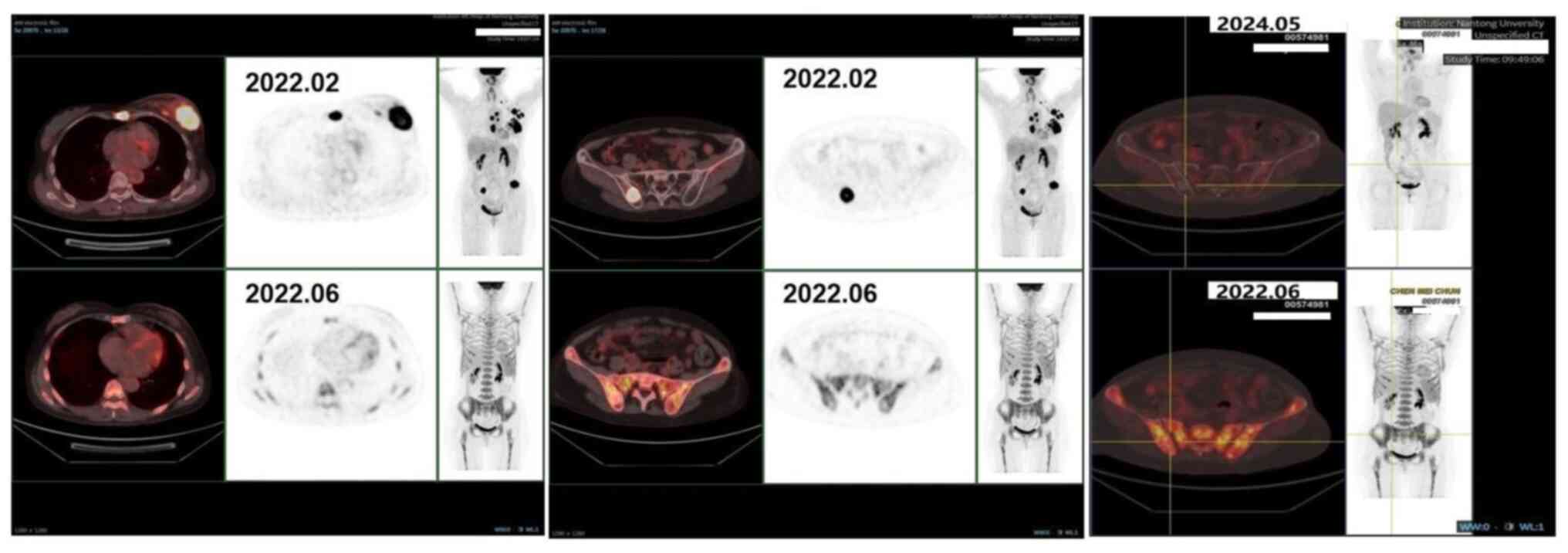
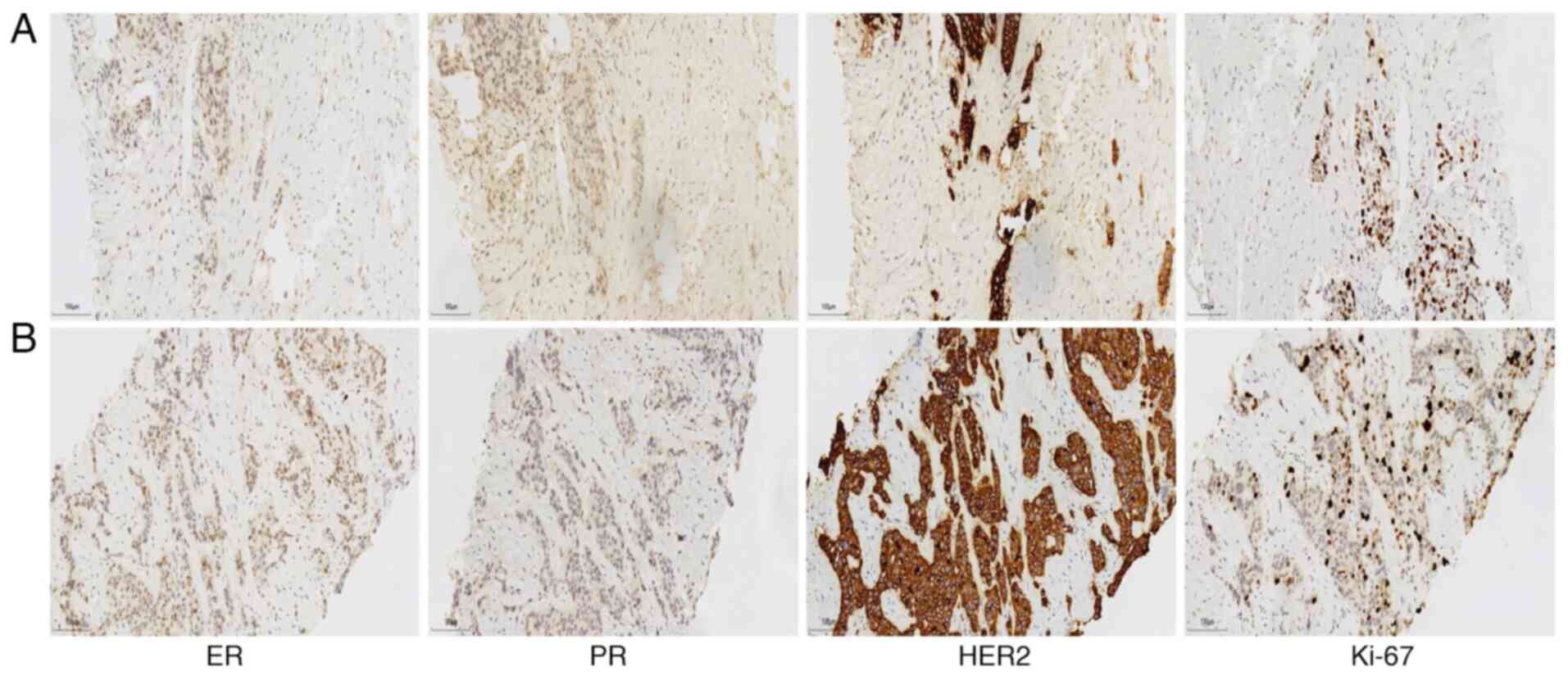
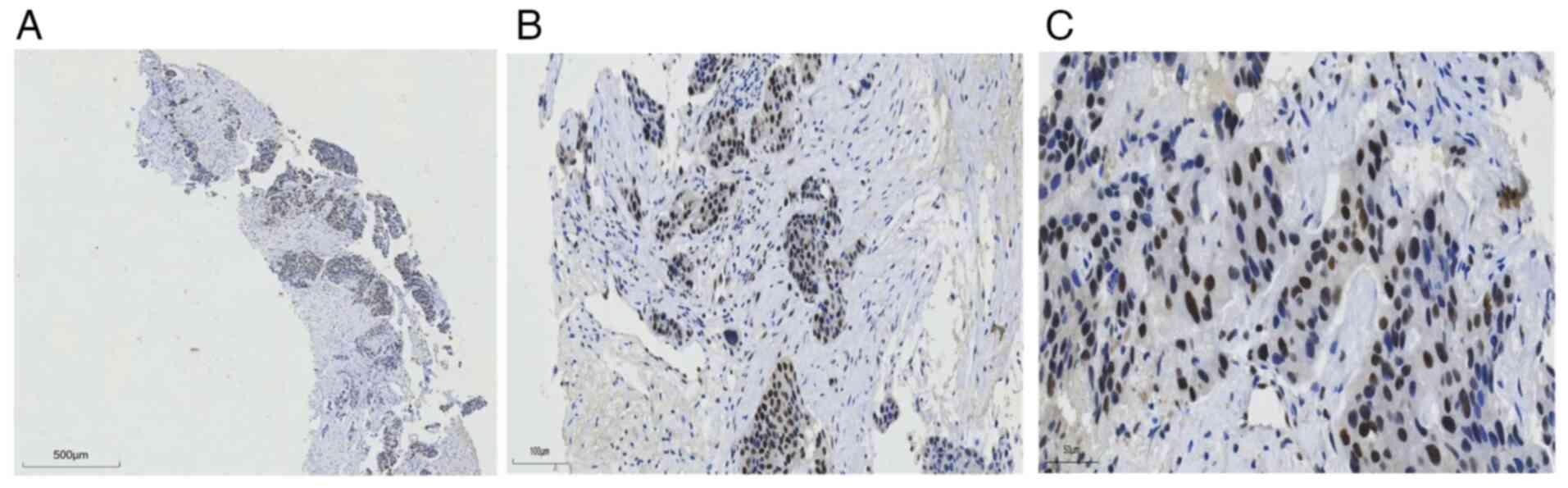
|
1
|
Chen H, Gui X, Zhou Z, Su F, Gong C, Li S,
Wu W, Rao N, Liu Q and Yao H: Distinct ER and PR expression
patterns significantly affect the clinical outcomes of early
HER2-positive breast cancer: A real-world analysis of 871 patients
treated with neoadjuvant therapy. Breast. 75:1037332024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai X, Cheng H, Bai Z and Li J: Breast
cancer cell line classification and its relevance with breast tumor
subtyping. J Cancer. 8:3131–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vici P, Pizzuti L, Sperduti I, Frassoldati
A, Natoli C, Gamucci T, Tomao S, Michelotti A, Moscetti L, Gori S,
et al: ‘Triple positive’ early breast cancer: an observational
multicenter retrospective analysis of outcome. Oncotarget.
7:17932–17944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larionov AA: Current therapies for human
epidermal growth factor receptor 2-positive metastatic breast
cancer patients. Front Oncol. 8:892018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goel S, Wang Q, Watt AC, Tolaney SM,
Dillon DA, Li W, Ramm S, Palmer AC, Yuzugullu H, Varadan V, et al:
Overcoming therapeutic resistance in HER2-positive breast cancers
with CDK4/6 inhibitors. Cancer Cell. 29:255–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tolaney SM, Goel S, Nadal J, Denys H,
Borrego MR, Litchfield LM, Liu J, Appiah AK, Chen Y and André F:
Overall survival and exploratory biomarker analyses of abemaciclib
plus trastuzumab with or without fulvestrant versus trastuzumab
plus chemotherapy in HR+, HER2+ metastatic breast cancer patients.
Clin Cancer Res. 30:39–49. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YJ, Li X, Hydbring P, Sanda T,
Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer
H and Sicinski P: The requirement for cyclin D function in tumor
maintenance. Cancer Cell. 22:438–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts PJ, Bisi JE, Strum JC, Combest AJ,
Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM and Sharpless NE:
Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer
therapy. J Natl Cancer Inst. 104:476–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witkiewicz AK, Cox D and Knudsen ES:
CDK4/6 inhibition provides a potent adjunct to Her2-targeted
therapies in preclinical breast cancer models. Genes Cancer.
5:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan M, Niu L, Lv H, Zhang M, Wang J, Liu
Z, Chen X, Lu Z, Zhang C, Zeng H, et al: Dalpiciclib and pyrotinib
in women with HER2-positive advanced breast cancer: A single-arm
phase II trial. Nat Commun. 14:62722023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sawaki M, Shien T and Iwata H:
TNMclassification of malignant tumors (Breast Cancer Study Group).
Jpn J Clin Oncol. 49:228–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J and Jiang Z: Chinese society of
clinical oncology breast cancer (CSCO BC) guidelines in 2022:
Stratification and classification. Cancer Biol Med. 19:769–773.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Zhou F, Zou J, Fang Y, Liu Y, Li
L, Hou J, Wang G, Wang H, Lai X, et al: Clinical considerations of
CDK4/6 inhibitors in HER2 positive breast cancer. Front Oncol.
13:13220782024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez EA, Barrios C, Eiermann W, Toi M, Im
YH, Conte P, Martin M, Pienkowski T, Pivot XB, Burris HA III, et
al: Trastuzumab emtansine with or without pertuzumab versus
trastuzumab with taxane for human epidermal growth factor receptor
2-positive advanced breast cancer: Final results from MARIANNE.
Cancer. 125:3974–3984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: Results from the randomized phase III TAnDEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston S, Pippen J, Pivot X, Lichinitser
M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ,
et al: Lapatinib combined with letrozole versus letrozole and
placebo as first-line therapy for postmenopausal hormone
receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glaviano A, Wander SA, Baird RD, Yap KCH,
Lam HY, Toi M, Carbone D, Geoerger B, Serra V, Jones RH, et al:
Mechanisms of sensitivity and resistance to CDK4/CDK6 inhibitors in
hormone receptor-positive breast cancer treatment. Drug Resist
Updat. 76:1011032024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pilehvari A, Kimmick G, You W, Bonilla G
and Anderson R: Disparities in receipt of 1-st line CDK4/6
inhibitors with endocrine therapy for treatment of hormone receptor
positive, HER2 negative metastatic breast cancer in the real-world
setting. Breast Cancer Res. 26:1442024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar R, Gururaj AE, Vadlamudi RK and
Rayala SK: The clinical relevance of steroid hormone receptor
corepressors. Clin Cancer Res. 11:2822–2831. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iqbal N and Iqbal N: Human epidermal
growth factor receptor 2 (HER2) in cancers: Overexpression and
therapeutic implications. Mol Biol Int. 2014:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Sullivan CC, Suman VJ and Goetz MP: The
emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv
Med Oncol. 11:1758835919887662019.
|
|
28
|
Vinciguerra GL, Sonego M, Segatto I,
Dall'Acqua A, Vecchione A, Baldassarre G and Belletti B: CDK4/6
Inhibitors in combination therapies: Better in company than alone:
A mini review. Front Oncol. 12:8915802022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huo S, Xue J, Wang S, Shan H, Chen G, Niu
N, Wang Y, Qiu F, Zhao Y, Xing F, et al: A pilot trial of
neoadjuvant pyrotinib plus trastuzumab, dalpiciclib, and letrozole
for triple-positive breast cancer. MedComm (2020). 5:e5052024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciruelos E, Villagrasa P, Pascual T,
Oliveira M, Pernas S, Paré L, Escrivá-de-Romaní S, Manso L, Adamo
B, Martínez E, et al: Palbociclib and trastuzumab in HER2-Positive
advanced breast cancer: Results from the phase II SOLTI-1303
PATRICIA Trial. Clin Cancer Res. 26:5820–5829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue Y and Zhai J: Strategy of combining
CDK4/6 inhibitors with other therapies and mechanisms of
resistance. Int J Clin Exp Pathol. 17:189–207. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cen L, Carlson BL, Schroeder MA, Ostrem
JL, Kitange GJ, Mladek AC, Fink SR, Decker PA, Wu W, Kim JS, et al:
p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase
inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol.
14:870–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Ba J, Kang Y, Gong Z, Liang T,
Zhang Y, Qi J and Wang J: Recent progress in CDK4/6 inhibitors and
PROTACs. Molecules. 28:80602023. View Article : Google Scholar : PubMed/NCBI
|