic regulator with implications
for diabetes, obesity, and cancer. Cells. 8:12072019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phan AT: Adrenal cortical carcinoma-review
of current knowledge and treatment practices. Hematol Oncol Clin
North Am. 21:489–507. viii–ix. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Angelousi A, Kassi E and Kaltsas GA:
Adrenocortical Carcinoma. Endotext [Internet], MDText.com, Inc.,
South Dartmouth, MDText.com, Inc. 2025.
|
|
24
|
Fassnacht M, Dekkers OM, Else T, Baudin E,
Berruti A, de Krijger R, Haak HR, Mihai R, Assie G and Terzolo M:
European society of endocrinology clinical practice guidelines on
the management of adrenocortical carcinoma in adults, in
collaboration with the European network for the study of adrenal
tumors. Eur J Endocrinol. 179:G1–G46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fassnacht M, Terzolo M, Allolio B, Baudin
E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A,
Jarzab B, et al: Combination chemotherapy in advanced
adrenocortical carcinoma. N Engl J Med. 366:2189–2197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng JH, Li HZ, Ji ZG, Zhang Y and Liu GH:
Comprehensive treatment of adrenal cortical carcinoma. Beijing Da
Xue Xue Bao Yi Xue Ban. 51:298–301. 2019.(In Chinese). PubMed/NCBI
|
|
27
|
Yeap BT, Teah KM, Tan JBG and Azizan N:
Case report: A giant hemorrhagic adrenocortical carcinoma causing
cardiorespiratory embarrassment. Ann Med Surg (Lond).
71:1029962021. View Article : Google Scholar : PubMed/NCBI
|