Introduction
Diffuse midline gliomas (DMG) with the H3K27M
mutation are primary malignant tumors located in the linear
structures of the brain; according to the CBTRUS statistical
report, DMG is rare in adults, with an annual incidence rate of
~0.1 cases per 1 million people, accounting for 2–3% of all gliomas
in adults. H3K27M mutation is a core molecular hallmark of DMG, and
>70% of patients with DMGs carry this mutation. The prognosis
for this disease is extremely poor, and the mortality rate is
extremely high, with a median overall survival of only 12 months
for newly diagnosed patients and a 5-year survival rate of only 1%
(1). Other molecular types of DMG
include the EZHIP overexpression type, EGFR mutant type and H3G34
mutant type. In addition to use of conventional surgery,
radiotherapy and chemotherapy, the exploration of new approaches is
essential to improve patient prognosis. Currently, there are few
reports on the diagnosis and treatment of H3K27M mutant diffuse
midline glioma (2). In the present
study, a patient with adult H3 K27M mutant diffuse midline glioma
was admitted to the Department of Radiotherapy of Oncology,
Shenzhen People's Hospital (Shenzhen, China) and was administered a
combination of treatment strategies such as surgery, radiotherapy,
chemotherapy, electric field therapy, immunotherapy and targeted
therapy, which is a new innovative direction for treatment.
Case report
A 20-year-old woman presented to Shenzhen People's
Hospital in September 2021 with a deviated angle of the mouth and
left-sided limb dysfunction. Subsequently, a magnetic resonance
imaging (MRI) examination was performed at the Imaging Department
of Shenzhen People's Hospital (Shenzhen, China), which showed
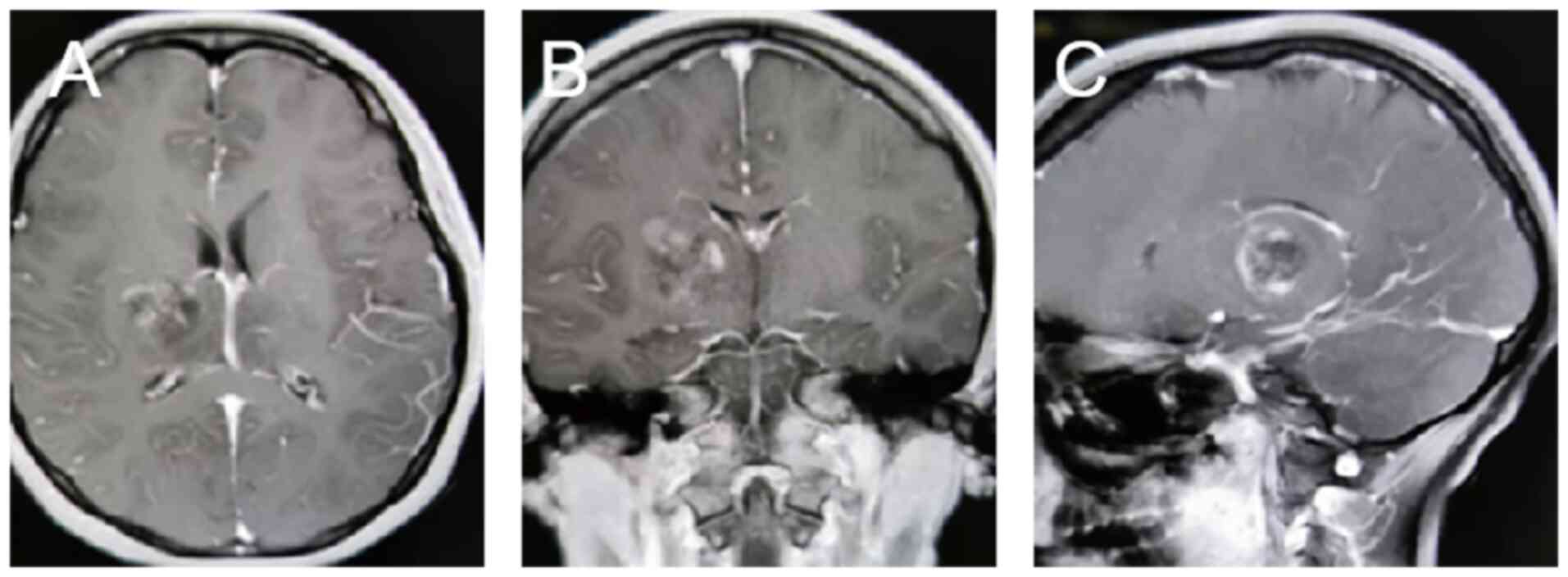
bilateral thalamic masses (Fig. 1).
The thalamus assists in maintaining consciousness and perceptual
functions. Surgical procedures can damage the structures of the
thalamus, leading to complications such as impaired memory,
language or motor function, and the high risk of surgery requires
extra care in the surgeon's evaluation. On the recommendation of
the surgeon, the patient underwent microscopic resection of the
right thalamic mass. Postoperative pathology showed a DMG of the
right thalamus, with H3K27M mutation, at World Health Organization
(WHO) grade 4 (3). The
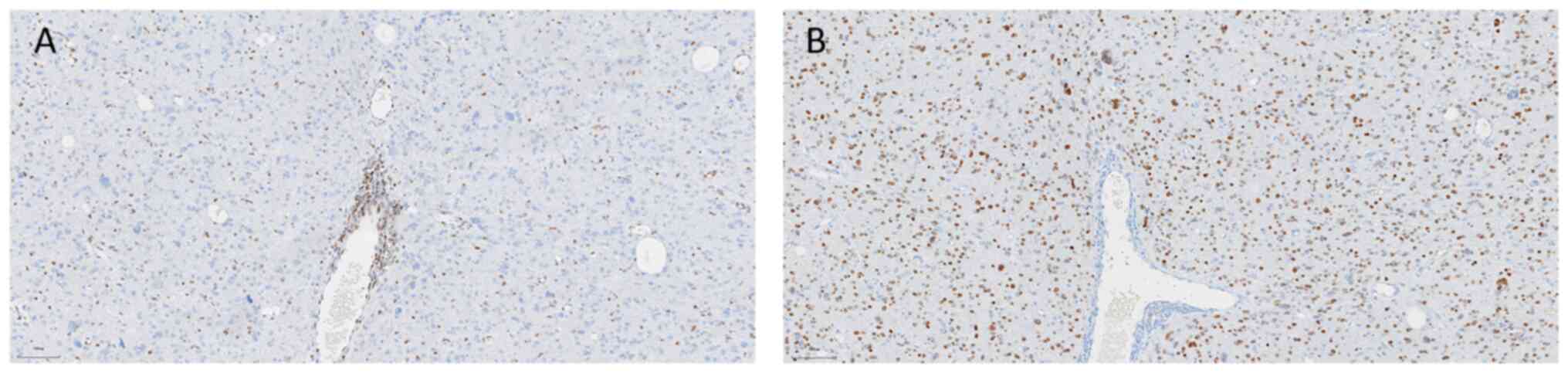
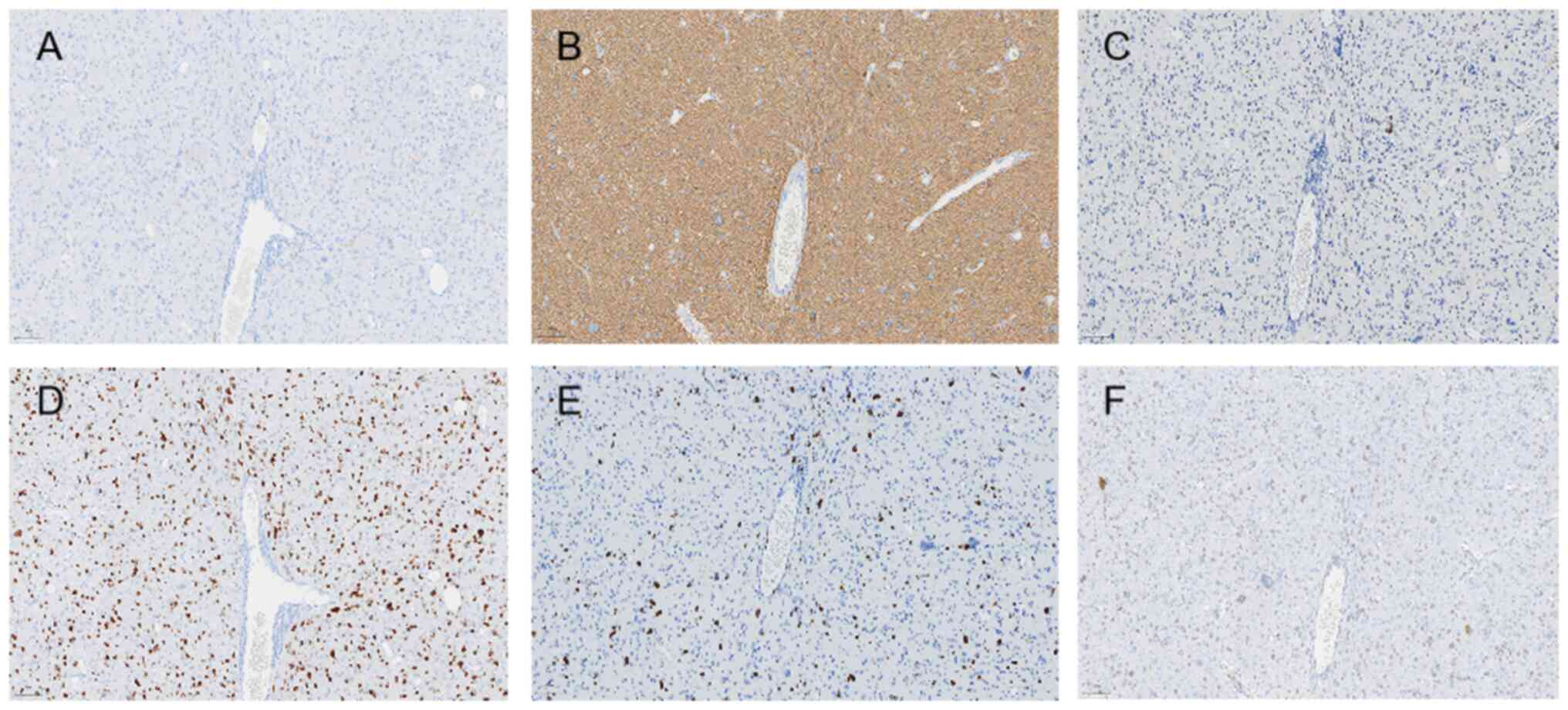
immunohistochemistry protocol was as follows: Paraffin-embedded
tissue sections were deparaffinized and hydrated sequentially in
xylene, anhydrous ethanol, gradient ethanol and distilled water.
The sections were then placed in EDTA antigen repair buffer, heated
at 95–100°C for 20 min, cooled for 10 min and rinsed three times (3
min/wash) with PBS. Next, 3% peroxidase blocker was added at 36°C
for 10 min, and the sections were rinsed three times with PBS (3
min/wash). Primary antibodies were added to the sections and
incubated at 4°C overnight (12 h). Sections were then preheated at
37°C for 30 min and washed three times (5 min/wash) with PBS (cat.
no. 950–300; Roche Diagnostics). A secondary antibody (horseradish
peroxidase-conjugated; 1:100; cat. no. 760-500; Roche Diagnostics)
was added and incubated in an oven at 37°C for 8 min, then washed
three times (5 min/wash) with PBS. After removing the PBS solution,
freshly prepared DAB color development solution was added and
incubated for 3–5 min. After rinsing with tap water, the sections
were incubated with hematoxylin stain for 10 to 30 sec, and then
rinsed with PBS. Finally, the sections were dehydrated, cleared and
sealed. Light microscopic examination (Leica Biosystems)
demonstrated the following results: H3K27M(+) (1:100; cat. no.
ZA-0321), H3K27me3(−) (1;100; cat. no. ZA-0327), isocitrate
dehydrogenase 1 (IDH1)(−) (1:50; cat. no. TW-0821), glial
fibrillary acidic protein(+) (1:1,000; cat. no. MX-047), p53(−)
(1;100; cat. no. BPM6168), oligodendrocyte transcription
factor-2(+) (1:100; cat. no. EP-112), Ki-67(+)(50%) (1;100; cat.
no. BP-6045), transcriptional regulator ATRX(+) (1:200; cat. no.
MX-071) (all Roche Diagnostics). O6 methylguanine DNA
methyltransferase (MGMT) promoter methylation negativity (cat. no.
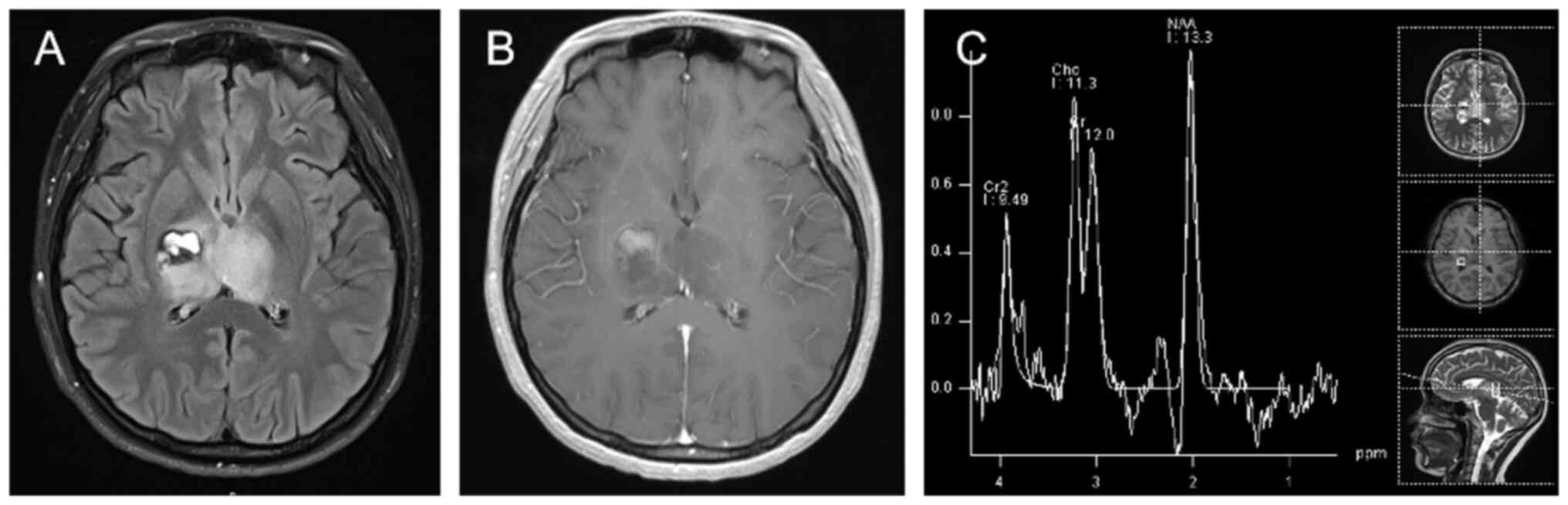
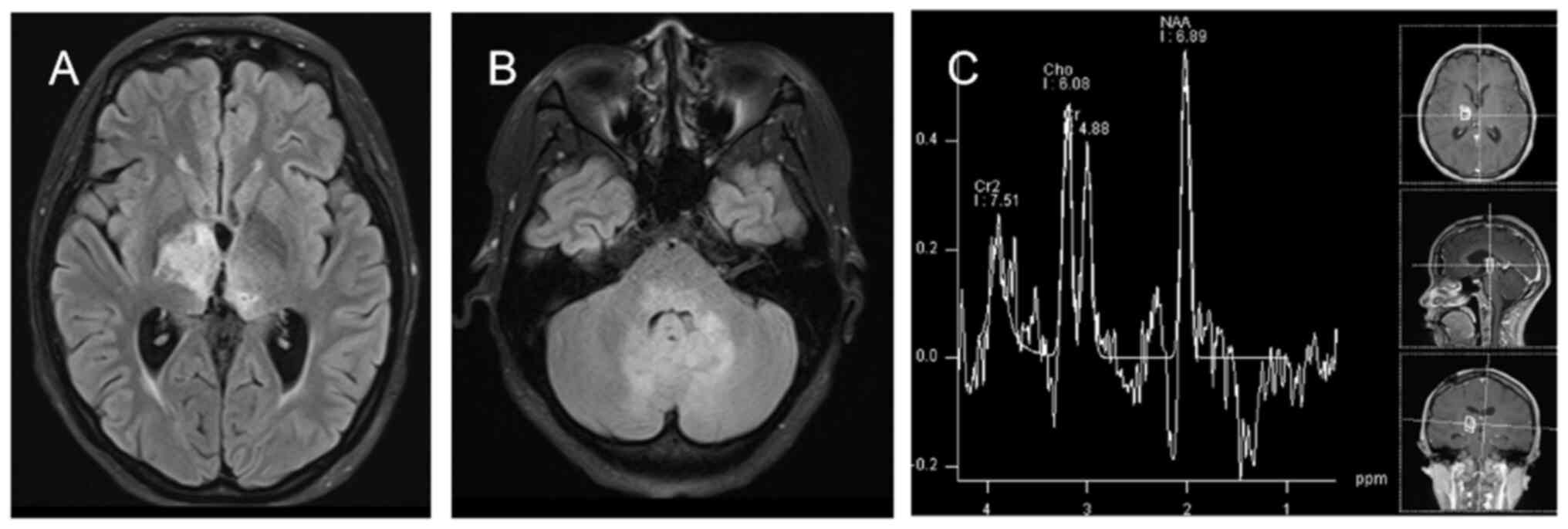
MX-0361), and a lack of 1p/19q co-deletion (Figs. 2 and 3). At 1-month post-surgery, head MRI
follow-up showed a thalamic glioma on the left side and
postoperative changes on the right side of the lesion; Magnetic
resonance spectroscopy (MRS) showed that MRS changes in the left
thalamic lesion were consistent with the tumor lesion, and MRS
around the right thalamic surgical area did not show any
significant abnormality (Fig.
4).
As the tumor could not be completely removed by
surgery, the patient started postoperative adjuvant radiotherapy in
November 2021, following the Radiation Therapy Oncology Group
principles of radiotherapy target delineation for high-grade
gliomas (4). The prescribed dose
for the first phase was 46 Gy/23 fractions and the prescribed dose
for the second phase of treatment was 14 Gy/7 fraction. During
radiotherapy, concurrent temozolomide chemotherapy (75
mg/m2, orally, after 4 h of fasting, once a day) was
administered. At 4 weeks post-synchronous radiotherapy and
chemotherapy, the patient entered the adjuvant chemotherapy stage
and orally took 150–200 mg/m2 temozolomide daily for 5
consecutive days, repeating every 28 days for a total of 12 cycles.
Notably, the treatment was combined with maintenance with
tumor-treating fields (TTFields) during and after radiotherapy.
During the follow-up period, the patient underwent a
brain-enhanced MRI follow-up assessment every 3 months, and the
efficacy was analyzed according to the Response Assessment Criteria
in Neuro-Oncology (RANO) (5). As of
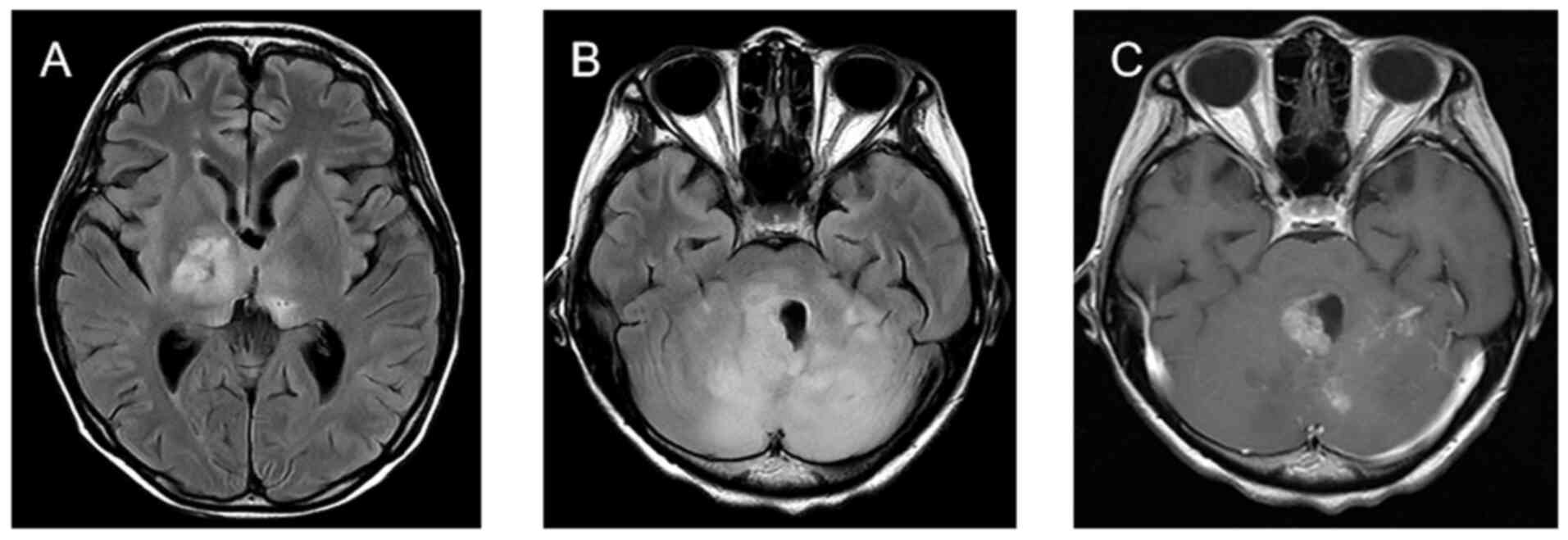
June 2023, the patient developed the symptom of unsteady walking,
and the follow-up cranial MRI showed significant changes in the
long T1 and long T2 abnormal signals in the bilateral thalamus,
bilateral cerebellar hemispheres and cerebral pons (Fig. 5). Tumor progression was considered
after a multidisciplinary discussion based on RANO criteria.
In August 2023, the patient was started on
maintenance therapy with bevacizumab (5 mg/kg once every 2 weeks)
in combination with TTFields. After 2 months, follow-up brain
enhancement MRI showed a slightly larger intracranial
space-occupying lesion than before (in June 2023, the size of the
lesion in the thalamus was 20×17 mm, and the size of the lesion in
the cerebellar hemispheres and pontine brain was 28×21 mm; in
October 2023, the size of the lesion in the thalamus was 30×20 mm,
and the size of the lesion in the cerebellar hemispheres and pons
was 28×25 mm), indicating that the patient's disease was poorly
controlled. In October 2023, the treatment regimen was changed to
bevacizumab (10 mg/kg every 2 weeks) and pembrolizumab (200 mg
every 3 weeks) immunotherapy in combination with TTFields. In
December 2023, follow-up brain-enhanced MRI showed slow progression
of the lesion. The efficacy was assessed as stable disease (SD)
according to the RANO criteria. However, the patient's family
wanted to achieve more effective tumor suppression, so they
purchased their own targeted drugs, ONC201 (510 mg orally once a
week) and ONC206 (60 mg orally once a week) (both Lindburg
Pharmacy, Inc.), which are not currently indicated for use in
China, and used them for personalized combination therapy with
bevacizumab, pembrolizumab and TTFields in December 2023. In
January 2024, a follow-up cranial MRI showed that the bilateral
thalamus, dorsal pontine, cerebellar vermis and bilateral
cerebellar hemispheric lesions had not changed, showing temporary
control of the lesion.
In February 2024, due to aspiration pneumonia, the
patient suspended the treatment of bevacizumab and pembrolizumab
but still insisted on the treatment using TTFields, ONC201 and
ONC206. In March 2024, the patient developed symptoms of choking on
drinking water, difficulty in swallowing and weakness while
coughing up sputum. In March 2024, a follow-up cranial MRI showed
that the foci of the bilateral thalamus, dorsal pontine, cerebellar
earth and bilateral cerebellar hemispheres had increased in size
(Fig. 6) compared with the previous
measurements (the size of the lesion in the thalamus was 30×20 mm,
and the size of the lesion in the cerebellar hemispheres and pons
was 33×29 mm), and tumor progression was considered. The patient
subsequently received respiratory treatment for a lung infection,
and anti-tumor therapy was suspended. The patient succumbed to
respiratory failure in March 2024.
Discussion
H3K27M mutant glioma is a relatively newly
discovered disease. This mutation was first reported in 2012 and
typically occurs in H3.1 or H3.3 subtypes, serving as the
initiating mutation in tumors (6).
This mutation coexists with multiple genetic changes, but does not
coexist with IDH and EGFR mutations. H3K27M mutant tumors usually
have unmethylated MGMT, leading to poor efficacy of temozolomide in
radiotherapy. This mutation also leads to H3K27 trimethylation loss
and H3K27 acetylation increase, affecting gene expression. In 2016,
the WHO classified H3K27M mutant tumors as a unique form of grade
IV glioma. The updated 2021 classification has been further refined
to include prognostically relevant molecular annotation for H3K27
(3,7). The H3K27M mutation is associated with
poor survival rates, and affects children and young adults. Up to
90% of cases in pediatric diffuse intrinsic pontine glioma (DIPG)
carry this mutation, and 15–60% of cases in adult DMG also have
this mutation (8).
Radiotherapy is currently the only standard of care
that temporarily relieves the symptoms of DMG, delays disease
progression and prolongs median survival time (9); however, there is still insufficient
evidence to determine the optimal radiotherapy target area and
dose. In 2017, the Chinese consensus of experts on radiotherapy for
gliomas recommended a total radiation dose of 45–54 Gy (1.8–2.0
Gy/dose). Depending on the specific circumstances (such as high
malignancy), 54–60 Gy (1.8 to 2.0 Gy/dose) or 39 Gy (3.0 Gy/dose)
could also be selected (10). The
Korean Society of Neuro-Oncology guideline 2021.1 for adult DMGs
recommends a total dose of 54 to 60 Gy, and the therapeutic target
range includes 1–2 cm of tumor outgrowth for conventional segmental
irradiation (11). In the present
case, the patient's tumor was located in the bilateral thalamus and
the tumor could not be entirely removed by surgery. Postoperative
radiotherapy was the primary treatment method. Therefore, the
radiotherapy plan was developed according to the RTOG high-grade
glioma radiotherapy target area delineation method (4). The total dose of radiotherapy was up
to 60 Gy, and the patient's whole radiotherapy process went
smoothly without any prominent radiotherapy toxicities or side
effects. It is well known that the high expression of MGMT in DMG
with H3K27M mutation can lead to resistance to temozolomide in
patients (12,13). In addition, the presence of the
blood-brain barrier limits the entry of most antitumor drugs into
the brain (14,15). However, a retrospective study using
radiotherapy with synchronized temozolomide followed by adjuvant
temozolomide chemotherapy for the treatment of DMGs in children
showed that the median time to progression after treatment was 10.2
months. The 1-year progression-free survival (PFS) rate was 41.7%,
with a median survival time of 13.5 months, suggesting that
chemotherapy improves outcomes (16). In the present case, the patient was
treated postoperatively with radiotherapy synchronized with
temozolomide chemotherapy followed by a sequential 12-cycle long
course of temozolomide adjuvant chemotherapy, and the patient's
disease did not progress throughout the entire course of
chemotherapy. Therefore, whether the combination of radiotherapy
and chemotherapy would have a long-term survival effect still
requires further study.
Tumor-treating fields (TTFields) is a portable,
non-invasive anticancer therapy whose primary antitumor mechanism
of action is the use of alternating electric fields to selectively
inhibit the mitosis of tumor cells, thus achieving the purpose of
tumor control. Currently, TTFields is mainly approved for the
treatment of glioblastoma. In the international EF-14 trial, the
median overall survival (OS) time of the combined treatment group
of electric field therapy + radiotherapy was 20.5 months, while
that of the control group was 15.6 months (17). However, there needs to be more large
clinical studies reporting the efficacy of TTFields in the
treatment of DMGs, and there have only been case reports of this
treatment demonstrating efficacy. In one case, a 3-year-old child
with DMG who was treated with TTFields in combination with TMZ
after concurrent chemoradiotherapy achieved PFS for 9 months, with
gradual improvement over time (18). In the present case, the patient was
treated with adjuvant TTFields throughout the postoperative period,
and the patient's PFS time reached 21 months, far exceeding that of
other studies using radiotherapy alone (16). The treatment also did not add
additional toxic severe side effects. Patients with DMG may achieve
more significant survival benefits in combination with TTFields
based on radiotherapy.
DMGs with H3K27M mutation pose an excellent
challenge for complete surgical tumor removal due to their growth
in midline locations such as the brainstem. However, in recent
years, with the rapid development of targeted therapies and
immunotherapies, it has been found that stereotactic biopsies are
safe with the ability to obtain enough tissue to generate valuable
molecular information, which can help in the research of new
antitumor drugs (19). The biopsies
are therefore widely used in the clinic. Studies have shown that
gliomas have a variety of immunosuppressive factors, including
programmed cell death ligand 1 and indoleamine 2,3-dioxygenase, and
that elevated levels of these immunosuppressive factors can hinder
antigen presentation (20,21). Currently, various immune checkpoint
inhibitors are widely used in the treatment of clinical solid
tumors and have achieved sound therapeutic effects (20,21).
For the application of immune checkpoint inhibitors in DMG, an
American single-center clinical trial explored the efficacy of
immunotherapy for DIPG after relapse. The results showed that the
combination of radiotherapy and immunotherapy or immunotherapy
alone prolonged the survival of patients by at least 14 months
compared with no treatment. All treatment was well tolerated, with
no acute or late toxic reactions. Thus, clinically, combination
immunotherapy is expected to improve patient survival (22).
Combinations of targeted therapies and
immunotherapies, among others, for the treatment of DMGs, are also
currently being explored with the aim of evaluating whether
potential synergistic effects can be exploited to improve the
outcome of these highly aggressive tumors. Shandong Cancer Hospital
reported that an adult patient with DMG responded to treatment with
olaparib in combination with bevacizumab and achieved complete
remission with an OS time of 16 months (23). Recently, ONC201 and its analogous
compound ONC206, an orally administered small molecule that crosses
the blood-brain barrier and enhances the activity of TNF-related
apoptosis-inducing ligand to inhibit tumor growth and induce cancer
cell death, have also been the subject of intense research
(22). In a study published by
Venneti et al (24), ONC201
demonstrated significant antitumor activity in both non-recurrent
and recurrent DMGs, with a median OS time of 21.7 months in
patients with non-recurrent tumors and 9.3 months in patients with
recurrent tumors. The objective remission rate was 36.8% in
patients with non-recurrent tumors and 40.9% in patients with
recurrent tumors (24). In the
present case, bevacizumab targeted therapy was added after the
first disease progression in August 2023, together with maintenance
TTFields. The treatment was changed to a bevacizumab combined with
pembrolizumab regimen in October 2023 after consideration of the
lack of significant efficacy. After the combination therapy
resulted in SD, the patient was again administered onc201 and
onc206 targeted drugs under the existing combination therapy
regimen in December 2023. Imaging again evaluated the patient as
exhibiting SD in January 2024, and under the combination therapy
strategy of TTFields + targeted therapy + immunotherapy, the
patient's PFS time reached 5 months, suggesting that the
combination therapy strategy is beneficial in improving the
prognosis of patients with DMG.
In conclusion, DMG, a highly aggressive brain tumor
with a median survival time of <1 year, poses a major
therapeutic challenge for clinicians and researchers. Precision
medicine approaches, such as electric field therapy, immunotherapy
and targeted therapies, may have great potential to improve the
prognosis of patients with this deadly disease. The present patient
achieved a long-term survival benefit of >29 months of OS
following use of a comprehensive treatment strategy, suggesting
that exploiting the potential synergistic effects of multiple
treatment modalities may improve the prognosis of this highly
aggressive type of tumor. It is hoped that more clinical studies in
the future will explore the possibility of finding better solutions
to treat this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LG, ZHL and JZZ treated the patient. LJW and MQS
analyzed the data, conducted the histopathological evaluation and
assisted in writing the manuscript. LJW and MQS confirm the
authenticity of all the raw data. All authors contributed to the
article and approved the submitted version.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shenzhen People's Hospital (Shenzhen, China; approval no.
LL-KY-2024154-01), and the study complied with the ethical
standards set forth in the 1964 Declaration of Helsinki.
Patient consent for publication
Written informed consent for publication of this
case report and any accompanying images was obtained from the
relatives of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angelico G, Mazzucchelli M, Attanasio G,
Tinnirello G, Farina J, Zanelli M, Palicelli A, Bisagni A,
Barbagallo GMV, Certo F, et al: H3K27me3 loss in central nervous
system tumors: Diagnostic, prognostic, and therapeutic
implications. Cancers (Basel). 16:34512024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McNamara C, Mankad K, Thust S, Dixon L,
Limback-Stanic C, D'Arco F, Jacques TS and Löbel U: 2021 WHO
classification of tumours of the central nervous system: A review
for the neuroradiologist. Neuroradiology. 64:1919–1950. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabrera AR, Kirkpatrick JP, Fiveash JB,
Shih HA, Koay EJ, Lutz S, Petit J, Chao ST, Brown PD, Vogelbaum M,
et al: Radiation therapy for glioblastoma: Executive summary of an
American society for radiation oncology evidence-based clinical
practice guideline. Pract Radiat Oncol. 6:217–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen PY, van den Bent M, Youssef G,
Cloughesy TF, Ellingson BM, Weller M, Galanis E, Barboriak DP, de
Groot J, Gilbert MR, et al: RANO 2.0: Update to the response
assessment in neuro-oncology criteria for high- and low-grade
gliomas in adults. J Clin Oncol. 41:5187–5199. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwartzentruber J, Korshunov A, Liu XY,
Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA,
Tönjes M, et al: Driver mutations in histone H3.3 and chromatin
remodelling genes in paediatric glioblastoma. Nature. 482:226–231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeWitt JC, Mock A and Louis DN: The 2016
WHO classification of central nervous system tumors: What
neurologists need to know. Curr Opin Neurol. 30:643–649. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo Greco MC, Marano G, La Rocca M,
Acquaviva G, Milazzotto R, Liardo RLE, Basile A, Foti PV, Palmucci
S, David E, et al: Latest advancements in the management of
H3K27M-mutant diffuse intrinsic pontine glioma: A narrative review.
Cancers (Basel). 17:4202025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akdemir EY, Odia Y, Hall MD, Mehta MP and
Kotecha R: An update on H3K27M-altered diffuse midline glioma:
Diagnostic and therapeutic challenges in clinical practice. Pract
Radiat Oncol. 14:443–451. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
China Society for Radiation Oncology, .
Expert consensus of China on radiation therapy for gliomas in 2017.
Chin J Radiat Oncol. 27:123–131. 2018.(In Chinese).
|
|
11
|
Yoon HI, Wee CW, Kim YZ, Seo Y, Im JH, Dho
YS, Kim KH, Hong JB, Park JS, Choi SH, et al: The Korean society
for neuro-oncology (KSNO) guideline for adult diffuse midline
glioma: Version 2021.1. Brain Tumor Res Treat. 9:1–8. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abe H, Natsumeda M, Kanemaru Y, Watanabe
J, Tsukamoto Y, Okada M, Yoshimura J, Oishi M and Fujii Y: MGMT
expression contributes to temozolomide resistance in H3K27M-mutant
diffuse midline gliomas and MGMT silencing to temozolomide
sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo).
58:290–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guerra-García P, Marshall LV, Cockle JV,
Ramachandran PV, Saran FH, Jones C and Carceller F: Challenging the
indiscriminate use of temozolomide in pediatric high-grade gliomas:
A review of past, current, and emerging therapies. Pediatr Blood
Cancer. 67:e280112020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao M, Wu Y and He Q: Recent advances in
targeted drug delivery for the treatment of glioblastoma.
Nanoscale. 16:8689–8707. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberoi RK, Parrish KE, Sio TT, Mittapalli
RK, Elmquist WF and Sarkaria JN: Strategies to improve delivery of
anticancer drugs across the blood-brain barrier to treat
glioblastoma. Neuro Oncol. 18:27–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Nunno V, Lombardi G, Simonelli M,
Minniti G, Mastronuzzi A, Di Ruscio V, Corrà M, Padovan M, Maccari
M, Caccese M, et al: The role of adjuvant chemotherapy in patients
with H3K27 altered diffuse midline gliomas: A multicentric
retrospective study. J Neurooncol. 167:145–154. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ali AS, Lombardo J, Niazi MZ, Miller RC,
Alnahhas I, Martinez NL, Andrews DW, Judy KD and Shi W: Concurrent
chemoradiation and tumor treating fields (TTFields, 200 kHz) for
patients with newly diagnosed glioblastoma: Patterns of progression
in a single institution pilot study. J Neurooncol. 160:345–350.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gött H, Kiez S, Dohmen H, Kolodziej M and
Stein M: Tumor treating fields therapy is feasible and safe in a
3-year-old patient with diffuse midline glioma H3K27M-a case
report. Childs Nerv Syst. 38:1791–1796. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ehteda A, Simon S, Franshaw L, Giorgi FM,
Liu J, Joshi S, Rouaen JRC, Pang CNI, Pandher R, Mayoh C, et al:
Dual targeting of the epigenome via FACT complex and histone
deacetylase is a potent treatment strategy for DIPG. Cell Rep.
35:1089942021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang EI, Sayour EJ, Flores CT, Grant G,
Wechsler-Reya R, Hoang-Minh LB, Kieran MW, Salcido J, Prins RM,
Figg JW, et al: The current landscape of immunotherapy for
pediatric brain tumors. Nat Cancer. 3:11–24. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Estevez-Ordonez D, Gary SE, Atchley TJ,
Maleknia PD, George JA, Laskay NMB, Gross EG, Devulapalli RK and
Johnston JM: Immunotherapy for pediatric brain and spine tumors:
Current state and future directions. Pediatr Neurosurg. 58:313–336.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kline C, Liu SJ, Duriseti S, Banerjee A,
Nicolaides T, Raber S, Gupta N, Haas-Kogan D, Braunstein S and
Mueller S: Reirradiation and PD-1 inhibition with nivolumab for the
treatment of recurrent diffuse intrinsic pontine glioma: A
single-institution experience. J Neurooncol. 140:629–638. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Xu J, Luo N, Qi C and Tao R:
Successful treatment of an adult patient with diffuse midline
glioma employing olaparib combined with bevacizumab. Invest New
Drugs. 39:1432–1435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Venneti S, Kawakibi AR, Ji S, Waszak SM,
Sweha SR, Mota M, Pun M, Deogharkar A, Chung C, Tarapore RS, et al:
Clinical efficacy of ONC201 in H3K27M-mutant diffuse midline
gliomas is driven by disruption of integrated metabolic and
epigenetic pathways. Cancer Discov. 13:2370–2393. 2023. View Article : Google Scholar : PubMed/NCBI
|