Introduction
Breast cancer (BC) is a highly heterogeneous disease
comprising genetically and epigenetically distinct subtypes with
varying clinical features (1).
Based on the expression of estrogen receptor (ER), progesterone
receptor (PR) expression and human epidermal growth factor receptor
2 (HER2), or HER2 amplification, BC is classified into five
subtypes: Luminal A, Luminal B, HER2+, triple-positive
and triple-negative subtypes (2).
A total of >50% of patients with HER2+
BC also express ER and/or PR (3).
HR+/HER2+ BC can be categorized as
HER2+ BC combined with ER+ or PR+,
or both ER+ and PR+ [triple-positive BC
(TPBC)]. There has been significant progress in the treatment of
HER2+ BC, and combination chemotherapy with pertuzumab
and trastuzumab improves the median survival time of metastatic
HER2+ patients to >5 years (4). The biology of
HER2+/HR+ BC differs from that of
HER2+/HR− BC. For
HER2+/HR+ tumors, the current consensus
recommends endocrine therapy after the completion of targeted
therapy and chemotherapy. However, patients with HER2+
advanced BC have few effective treatment options beyond anti-HER2
therapy and chemotherapy. The addition of a cyclin-dependent kinase
(CDK)4/6 inhibitor (CDK4/6i) combined with endocrine therapy has
been reported to have notable efficacy in the treatment of in
HR+/HER2+ advanced BC (5). Preclinical studies have reported that
CDK4/6is are effective against HER2+ cell lines
(5,6). In animal models, the combination of
anti-HER2 therapy and CDK4/6is has been reported to be more
effective than either drug alone, and it re-sensitized resistant
HER2+ BCs to anti-HER2 therapy (6). Furthermore, recent clinical trials
have evaluated the clinical efficacy of administering CDK4/6is,
aromatase inhibitors or fulvestrant to anti-HER2 therapy and
chemotherapy to patients with HR+/HER2+ BC,
showing improved median overall survival time in women with
HR+/HER2+ advanced BC when compared with
chemotherapy + trastuzumab (7,8).
CDKs are a family of serine-threonine kinases that
serve an vital role in regulating cell cycle progression (8). Among the cyclin classes, D-type
cyclins are important in cancer due to their role as the final
recipients of many oncogenic pathways. This family consists of
cyclins D1, D2 and D3, which are expressed in an overlapping and
redundant manner in all proliferating cell types (9). D-cyclins bind to and activate CDK4 and
CDK6, forming complexes that phosphorylate the retinoblastoma (RB)
tumor suppressor protein (pRB) and pRB-like proteins (p107 and
p130). This process activates E2F transcription factors, which
induce target genes necessary for DNA synthesis during the S phase.
Meanwhile, the cyclin D-CDK4/6 complex activates the CDK2 by
sequestering the cell cycle inhibitors p27Kip1 and p21Cip1,
facilitating G1 phase progression (10). The cyclin D/CDK4/6 complex is
located downstream of the HER2 pathway. In mouse models,
pharmaceutical inhibition of CDK4/6 has been reported to antagonize
HER2-driven mammary tumor growth (11). Therefore, using CDK4/6is for the
treatment of the HER2+ BC subtype is reasonable
(12). Historically, cell cycle
inhibitors have been used primarily for
ER+/HER2− BC, and they have attracted much
attention in the research of HER2+ BC. However,
preclinical and clinical studies suggest that CDK4/6is may provide
new therapeutic strategies for HER2+ BC in the future
(13).
The present report describes the case of a
60-year-old female patient diagnosed with unresectable advanced
HR+/HER2+ BC. The patient underwent systemic
treatment with an aromatase inhibitor, CDK4/6is and anti-HER2
monoclonal antibodies, achieving a favorable clinical response to
treatment. The present case highlights the potential of this
therapeutic approach.
Case report
A 60-year-old female patient discovered a mass in
the left breast and subsequently developed chest tightness in
January 2021. The patient presented to the Affiliated Hospital of
Nantong University (Nantong, China) for evaluation, and computed
tomography (CT) revealed multiple nodules in both lungs, a mass in
the left breast, multiple enlarged lymph nodes in the left axilla
and an area of non-uniform density in the right sixth rib (data not
shown). Given these findings and clinical characteristics, a
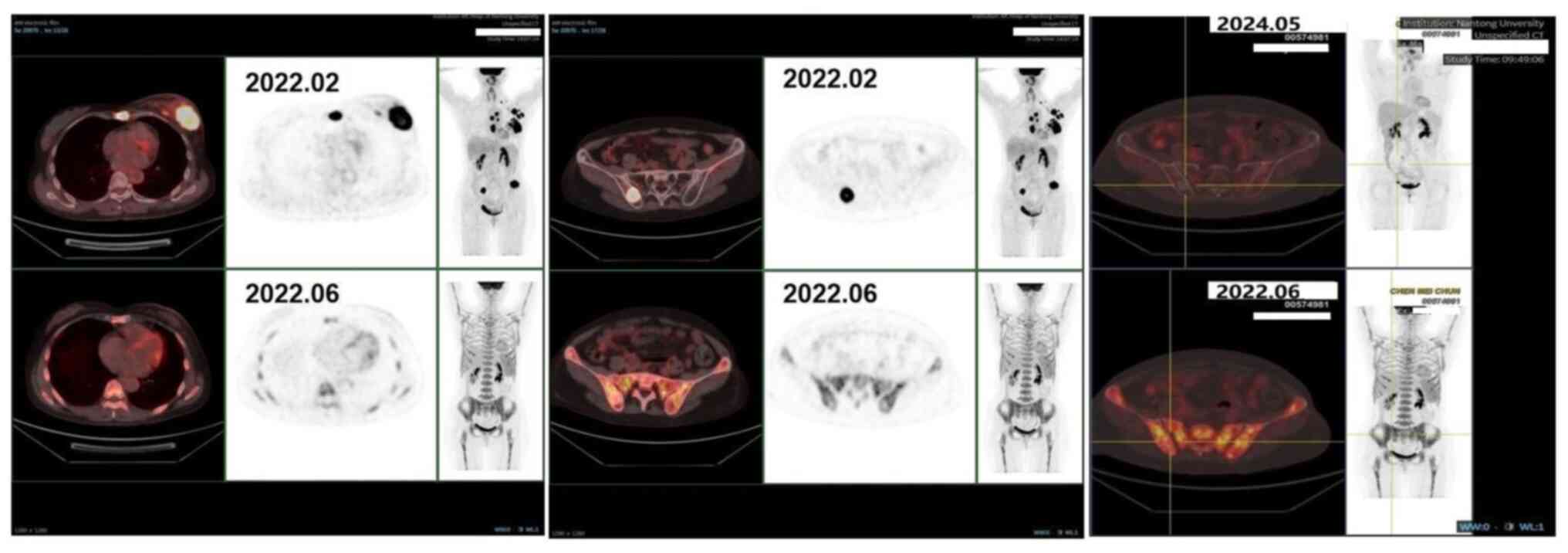
positron emission tomography (PET)-CT scan was performed in
February 2022, which indicated multiple metastases originating from
left BC (Fig. 1). The next day, a
needle biopsy was performed on the left breast mass and the left
axillary lymph node. Pathological examination of the left breast
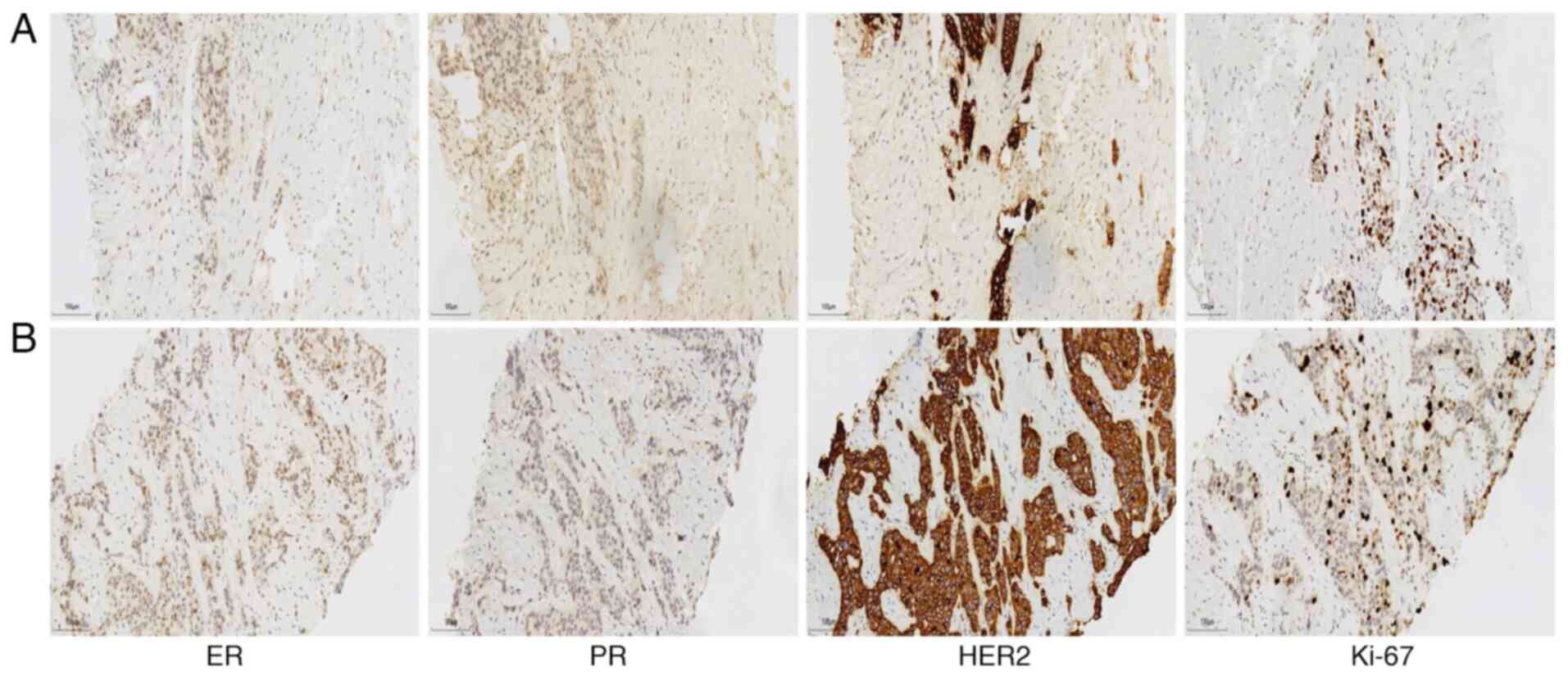
mass revealed invasive ductal carcinoma. Moreover,
immunohistochemical analysis was performed on 10% formalin-fixed
(room temperature for ≤12 h) and paraffin-embedded tissue sections,
with deaffinity and rehydration using alcohol xylene. Sections were
heated in a microwave in a sodium citrate buffer (0.01 M, pH 6.0)
for antigen retrieval. Thereafter, the sections were cultured with
5% BSA and blocked at room temperature for 30 min to inhibit
endogenous peroxidase activity and then incubated with rabbit
anti-ER (cat. no. 790-4325), anti-PR (cat. no. 790-4296) and
anti-HER2 (cat. no. 790-4493) (all Roche Diagnostics) for 1 h at
room temperature. The results demonstrated tumor staining positive
for ER (10%), negative for PR, positive for HER2 (3+), and a Ki-67
proliferation index of 30% (Fig.
2A). Similarly, the left axillary lymph node biopsy revealed
tumor staining positive for ER (30%), negative for PR, positive for
HER2 (3+), and a Ki-67 proliferation index of 30% (Fig. 2B). These results, combined with
those from the pathological examination and PET-CT scan, the
patient was diagnosed with left-sided invasive BC (maximum breast
mass diameter, 4.7 cm), accompanied by metastases to the left
supraclavicular and parasternal regions, left anterior superior
chest wall muscle space, multiple axillary lymph nodes, sternal
body and bilateral iliac bones. According to American Joint
Committee on Cancer staging, the clinical staging was cT2N3M1,
stage IV and Luminal B HER2+ subtype (14).
According to the 2022 Chinese Society of Clinical
Oncology guidelines for BC (15),
the patient received six cycles of docetaxel + trastuzumab+
pertuzumab (THP) therapy as first-line treatment from February 2022
to July 2022. The regimen included docetaxel (75 mg/m2;
day 1), trastuzumab (8 mg/kg for the first cycle and 6 mg/kg for
subsequent cycles; day 1) and pertuzumab (840 mg for the first
cycle and 420 mg for subsequent cycles; day 1), administered every
21 days. Subcutaneous injection of denosumab (120 mg, every 3
weeks) was concurrently administered to prevent bone-related
events.
PET-CT performed in June 2022 demonstrated a partial
response to treatment (Fig. 1).
Given this favorable treatment response, the patient transitioned
to maintenance therapy, consisting of exemestane (25 mg oral daily)
and palbociclib (125 mg oral daily for 21 days, followed by a 7-day
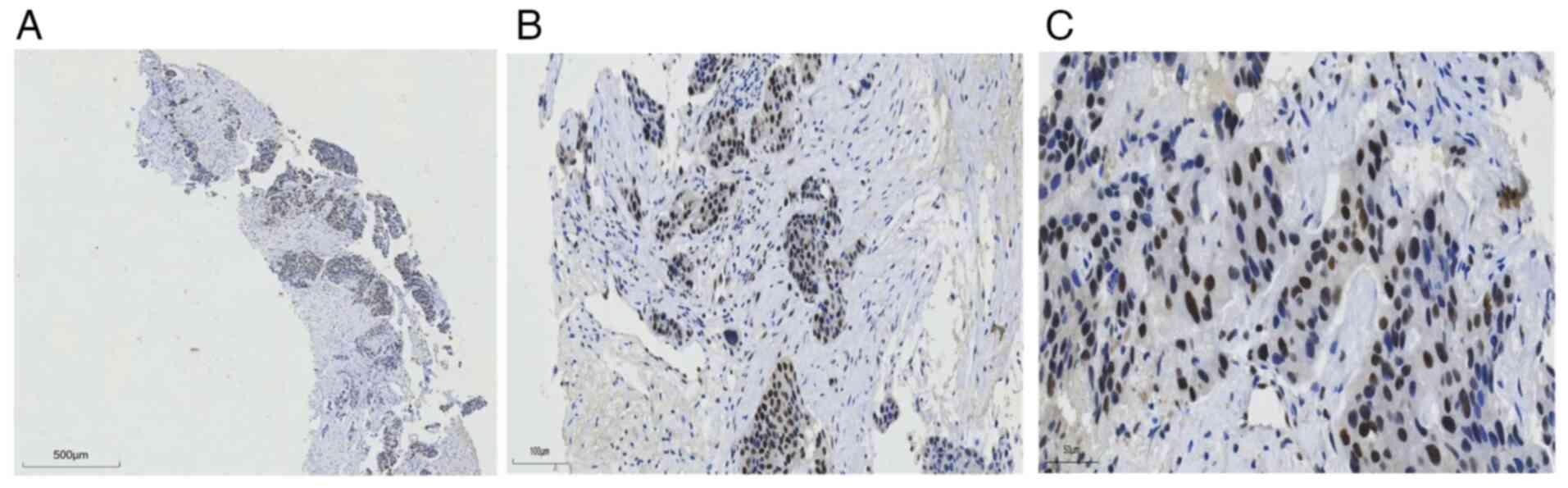
break). RB protein expression was assessed by immunohistochemistry
to predict the efficacy of the CDK4/6is (Fig. 3). Due to persistent grade IV
leukopenia after one cycle of 125 mg palbociclib, the dose was
reduced to 100 mg daily. The patient continued trastuzumab and
pertuzumab during maintenance therapy with exemestane and
palboxiclib.
In May 2024, the patient underwent PET-CT again, and
the results indicated a sustained good response. Based on the
previous PET-CT results, the last result was rated as a continuous
partial response (Fig. 1).
According to the RECIST 1.1 criteria (16), the patient achieved a
progression-free survival (PFS) of ≥30 months as of the last
follow-up in September 2024 (Fig.
4).
Discussion
HER2+ BC accounts for 15–20% of all BCs
and is considered a more aggressive subtype (17). A total of >50% of
HER2+ tumors also express hormonal receptors (4). Chemotherapy with a taxane +
trastuzumab and pertuzumab is currently the frontline regimen for
patients with advanced HER2+ BC based on the results of
the CLEOPATRA trial. Trastuzumab emtansine (T-DM1) is the
second-line option for current treatment (18,19).
Furthermore, the results of the MARIANNE trial reported that the
median overall survival rate was similar across the different
treatment groups in the study: Trastuzumab + a taxane, 50.9 months;
T-DM1, 53.7 months; and T-DM1 + pertuzumab, 51.8 months (19). None of the subgroups showed a
significant benefit with one treatment regimen in comparison with
the others. Therefore, T-DM1 is currently recommended as an
appropriate choice for patients deemed unsuitable for taxane-based
therapy. Moreover, data from the CLEOPATRA trial currently reports
the longest PFS for patients with advanced breast cancer. Other
clinical trials such as TANDEM, EGF30008 and ELECTRA have reported
that combining HER2-targeted therapies with endocrine treatment
notably prolongs PFS in metastatic TPBC (20,21).
Based on these findings, it is recommended to incorporate hormone
agents to HER2-targeted therapy following the completion of
cytotoxic chemotherapy. Furthermore, preclinical evidence suggests
that crosstalk between HER2 and ER signaling pathways contributes
to resistance to hormonal therapy in BC (22). Meanwhile, multiple trials have
reported that including CDK4/6is in endocrine treatment markedly
improves PFS in metastatic cases (22,23).
CDK4/6 has emerged as a promising target in
HER2+ BC, as the cyclin D1/CDK4/6/pRB axis is also a key
pathway influencing the efficacy of HER2-targeted therapies
(6). ER and PR, which are
classified as steroid hormone receptors (also known as nuclear
receptors), directly bind to specific DNA sequences in the
regulatory regions of target genes to regulate transcription,
promoting cell division, proliferation and invasion (24). Even estrogens, which engage in
nongenomic ER activity outside the nucleus, have been reported to
activate the HER2 signaling pathway (25). The elucidation of the CDK4/6-cyclin
D-RB pathway, implicated in the pathogenesis of BC and other
tumors, has led to the development of CDK4/6is to induce G1 cell
cycle arrest and apoptosis. These inhibitors associate with cyclin
D to form complexes that promote Rb protein phosphorylation and
inactivation, driving cell division. Through the inhibition of
CDK4/6, Rb is dephosphorylated, leading to cell cycle arrest
(26). CDK4/6is (palbociclib,
ribociclib and abemaciclib) have been extensively studied across
several tumors, and CDK4/6 has also been implicated in resistance
mechanisms to HER2-targeted therapies (27).
Furthermore, molecular studies indicate that the
cyclin D-CDK4/6 pathway is often overactivated in HR+ BC
(28). The amplification of the
cyclin D1 oncogene (CCND1), CDK4 or deletions of the tumor
suppressor genes CDK inhibitor 2A (encoding p16INK4a and p14ARF)
have been observed in BC. Additionally, ER, which is the primary
driver of tumor growth and survival in HR+ BC, directly
targets CCND1 (22).
Evidence suggests that complex crosstalk between
HER2 and ER signaling contributes to poor responses to standard
therapies in patients with TPBC; therefore, it is reasonable to
consider targeting both types of signaling pathways.
Trastuzumab-treated resistant tumors exhibit CDK4/6-dependent
proliferation (6). Marked progress
has been made in the treatment of metastatic (M)BC, particularly in
ER+ subtypes, where CDK4/6is are now recommended as
standard additions to endocrine therapy (8). Considering the multifaceted signaling
mechanisms in TPBC, combination therapies targeting both HER2 and
ER signaling may be more effective in blocking the complex network.
Moreover, HER2 and ER signaling converge on RB1, and inhibition of
cyclin D1 and CDK4 has been reported to reverse resistance to
HER2-targeted therapies in HER2+ BC. Although combined
HER2 and CDK4/6 inhibition does not significantly increase tumor
cell apoptosis, in vitro and in vivo studies have
reported that this approach reduces cellular proliferation by
inducing G1 cell cycle arrest (6).
Recently, a pilot trial investigating neoadjuvant
pyrotinib combined with trastuzumab, dalpiciclib and letrozole in
patients with TPBC reported a promising pathological response with
an acceptable safety profile (29).
Furthermore, the MONARCHER trial reported that abemaciclib, in
combination with fulvestrant and trastuzumab, markedly improved PFS
and overall survival in patients with HR+ and
HER2+ advanced BC compared with standard-of-care
chemotherapy + trastuzumab (7).
Similarly, the SOLTI-1303 PATRICIA trial reported that palbociclib
in combination with trastuzumab was safe and exhibited promising
survival outcomes in patients with ER+/HER2+
advanced BC (30). These results
suggest that combination therapy involving CDK4/6is, HER2-targeted
therapies and endocrine therapy may be a promising approach for
TPBC. However, the efficacy of CDK4/6is is often dependent on RB
protein expression (31).
Therefore, monitoring of RB protein loss or other RB mutations is
recommended both before and after treatment progression with
CDK4/6is. Low RB1 protein expression has been reported to reduce
the effectiveness of CDK4/6-Cyclin D complex inhibition, leading to
the tumor resistance to CDK4/6 inhibitors (32). A study using glioblastoma xenograft
cells reported that an A193T missense mutation in RB exon 2, which
reduced RB1 protein levels, was associated with resistance to
CDK4/6is (32). Therefore, further
exploration and analysis of the expression and regulatory
mechanisms of RB1 may help to overcome the resistance and expand
the application of CDK4/6is in TPBC. Although the patient in the
present case achieved encouraging results, there are still certain
limitations in the application of CDK4/6is for HER2+ and
HR+ BC, such as the high cost of CDK4/6is and the lack
of large-scale clinical trials specifically targeting
HER2+ and HR+ subtypes (29). Furthermore, the underlying causes of
heterogeneity within these subtypes require further
investigation.
Treatment-related adverse events and comorbidities
are critical to maintain effective and timely treatment, as they
affect the quality of life of patients. The development of
third-generation CDKis has led to marked improvements in
selectivity, activity and toxicity profiles (33). For example, maintenance therapy with
CDK4/6is is associated with fewer adverse events compared with
chemotherapy, such as gastrointestinal reactions, liver function
abnormalities and fatigue. However, attention must still be paid to
potential adverse effects of CDK4/6is. In the present case, the
patient experienced grade 3–4 reductions in white blood cell
counts, increasing the risk of infection during treatment. Although
the white blood cell levels recovered during the withdrawal of
CDK4/6is, the risk of infection remained substantial during the
period. Therefore, it is essential to manage the adverse reactions
throughout the treatment course. Currently, CDK4/6is have been
considered as one of the most promising therapies for
HER2+ MBC and are likely to become standard clinical
practice in the near future. Furthermore, the results of the
present case support the feasibility and efficacy of combining
CDK4/6is with HER2-targeted and endocrine therapies, providing a
solid foundation for future clinical applications.
In conclusion, the present report details the case
of a patient with HR+/HER2+ MBC who achieved
a favorable outcome after six cycles of THP therapy, followed by
treatment with exemestane (25 mg once daily) and palbociclib
(initially 125 mg once daily for 21 days, followed by a 7-day
break, then adjusted to 100 mg once daily). To date, the patient
has achieved a PFS of ≥30 months. Therefore, the present clinical
case helps to further recognize that the classic paradigm of dual
target therapy, combining with endocrine therapy and CDK4/6is,
demonstrates superior super efficacy in patients with
HR+/HER2+ BC, especially in those with high
RB expression, providing valuable insights for the clinical
practice in the treatment of HR+/HER2+
BC.
Acknowledgements
Not applicable.
Funding
Funding was received from The Foundation of Affiliated Hospital
of Nantong University (grant nos. BSH202203 and Tdb2107) and The
Science and Technology Project of Nantong City (grant no.
MSZ2024013).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FL made recommendations for treatment and conceived
the idea of the study. YHC designed and drafted the manuscript. JLZ
obtained medical images and collected the data. HXD and YHC
analyzed the data and revised the manuscript. FL and YHC confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Affiliated Hospital of Nantong University (approval no.
2018-K020).
Patient consent for publication
The patient in the present report agreed to
participate in the study and provided written informed consent for
publication of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen H, Gui X, Zhou Z, Su F, Gong C, Li S,
Wu W, Rao N, Liu Q and Yao H: Distinct ER and PR expression
patterns significantly affect the clinical outcomes of early
HER2-positive breast cancer: A real-world analysis of 871 patients
treated with neoadjuvant therapy. Breast. 75:1037332024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai X, Cheng H, Bai Z and Li J: Breast
cancer cell line classification and its relevance with breast tumor
subtyping. J Cancer. 8:3131–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vici P, Pizzuti L, Sperduti I, Frassoldati
A, Natoli C, Gamucci T, Tomao S, Michelotti A, Moscetti L, Gori S,
et al: ‘Triple positive’ early breast cancer: an observational
multicenter retrospective analysis of outcome. Oncotarget.
7:17932–17944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larionov AA: Current therapies for human
epidermal growth factor receptor 2-positive metastatic breast
cancer patients. Front Oncol. 8:892018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goel S, Wang Q, Watt AC, Tolaney SM,
Dillon DA, Li W, Ramm S, Palmer AC, Yuzugullu H, Varadan V, et al:
Overcoming therapeutic resistance in HER2-positive breast cancers
with CDK4/6 inhibitors. Cancer Cell. 29:255–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tolaney SM, Goel S, Nadal J, Denys H,
Borrego MR, Litchfield LM, Liu J, Appiah AK, Chen Y and André F:
Overall survival and exploratory biomarker analyses of abemaciclib
plus trastuzumab with or without fulvestrant versus trastuzumab
plus chemotherapy in HR+, HER2+ metastatic breast cancer patients.
Clin Cancer Res. 30:39–49. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YJ, Li X, Hydbring P, Sanda T,
Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer
H and Sicinski P: The requirement for cyclin D function in tumor
maintenance. Cancer Cell. 22:438–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts PJ, Bisi JE, Strum JC, Combest AJ,
Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM and Sharpless NE:
Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer
therapy. J Natl Cancer Inst. 104:476–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witkiewicz AK, Cox D and Knudsen ES:
CDK4/6 inhibition provides a potent adjunct to Her2-targeted
therapies in preclinical breast cancer models. Genes Cancer.
5:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan M, Niu L, Lv H, Zhang M, Wang J, Liu
Z, Chen X, Lu Z, Zhang C, Zeng H, et al: Dalpiciclib and pyrotinib
in women with HER2-positive advanced breast cancer: A single-arm
phase II trial. Nat Commun. 14:62722023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sawaki M, Shien T and Iwata H:
TNMclassification of malignant tumors (Breast Cancer Study Group).
Jpn J Clin Oncol. 49:228–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J and Jiang Z: Chinese society of
clinical oncology breast cancer (CSCO BC) guidelines in 2022:
Stratification and classification. Cancer Biol Med. 19:769–773.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Zhou F, Zou J, Fang Y, Liu Y, Li
L, Hou J, Wang G, Wang H, Lai X, et al: Clinical considerations of
CDK4/6 inhibitors in HER2 positive breast cancer. Front Oncol.
13:13220782024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez EA, Barrios C, Eiermann W, Toi M, Im
YH, Conte P, Martin M, Pienkowski T, Pivot XB, Burris HA III, et
al: Trastuzumab emtansine with or without pertuzumab versus
trastuzumab with taxane for human epidermal growth factor receptor
2-positive advanced breast cancer: Final results from MARIANNE.
Cancer. 125:3974–3984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: Results from the randomized phase III TAnDEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston S, Pippen J, Pivot X, Lichinitser
M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ,
et al: Lapatinib combined with letrozole versus letrozole and
placebo as first-line therapy for postmenopausal hormone
receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glaviano A, Wander SA, Baird RD, Yap KCH,
Lam HY, Toi M, Carbone D, Geoerger B, Serra V, Jones RH, et al:
Mechanisms of sensitivity and resistance to CDK4/CDK6 inhibitors in
hormone receptor-positive breast cancer treatment. Drug Resist
Updat. 76:1011032024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pilehvari A, Kimmick G, You W, Bonilla G
and Anderson R: Disparities in receipt of 1-st line CDK4/6
inhibitors with endocrine therapy for treatment of hormone receptor
positive, HER2 negative metastatic breast cancer in the real-world
setting. Breast Cancer Res. 26:1442024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar R, Gururaj AE, Vadlamudi RK and
Rayala SK: The clinical relevance of steroid hormone receptor
corepressors. Clin Cancer Res. 11:2822–2831. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iqbal N and Iqbal N: Human epidermal
growth factor receptor 2 (HER2) in cancers: Overexpression and
therapeutic implications. Mol Biol Int. 2014:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Sullivan CC, Suman VJ and Goetz MP: The
emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv
Med Oncol. 11:1758835919887662019.
|
|
28
|
Vinciguerra GL, Sonego M, Segatto I,
Dall'Acqua A, Vecchione A, Baldassarre G and Belletti B: CDK4/6
Inhibitors in combination therapies: Better in company than alone:
A mini review. Front Oncol. 12:8915802022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huo S, Xue J, Wang S, Shan H, Chen G, Niu
N, Wang Y, Qiu F, Zhao Y, Xing F, et al: A pilot trial of
neoadjuvant pyrotinib plus trastuzumab, dalpiciclib, and letrozole
for triple-positive breast cancer. MedComm (2020). 5:e5052024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciruelos E, Villagrasa P, Pascual T,
Oliveira M, Pernas S, Paré L, Escrivá-de-Romaní S, Manso L, Adamo
B, Martínez E, et al: Palbociclib and trastuzumab in HER2-Positive
advanced breast cancer: Results from the phase II SOLTI-1303
PATRICIA Trial. Clin Cancer Res. 26:5820–5829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue Y and Zhai J: Strategy of combining
CDK4/6 inhibitors with other therapies and mechanisms of
resistance. Int J Clin Exp Pathol. 17:189–207. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cen L, Carlson BL, Schroeder MA, Ostrem
JL, Kitange GJ, Mladek AC, Fink SR, Decker PA, Wu W, Kim JS, et al:
p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase
inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol.
14:870–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Ba J, Kang Y, Gong Z, Liang T,
Zhang Y, Qi J and Wang J: Recent progress in CDK4/6 inhibitors and
PROTACs. Molecules. 28:80602023. View Article : Google Scholar : PubMed/NCBI
|