Introduction
Colorectal cancer is the third-most common cancer in
the world and second in terms of cancer-related deaths (1). The disease is widespread in developed
countries, but its incidence is increasing in middle- and
low-income countries (1). For
early-stage rectal cancer, surgery is the main treatment. However,
for locally advanced rectal cancer (LARC), total mesorectal
excision after neoadjuvant chemoradiotherapy (nCRT) or radiotherapy
(RT) is recommended. The neoadjuvant treatment approach effectively
reduces the local recurrence rate, and compared to adjuvant
treatment, acute side effects are less common (2). There is still no consensus view on the
effect of adjuvant chemotherapy (ChT) in patients with rectal
cancer (3). The optimal timing of
RT and ChT, as well as the type of ChT, is controversial (4).
The identification of prognostic factors with an
impact on the survival of patients with rectal cancer may allow
individualized treatment and an improvement in the quality of life.
TNM stage is the most important known prognostic factor for
colorectal cancer (5). However, in
patients with the same pathological stage, especially in stages II
and III, there are significant differences in clinical outcomes and
prognosis (6). For patients with
stage IIIA and IIIC, the reported 5-year survival rate varies from
80 to 30% (7). Therefore, in order
to improve the accuracy of predicting the prognosis of patients
with rectal cancer, it is necessary to identify additional
prognostic factors that may allow the treatment to be tailored to
the risk profile and preferences of the individual.
Several studies have shown that factors such as CEA
level, number of metastatic lymph nodes, response to neoadjuvant
therapy, neoadjuvant rectal score (NAR score), lymphovascular
invasion (LVI), perineural invasion (PNI) and circumferential
resection margin are associated with overall survival (OS) and
disease-free survival (DFS) (8–12).
Prognostic factors have been used to manage the follow-up of
patients and optimize adjuvant therapy; however, there needs to be
more consensus on the factors with the most significant impact
(12). It is important to develop
accurate models to predict prognosis and identify risk factors,
especially for patients at high risk of recurrence or metastasis.
For this purpose, various risk-scoring models and nomograms have
been developed in the literature. However, risk-scoring models that
assess clinical and pathological factors together are uncommon.
The present study aimed to retrospectively analyze
the treatment outcomes of patients with LARC treated with nCRT and
develop a scoring model that predicts local control (LC) and
survival, using clinical and pathological factors affecting
prognosis.
Patients and methods
Patient characteristics
A total of 115 patients with LARC who were treated
between February 2010 and December 2020 at Istanbul
University-Cerrahpasa, Cerrahpasa Medical Faculty (Istanbul,
Turkey) were included in the present study. The inclusion criteria
were as follows: i) Histological confirmation of stage II–III
rectal adenocarcinoma, according to the American Joint Committee on
Cancer staging system, 8th edition; ii) receipt of nCRT; iii)
complete clinicopathological and follow-up data; and iv) no other
malignancies. The exclusion criteria were as follows: i) Receipt of
postoperative RT; ii) receipt of palliative RT; and iii) missing or
incomplete clinical data. All patients were treated with
intensity-modulated RT concomitant with ChT followed by total
mesorectal excision. Age, sex, clinical tumor (cT) stage, clinical
node stage, tumor distance from the anal verge, tumor histological
grade, tumor diameter at colonoscopy and MRI, preoperative ChT
regimen, postoperative pathological findings [Mandard regression
grade (13), pathological tumor
(pT), pathological node (pN), resection grade, circumferential
resection margin, LVI and PNI], adjuvant ChT and follow-up data
(local-regional recurrence and metastasis) were all evaluated
retrospectively.
Treatment
Patients were evaluated with a digital rectal
examination, routine blood examination, colonoscopy, pelvic MRI,
thorax CT and/or positron emission tomography (PET)/CT imaging
before treatment. Target volumes [gross tumor volume (GTV) and
involved lymph nodes] were identified through fusion with
fluorodeoxglucose-PET-CT and/or pelvic MRI. The high-risk clinical
target volume (CTV-HR) was created by including the mesorectum and
presacral area with a safety margin of 2.5 cm proximal and distal
to the GTV. Elective lymphatic areas (internal iliac, external
iliac and obturator lymphatic area) were included in the CTV-HR
according to tumor stage and location, and the standard-risk CTV
(CTV-SR) was created (14).
Planning target volumes (PTVs) were generated by expanding the CTVs
by 0.7 cm symmetrically. The PTV-SR was delivered a dose of 45 Gy
in 25 fractions, and the total dose to the PTV-HR was 50.4 or 54
Gy, with an additional 180 cGy per day administered in 3–5
fractions. Treatment plans for the patients were created using
volumetric modulated arc therapy or intensity-modulated RT methods
in the Varian's Eclipse v.15.6.3 Treatment Planning System. Target
volumes and doses to critical organs were assessed according to the
International Commission on Radiation Units and Measurements report
number 83 (15). Patient treatments
were performed using the Varian brand iX (Rapid Arc) model linear
accelerator (Varian Medical Systems, Inc.) at 6 MV photon energy,
and quality control of the approved treatment plans was performed.
All patients received capecitabine (1,650 mg/m2/day; 5
days per week for 5–6 weeks) or 5-fluorouracil (225
mg/m2/day, continuous infusion) concomitant with RT.
Follow-up
Throughout the preoperative CRT period, a weekly
evaluation of physical examination and blood parameters was
conducted. The tumor response after CRT was evaluated by
contrast-enhanced pelvic MRI 6 weeks after treatment. Postoperative
follow-up was performed every 3–6 months for the first 2 years,
every 6 months for 5 years and annually after 5 years, with a
medical history, complete physical examination, laboratory tests
and pelvic MRI. A colonoscopy was performed every 6 months for the
first 2 years and annually after 2 years.
Developing a risk-score model
Similar to the system used in the study by Morini
et al (16), points were
assigned based on specific parameters that affected LC, DFS and OS.
Parameters associated with decreased LC, DFS and OS in multivariate
analysis were awarded +2 points, while +1 point was assigned to
factors related to LC, DFS and OS in only univariate analysis. A
model was created to estimate LC and survival by calculating the
total score. In the scoring system, a low score indicates a good
prognosis, while a high score indicates a poor prognosis.
Statistical analysis
All analyses were performed using SPSS Statistics
version 20 (IBM Corp.). LC time was defined as the time from
diagnosis to local recurrence or last follow-up. DFS was defined as
the time from diagnosis to local recurrence, metastasis or final
follow-up. OS time was defined as the time from diagnosis to death
or last follow-up.
Descriptive statistical methods were used for
patient characteristics, univariate analysis was used for
demographic and clinical characteristics comparisons, the
Kaplan-Meier method was used for survival analysis, survival
differences were analyzed by log-rank test, and Cox regression
analysis was used for multivariate analysis. P≤0.05 was considered
to indicate a statistically significant difference. Using the
significant prognostic variables in the univariate and multivariate
statistical analyses, a risk-scoring model was developed to
estimate LC, DFS and OS. The predictive power of the developed
scoring model was evaluated using the receiver operating
characteristic (ROC) curve. The study was approved by the Istanbul
University-Cerrahpasa Ethics Committee (approval no.
E-83045809-064.01.01–626378) and all patients provided informed
written consent before treatment.
Results
Clinicopathological and demographic
characteristics
A total of 115 patients who received nCRT were
evaluated, of whom 81 (70.4%) were male. The median age of all
patients was 57 years (range, 28–85 years). The median tumor
diameter was 5 cm (range, 1–16 cm), and 87 patients (75.7%) had
tumors ≥5 cm. Tumors were located at a median distance of 6 cm
(range, 1–20 cm) from the anal verge, with the majority in the
lower rectum (n=54; 47.0%). Most of the patients were classified as
cT3N1/2 (79.1%) according to the American Joint Committee on Cancer
(8th edition) TNM system (17). A
total of 109 patients (94.8%) received 50.4 Gy/28 fractions RT and
95 patients (82.6%) received concurrent capecitabine. The
characteristics of the patients, the tumors and the treatment are
shown in Table I. In total, 81
patients (70.4%) underwent a low-anterior resection and 34 patients
(29.6%) underwent abdominoperineal surgery. A pathological complete
response was achieved in 21 patients (18.3%) after nCRT. The median
follow-up time was 70 months (range, 6–156 months). A total of 19
patients (16.5%) had locoregional recurrence, and 26 patients
(22.6%) had distant metastasis. At 2 and 5 years, the LC rates were
90.2 and 81.6%, the DFS rates were 85.7 and 72.2%, and the OS rates
were 94.7 and 70.2%, respectively (data not shown).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | No. (%) |
|---|
| Age,
yearsa |
|
|
≤60 | 71 (61.7) |
|
>60 | 44 (38.3) |
| Sex |
|
|
Female | 34 (29.6) |
|
Male | 81 (70.4) |
| cT stage |
|
| T2 | 6 (5.2) |
| T3 | 91 (79.1) |
| T4 | 18 (15.7) |
| cN stage |
|
| N0 | 24 (20.9) |
| N+ | 91 (79.1) |
| Location of the
tumor |
|
| Upper
rectum | 26 (22.6) |
| Middle
rectum | 35 (30.4) |
| Lower
rectum | 54 (47.0) |
| Tumor diameter,
cmb |
|
|
<5 | 28 (24.3) |
| ≥5 | 87 (75.7) |
| Tumor grade |
|
|
Well-differentiated | 64 (55.7) |
|
Undifferentiated | 51 (44.3) |
| RT dose,
Gyc |
|
|
50.4 | 109 (94.8) |
| 54 | 6 (5.2) |
| Concomitant
ChT |
|
|
Capecitabine | 95 (82.6) |
|
5-FU | 20 (17.4) |
Prognostic factors associated with LC,
DFS and OS
In the univariate analysis, patients with
pathological response Mandard grades 3–5, those positive for LVI
and PNI, and those not receiving postoperative ChT were found to
exhibit worse LC rates. Similarly, patients with pathological
response Mandard grades 3–5, those with a radial circumferential
resection margin >1 mm, those positive for LVI and PNI, patients
with pN+ disease, and individuals with no postoperative ChT had
worse DFS rates. Patients >60 years of age, with a tumor
diameter ≥5 cm, a tumor located in the lower rectum, pathological
response Mandard grades 3–5, presence of LVI and PNI, and pN+
disease had worse OS rates (P≤0.05) (Table II).
 | Table II.Univariate analysis results for LC,
DFS and OS rates. |
Table II.
Univariate analysis results for LC,
DFS and OS rates.
|
|
| LC | DFS | OS |
|---|
|
|
|
|
|
|
|---|
| Variable | No. | 2 years, % | 5 years, % | P-value | 2 years, % | 5 years, % | P-value | 2 years, % | 5 years, % | P-value |
|---|
| Age, years |
|
|
| 0.400 |
|
| 0.900 |
|
| 0.050 |
|
≤60 | 71 | 90.0 | 84.4 |
| 84.2 | 73.7 |
| 97.2 | 78.6 |
|
|
>60 | 44 | 90.6 | 77.7 |
| 88.2 | 70.2 |
| 90.9 | 57.8 |
|
| Sex |
|
|
| 0.900 |
|
| 0.200 |
|
| 0.200 |
|
Female | 34 | 91.1 | 80.4 |
| 91.1 | 80.4 |
| 96.6 | 74.4 |
|
|
Male | 81 | 89.9 | 82.0 |
| 83.3 | 68.6 |
| 93.8 | 68.4 |
|
| cT stage |
|
|
| 0.900 |
|
| 0.300 |
|
| 0.200 |
| T2 | 6 | 83.3 | 83.3 |
| 66.7 | 44.4 |
| 100.0 | 100.0 |
|
| T3 | 91 | 90.0 | 82.0 |
| 85.5 | 72.4 |
| 96.7 | 68.5 |
|
| T4 | 18 | 93.3 | 79.4 |
| 93.3 | 65.0 |
| 83.0 | 70.2 |
|
| cN stage |
|
|
| 0.900 |
|
| 0.200 |
|
| 0.600 |
| N0 | 24 | 95.8 | 83.1 |
| 78.9 | 64.5 |
| 95.7 | 63.9 |
|
| N+ | 91 | 92.1 | 81.2 |
| 87.5 | 74.2 |
| 94.5 | 74.7 |
|
| Location of the
tumor |
|
|
| 0.500 |
|
| 0.400 |
|
| 0.030 |
| Upper
rectum | 26 | 84.1 | 79.7 |
| 84.1 | 79.9 |
| 95.3 | 83.7 |
|
| Middle
rectum | 35 | 97.1 | 86.5 |
| 91.3 | 74.4 |
| 97.1 | 77.9 |
|
| Lower
rectum | 54 | 90.5 | 78.9 |
| 82.8 | 67.0 |
| 94.3 | 57.8 |
|
| Tumor diameter,
cm |
|
|
| 0.700 |
|
| 0.500 |
|
| 0.030 |
|
<5 | 28 | 92.7 | 82.9 |
| 85.3 | 66.3 |
| 96.2 | 72.6 |
|
| ≥5 | 87 | 89.3 | 81.2 |
| 85.8 | 73.8 |
| 94.2 | 61.4 |
|
| Pathological
response |
|
|
| 0.010 |
|
| 0.010 |
|
| 0.006 |
| Mandard
grades 1–2 | 40 | 95.0 | 95.0 |
| 92.4 | 85.3 |
| 100 | 83.0 |
|
| Mandard
grades 3–5 | 75 | 87.5 | 74.0 |
| 82.0 | 64.7 |
| 91.9 | 63.7 |
|
| Circumferential
resection margin, mm |
|
|
| 0.100 |
|
| 0.010 |
|
| 0.100 |
|
<1 | 19 | 91.3 | 83.4 |
| 90.4 | 76.8 |
| 96.9 | 74.4 |
|
| ≥1 | 96 | 83.6 | 72.0 |
| 60.4 | 48.3 |
| 83.9 | 50.3 |
|
| LVI |
|
|
| 0.020 |
|
| 0.010 |
|
| 0.006 |
|
Negative | 56 | 94.6 | 90.8 |
| 92.8 | 84.2 |
| 100 | 79.7 |
|
|
Positive | 59 | 85.7 | 72.0 |
| 78.6 | 60.0 |
| 89.7 | 91.1 |
|
| PNI |
|
|
| 0.006 |
|
| 0.001 |
|
| 0.001 |
|
Negative | 71 | 95.7 | 89.4 |
| 92.8 | 83.0 |
| 98.6 | 79.7 |
|
|
Positive | 44 | 80.9 | 67.3 |
| 73.8 | 53.1 |
| 88.4 | 53.3 |
|
| pT stage |
|
|
| 0.070 |
|
| 0.080 |
|
| 0.090 |
| T0 | 22 | 100 | 100 |
| 100 | 100 |
| 100 | 100 |
|
| T1 | 7 | 95.5 | 95.5 |
| 90.9 | 90.9 |
| 100 | 85.9 |
|
| T2 | 26 | 96.2 | 96.2 |
| 92.3 | 81.1 |
| 95.7 | 71.7 |
|
| T3 | 52 | 86.2 | 72.8 |
| 80.3 | 61.1 |
| 92.2 | 64.0 |
|
| T4 | 8 | 87.5 | 55.0 |
| 87.5 | 55.0 |
| 75.0 | 57.5 |
|
| pN stage |
|
|
| 0.060 |
|
| 0.020 |
|
| 0.004 |
| N0 | 86 | 92.8 | 85.7 |
| 89.1 | 77.9 |
| 95.3 | 73.9 |
|
|
N1-2 | 29 | 82.8 | 69.5 |
| 75.9 | 55.3 |
| 92.9 | 58.4 |
|
| Postoperative
ChT |
|
|
| 0.010 |
|
| 0.006 |
|
| 0.100 |
| No | 66 | 87.6 | 73.2 |
| 84.5 | 61.8 |
| 93.8 | 68.6 |
|
|
Yes | 49 | 93.7 | 93.7 |
| 87.3 | 87.3 |
| 95.9 | 72.5 |
|
Based on the multivariate analysis findings, it was
observed that patients with pathological response Mandard grades
1–2 to treatment exhibited improved LC. However, patients with PNI
had a worse DFS rate. In addition, the OS rate was significantly
worse in patients over 60 years of age, those with a tumor diameter
of 5 cm or more, those with pN2 disease and patients positive for
PNI (P≤0.05) (Table III).
 | Table III.Multivariate analysis results for LC,
DFS and OS. |
Table III.
Multivariate analysis results for LC,
DFS and OS.
| A, LC |
|---|
|
|---|
| Variable | RR | 95% CI | P-value |
|---|
| Pathological
response |
|
| 0.028 |
| Mandard
grades 1–2 | Reference |
|
|
| Mandard
grades 3–5 | 5.18 | 1.19–22.47 |
|
| PNI |
|
| 0.338 |
|
Negative | Reference |
|
|
|
Positive | 0.53 | 0.14–1.93 |
|
| LVI |
|
| 0.903 |
|
Negative | Reference |
|
|
|
Positive | 0.91 | 0.21–3.86 |
|
| Postoperative
ChT |
|
| 0.181 |
| No | 2.16 | 0.69–6.73 |
|
|
Yes | Reference |
|
|
|
| B, DFS |
|
|
Variable | RR | 95% CI | P-value |
|
| Pathological
response |
|
| 0.902 |
| Mandard
grades 1–2 | Reference |
|
|
| Mandard
grades 3–5 | 0.61 | 0.17–2.18 |
|
| PNI |
|
| 0.006 |
|
Negative | Reference |
|
|
|
Positive | 2.93 | 1.36–6.29 |
|
| LVI |
|
| 0.974 |
|
Negative | Reference |
|
|
|
Positive | 1.02 | 0.24–4.28 |
|
| pN stage |
|
| 0.085 |
| N0 | Reference |
|
|
| N1 | 0.02 | 0.06–0.85 |
|
| N2 | 0.28 | 0.07–1.10 |
|
| Circumferential
resection margin, mm |
|
| 0.118 |
|
<1 | 2.07 | 0.83–5.19 |
|
| ≥1 | Reference |
|
|
| Postoperative
ChT |
|
|
|
| No | 2.35 | 0.65–8.45 | 0.189 |
|
Yes | Reference |
|
|
|
| C, OS |
|
|
Variable | RR | 95% CI | P-value |
|
| Age, years |
|
| 0.030 |
|
≤60 | Reference |
|
|
|
>60 | 2.08 | 1.07–4.04 |
|
| Tumor diameter,
cm |
|
| 0.010 |
|
<5 | Reference |
|
|
| ≥5 | 2.46 | 1.20–5.04 |
|
| Location of the
tumor |
|
| 0.393 |
| Upper
rectum | Reference |
|
|
| Middle
rectum | 1.35 | 0.15–11.63 |
|
| Lower
rectum | 0.62 | 0.29–1.32 |
|
| Pathological
response |
|
| 0.936 |
| Mandard
grades 1–2 | Reference |
|
|
| Mandard
grades 3–5 | 0.688 | 0.17–2.67 |
|
| PNI |
|
| 0.009 |
|
Negative | Reference |
|
|
|
Positive | 2.55 | 1.26–5.16 |
|
| LVI |
|
| 0.985 |
|
Negative | Reference |
|
|
|
Positive | 0.990 | 0.340–2.881 |
|
| pN stage |
|
| 0.030 |
| N0 | Reference |
|
|
| N1 | 1.50 | 0.70–3.22 |
|
| N2 | 4.55 | 1.44–14.36 |
|
Stratification of risk groups
The predictive power of the developed scoring model
was evaluated using the ROC curve to give area under the curve
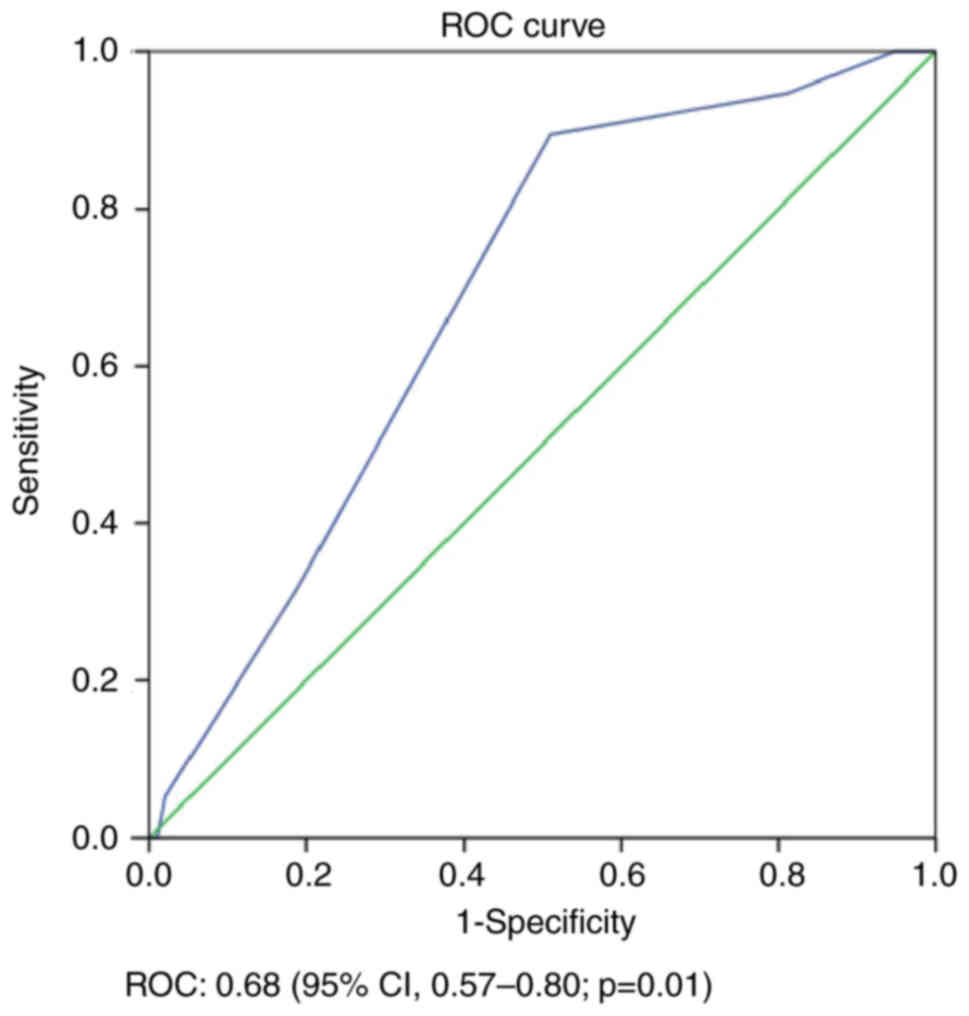
(AUC) values as follows: LC, 0.68 (95% CI, 0.57–0.80; P=0.01;
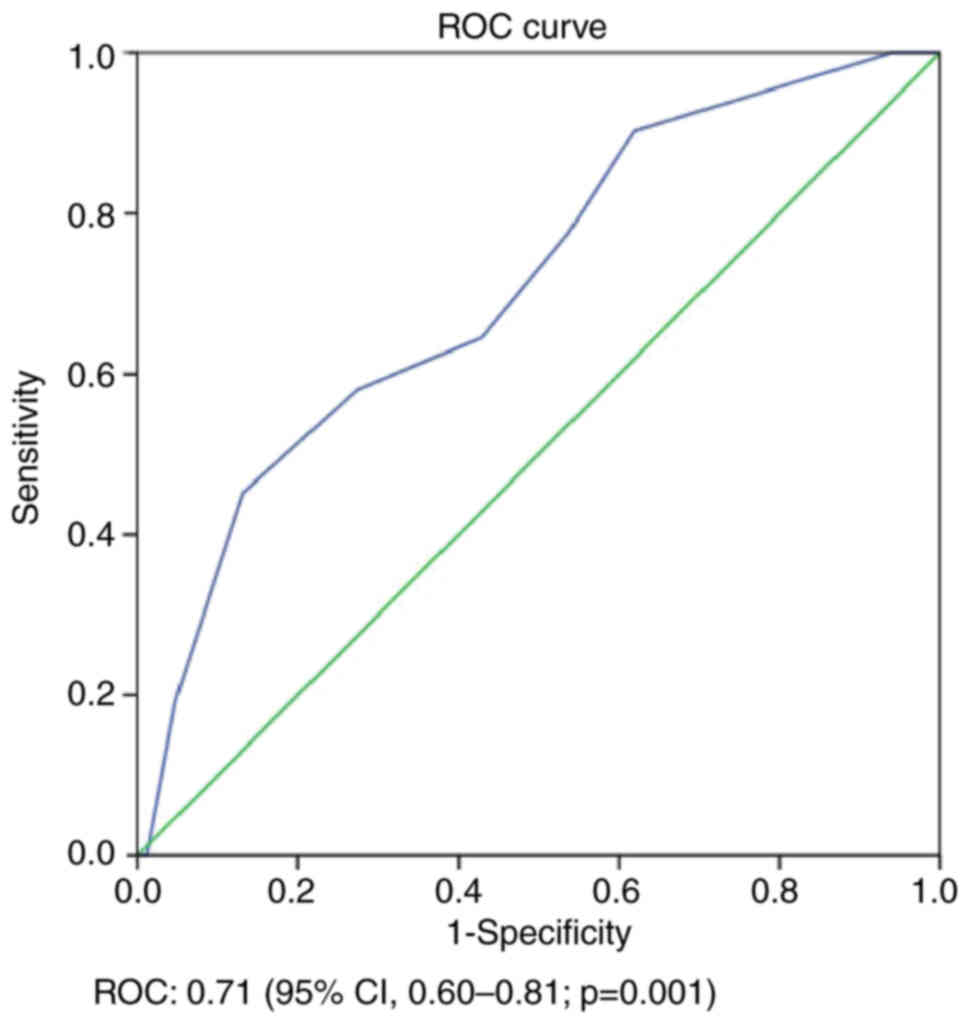
Fig. 1); DFS, 0.71 (95% CI,
0.60–0.81; P=0.001; Fig. 2); and
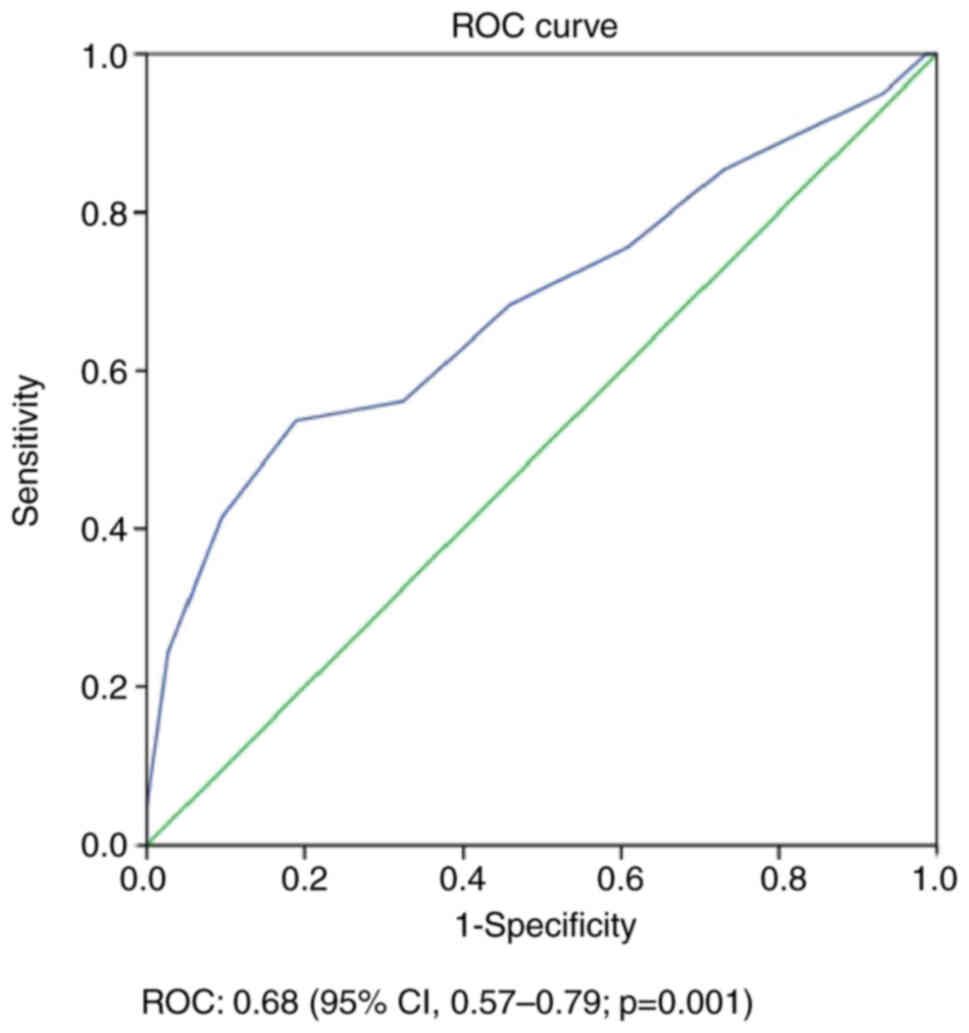
OS, 0.68 (95% CI, 0.57–0.79; P=0.001; Fig. 3). The total score of all patients
was stratified into three risk groups based on tertiles to assess
the ability of the risk scoring model to predict LC, DFS and OS.
The scores of groups 1, 2 and 3 for LC were 0–2, 2–4 and 4–5
points, respectively. The scores of groups 1, 2 and 3 for DFS were
0–2, 2–4 and 4–7 points, respectively, while for OS, the scores of
groups 1, 2 and 3 were 0–3, 3–6 and 6–11 points, respectively
(Table IV). Kaplan-Meier analysis
and log-rank tests demonstrated statistically and graphically that
the three risk groups differed significantly in terms of LC, DFS
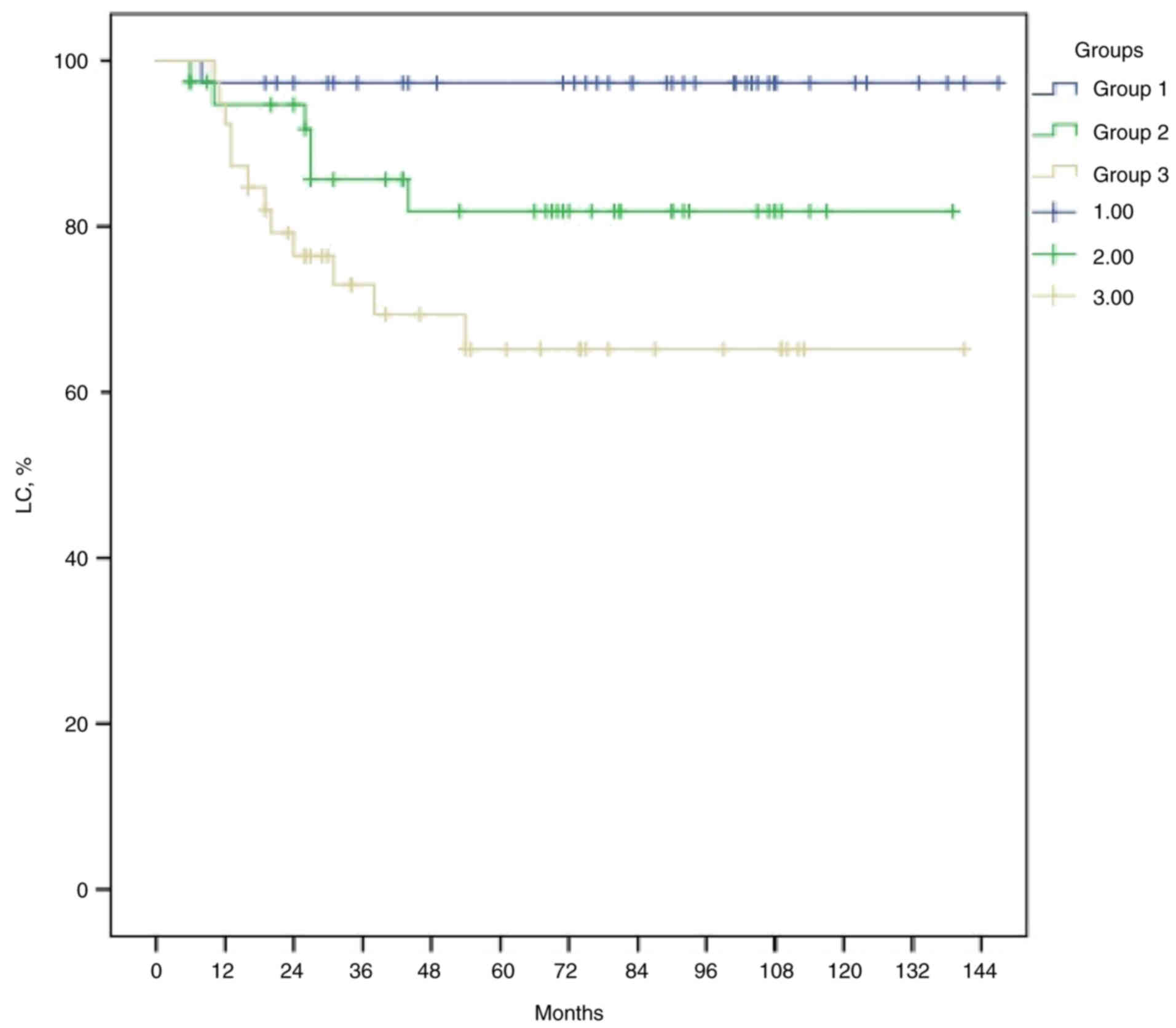
and OS. The median LC times for groups 1, 2 and 3 were 143.6, 97.2
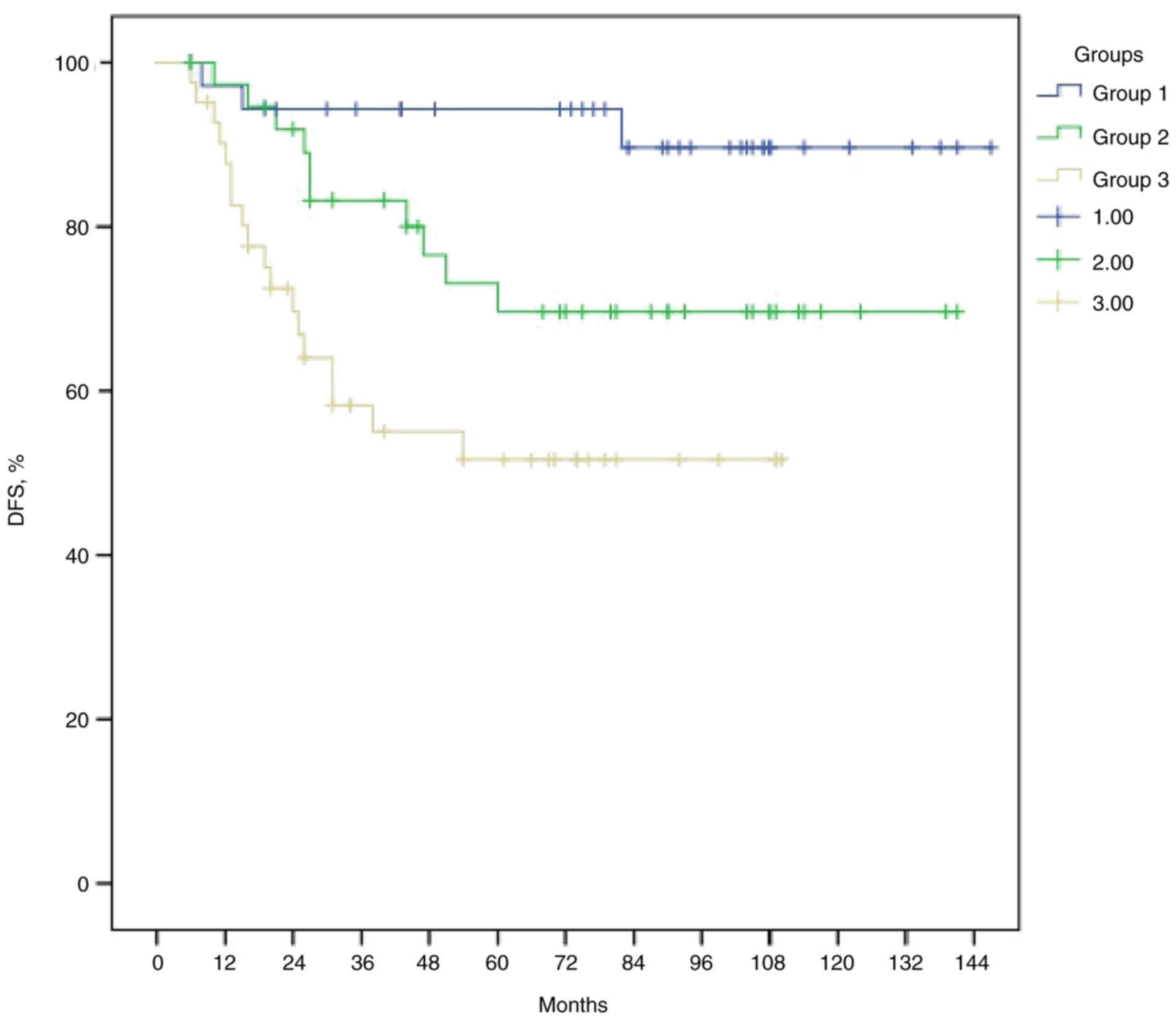
and 93.6 months, respectively (P=0.001; Fig. 4). The median DFS times for groups 1,
2 and 3 were 136.1, 108.5 and 67.2 months, respectively (P=0.001;
Fig. 5). The median OS times for
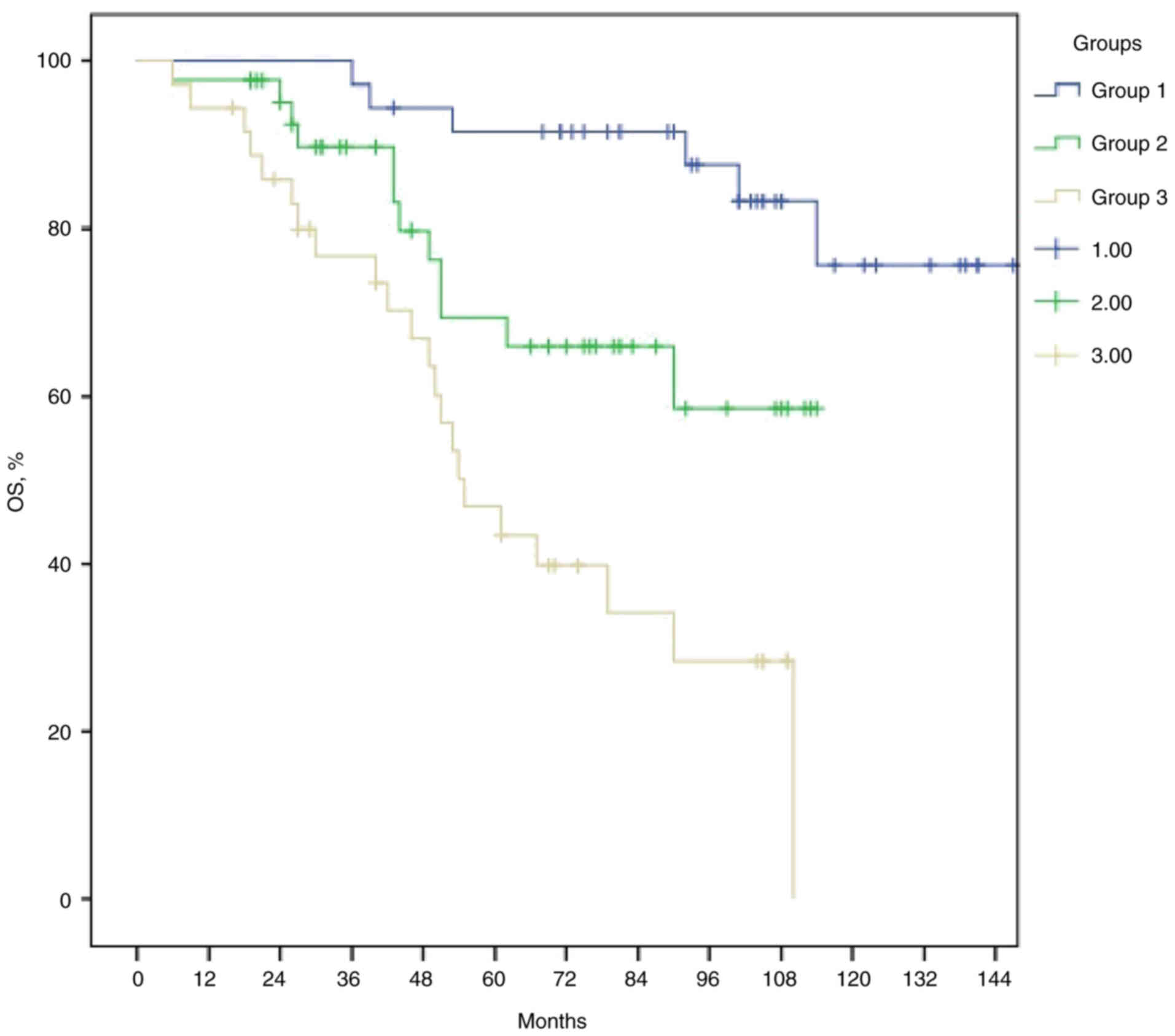
groups 1, 2 and 3 were 138.3, 87.2 and 64.6 months respectively
(P<0.001; Fig. 6). Patients in
group 1 had survival rates that were nearly twice as high as those
in the group 3. In addition, the study's sub-analysis obtained the
following results regarding OS: 86% of patients had a tumor
diameter of ≥5 cm, 72% exhibited multiple lymph node involvement
radiologically at baseline, 63% had pN+ disease, and 88% showed
PNI(+) after neoadjuvant treatment in group 3 (data not shown).
 | Table IV.Risk score points awarded. |
Table IV.
Risk score points awarded.
| Assessed
parameters | Point score
awarded |
|---|
| LC |
|
| Mandard
grades 3–5 | 2 |
|
LVI(+) | 1 |
|
PNI(+) | 1 |
|
Postoperative ChT(−) | 1 |
| DFS |
|
|
PNI(+) | 2 |
| Mandard
grades 3–5 | 1 |
|
LVI(+) | 1 |
|
pN(+) | 1 |
| CRM
<1 mm | 1 |
|
Postoperative ChT(−) | 1 |
| OS |
|
| Age
>60 years | 2 |
| Tumor
diameter ≥5 cm | 2 |
|
PNI(+) | 2 |
|
pN(+) | 2 |
| Lower
rectum | 1 |
| Mandard
grades 3–5 | 1 |
|
LVI(+) | 1 |
Discussion
TNM stage is the most important prognostic factor
known today in rectal cancer and has been the cornerstone of the
associated scoring and nomogram systems (5). However, patient- and tumor-specific
factors such as age, sex, tumor diameter, tumor location, lymph
node location, tumor response degree, peripheral circumferential
margin, LVI, PNI and other stages have been found to affect LC and
survival (18–20). For this reason, the present study
developed a prognostic risk-scoring model using clinical and
pathological factors to determine the prognosis and predict LC, DFS
and OS in patients with LARC.
Several risk scoring or nomogram systems have been
developed to predict prognosis or decide on adjuvant treatment for
rectal cancer (10,11,21,22).
One of the scoring systems is the NAR score, based on the TNM
staging system. The NAR score is calculated using the cT, ypT
(yield pathological T) and ypN (yield pathological N) stages. Baek
et al (10) compared the NAR
scoring system with tumor regression grade and pathological TNM
staging, and showed that the most important factor influencing
survival was the ypTNM stage (10).
Calculating the NAR score using the TNM staging system is practical
and easy. However, this scoring system does not consider a number
of important clinical and pathologic prognostic factors, such as
age, performance score, tumor location, tumor diameter, tumor
regression grade, LVI and PNI.
The degree of tumor response to neoadjuvant therapy
is one of the prognostic factors that has been discussed in risk
score models and nomograms in the literature over the last 10 years
(10,12,16).
In some studies, the degree of tumor response to neoadjuvant
therapy is one of the determinants of local recurrence risk and
even OS. Various tumor regression grading systems have been
developeDd to classify the pathological response to nCRT in
patients with LARC (13,23). Mandard et al (24) developed a five-category tumor
regression system to assess tumor response to CRT in patients with
oesophageal cancer by quantitatively evaluating residual tumor
cells versus inflammatory fibrosis. In subsequent studies, the
Mandard system was shown to be an effective classification system
for evaluating tumor response to nCRT and predicting prognosis in
patients with LARC (23,25). A meta-analysis of patients with a
pathological complete response to neoadjuvant treatment found that
local recurrence rates were low (1.6%) at a median follow-up time
of 46.4 months, and that the 5-year DFS and OS rates were 85 and
90%, respectively (26). The
present study classified the degree of tumor response to
neoadjuvant treatment according to the Mandard system. The LC rate
was statistically significantly lower in the Mandard grades 3–5
patient group compared with that in the Mandard grades 1–2 patient
group. In the pathological T0N0 group, local recurrence was
observed in only 1 out of 21 patients at a median follow-up time of
70 months, which is likely to be due to a larger tumor (>5 cm).
Complete pathological response was not a prognostic factor in the
risk score model due to the small number of patients with complete
pathological responses (pT0N0) after neoadjuvant therapy.
Another prognostic factor that should be more
broadly discussed in risk score models and nomograms is PNI. PNI
refers to the invasion of nerves by tumor cells. Tumor cells can
grow into, around or through the three layers of nerves
(endoneurium, perineurium and epineurium). Therefore, there are
different definitions in the literature. The most commonly used
definition is that >33% of the nerve periphery is surrounded by
tumor cells (20). The incidence of
PNI reported in the literature varies between 9 and 30% (20,27).
The incidence increases in advanced-stage disease. In one study, it
was reported to be ~10% in stage I–II, 30% in stage III and 40% in
stage IV disease (20). In the
present study, the rate of PNI was 38.3%. In a meta-analysis of
22,900 patients with colorectal cancer, PNI, depth of tumor
invasion, tumor grade, lymph node metastasis and extramural
invasion were shown to be prognostic factors. In addition, OS and
DFS rates were lower in patients with PNI (28). Similarly, in the present study, both
DFS and OS rates were significantly lower in patients with PNI.
Data from 2,795 patients in five large randomized
trials comparing preoperative CRT versus preoperative RT or
postoperative CRT/ChT have been used to develop nomograms to
predict local recurrence, distant metastases and OS. In addition to
pathological and clinical TNM staging as prognostic factors, sex,
age, tumor location, RT dose, concurrent and adjuvant ChT, and
surgical procedure were included in the analysis. The pathological
stage was found to be the most critical factor for the accurate
prediction of survival outcomes. In multivariate analysis, the
rates of LC and OS were higher, while distant metastasis rates were
lower in patients with low anterior resection, pT0, pN0 and
adjuvant ChT (11). Weiser et
al (22) also found that
postoperative pathological staging was more critical than clinical
staging in terms of prediction outcomes in patients receiving
neoadjuvant therapy to evaluate response to treatment and to choose
appropriate treatment (22). In the
univariate and multivariate analyses in the present study, clinical
T and N stages were not found to be prognostic factors for LC, DFS
and OS. However, pathological N stage was a prognostic factor for
DFS and OS in univariate and multivariate analysis, and OS was
higher in pN0 patients (P=0.03). Therefore, effective postoperative
ChT is needed, especially in patients with ypN2 disease, to improve
survival rates.
Lin's nomogram evaluated patients with stage II–III
rectal cancer, and ethnicity, sex, age, marital status, T stage,
tumor grade, tumor size, positive lymph node involvement rate, CEA
level and postoperative ChT were all factors found to be affecting
OS (29). For the first time, a
study reported that a tumor size >7 cm was an independent
prognostic factor for patients undergoing neoadjuvant CRT treatment
(23). There is a lack of consensus
among international guidelines regarding the specific cutoff value
for tumor size. A few studies have reported that a tumor size of 4
cm is a prognostic cut-off (30–32).
In the present study, multivariate analysis found that a tumor
diameter of ≥5 cm was significantly associated with a decreased OS
rate. Although tumor size may not represent the actual tumor volume
or burden, it is a convenient and rapid method to estimate tumor
volume in clinical practice. In the literature, the prognostic
significance of tumor volume has been investigated, and some
studies have reported that a small tumor volume is more important
than the size of the tumor (33,34).
In addition, Yeo et al (35)
showed that the decrease in tumor volume after neoadjuvant
treatment was a prognostic factor.
Weiser et al (22) developed a clinical risk calculation
model to determine recurrence-free survival and OS in patients
without a pathological complete response who were treated with
nCRT. Besides the TNM staging system and the NAR score, the
clinical risk calculation model included the number of positive
lymph nodes, the distance to the anal margin, VI and PNI as
predictors of relapse-free survival. For OS, age was also
evaluated. Recurrence-free survival and OS rates were lower in
patients with <5 cm distance to the anal verge, pathologically
advanced T stage, positive lymph node count >1, VI and PNI. In
addition, advanced age negatively affected survival. The study also
compared their scoring system with the NAR score and TNM staging
system. The recurrence probability and survival were predicted with
greater accuracy using their system compared with using the TNM
staging system and NAR score (22).
In the present risk score model, OS was significantly worse in
patients aged >60 years, with lower rectal tumors, pathological
lymph node involvement and PNI.
More accurate treatment outcome predictions can be
obtained when patient and tumor-specific clinical and pathological
factors other than the TNM staging system are added to the model.
In the present study, when patients in the group 3 were examined in
terms of OS according to the scoring system, 86% had a tumor
diameter ≥5 cm, 72% had radiological multiple lymph node
involvement at baseline, 63% had pN+ disease and 88% had PNI after
neoadjuvant treatment. The median survival time of these patients
in the group 3 was significantly lower than that of patients in the
group 1 and 2 (median OS times for groups 1, 2 and 3 were 138.3,
87.2 and 64.6 months, respectively; P<0.001). The addition of
ChT to neoadjuvant RT has a radiosensitizing role and has been
proven to have positive effects in terms of local recurrence
(36). However, it has been less
effective for OS. In particular, one of the novel treatment
strategies, total neoadjuvant treatment with maximal oncological
treatment before surgery, has been investigated in relation to the
effect on survival. Furthermore, the STELLAR study demonstrated an
OS benefit (4,37). Previous studies have shown that
total neoadjuvant treatment, in which systemic treatment is
intensified in the neoadjuvant period in distally located tumors
with clinical T4, N2 and extra mesorectal lymph node involvement,
contributes to DFS (38–40). In the present study, a significant
number of patients in the high-risk category had PNI, multiple
lymph node involvement or large tumors. With standard neoadjuvant
therapy, the survival rate for this group was poor. The advances in
medical treatment and RT have not significantly influenced DFS and
OS rates, and systemic metastases are seen in up to 30% of
high-risk cases (8). Total
neoadjuvant therapy (TNT) approaches that have proven effective can
be used in these patients.
Clinical prediction models can help determine the
surveillance of patients with rectal cancer and aid risk
stratification in clinical trials. In the present study, the
discriminative potential of the score model was measured by ROC
curves, and the AUCs were calculated. The risk scoring modeling had
reliable AUC values [LC, 0.68 (95% CI, 0.57–0.80; P=0.01); DFS,
0.71 (95% CI, 0.60–0.81; P=0.001); and OS, 0.68 (95% CI, 0.57–0.79;
P=0.001)]. These levels are suitable for clinical decision-making
but not optimal. These factors may help develop novel models. Model
accuracy can be improved by adding novel molecular factors related
to tumor biology to risk score modeling. The present study has
certain limitations, namely, it was a retrospective, single center
study, does not represent the general population, and the number of
patients was small. Additionally, novel molecular information, such
as microsatellite instability, was not included in the scoring
system. Since the scoring system consists of patients treated with
standard nCRT, it does not include total neoadjuvant treatment. It
was not possible to establish a training and validation cohort in
the present study, as there was an insufficient number of patients,
despite the fact that such a cohort has been established in the
relevant literature for some studies (22,29,41).
However, positively, the present study consists of a homogeneous
group of patients treated with the same RT dose, technique and ChT
regime by the same experienced team. The present scoring system was
similar to that of Morini et al (16). However, Morini's scoring system
investigated the effect of tumor regression, grading and LVI on
survival in patients with stage II–III rectal cancer, while the
present scoring system investigated a greater number of clinical
and pathological factors.
The present risk-scoring modeling determined the
difference between groups in terms of LC, DFS and OS. This may help
in decision-making for patient selection for different treatment
approaches and clinical trials, and it may provide a rationale for
individualized follow-up and treatment in high-risk patients.
Especially in patients with tumor size >5 cm, those with PNI and
those with multiple poor prognostic factors such as multiple lymph
node involvement, the addition of effective systemic therapy to RT
may lead to improved treatment outcomes and could be evaluated in
existing large randomized trials of TNT or adjuvant ChT for rectal
cancer. However, the external validation of the present risk score
model using large-scale prospective studies is required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Study conception and design was performed by TKC and
DCO. Data collection was performed by TKC and IFD. TKC and IFD
confirm the authenticity of all the raw data. Analysis and
interpretation of results were completed by TKC, GC and SAE. The
draft manuscript was prepared by TKC and DCO. All authors have read
and approved the final version of the manuscript
Ethics approval and consent to
participate
The present retrospective analysis was performed
with appropriate approval by Istanbul University-Cerrahpasa Ethics
Committee (dated Feburary 22, 2023; approval no.
E-83045809-064.01.01–626378). Informed written consent was obtained
from all individual participants included in the study.
Patient consent for publication
The authors affirm that human research participants
provided informed written consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xi Y and Xu P: Global colorectal cancer
burden in 2020 and projections to 2040. Transl Oncol.
14:1011742021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel Cd, Cervantes A and Arnold D; ESMO Guidelines Committee, :
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28:iv22–iv40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Deng Q, Cheng Y, Fu Z and Wu X:
Effect of adjuvant chemotherapy on the oncological outcome of
rectal cancer patients with pathological complete response. World J
Surg Oncol. 22:312024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson GG, Park J, Helewa RM, Goldenberg
BA, Nashed M and Hyun E: Total neoadjuvant therapy for rectal
cancer: A guide for surgeons. Can J Surg. 66:E1962023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neves ALF, Barbosa LER and Teixeira JPMdA:
Prognosis in colorectal cancer beyond TNM. J Coloproctology (Rio de
Janeiro). 40:404–411. 2020. View Article : Google Scholar
|
|
6
|
Zhang C, Yin S, Tan Y, Huang J, Wang P,
Hou W, Zhang Z and Xu H: Patient selection for adjuvant
chemotherapy in high-risk stage II colon cancer: A systematic
review and meta-analysis. Am J Clin Oncol. 43:279–287. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merkel S, Mansmann U, Papadopoulos T,
Wittekind C, Hohenberger W and Hermanek P: The prognostic
inhomogeneity of colorectal carcinomas stage III: A proposal for
subdivision of stage III. Cancer. 92:2754–2759. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong JW, Yang SX, Chen RP, Zhou YH, Ye
MS, Miao L, Xue ZX and Lu GR: Prognostic value of lymphovascular
invasion in patients with stage III colorectal cancer: A
retrospective study. Med Sci Monit. 25:6043–6050. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Destri G, Maugeri A, Ramistella A, La
Greca G, Conti P, Trombatore G, Vecchio GM, Magro GG, Barchitta M
and Agodi A: The prognostic impact of neoadjuvant chemoradiotherapy
on lymph node sampling in patients with locally advanced rectal
cancer. Updates Surg. 72:793–800. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baek JH, Baek DW, Kang BW, Kim HJ, Park
SY, Park JS, Choi GS and Kim JG: Prognostic impact of the
neoadjuvant rectal score as compared with the tumor regression
grade and yield pathologic TNM stage in patients with locally
advanced rectal cancer after neoadjuvant chemoradiotherapy. In
Vivo. 34:1993–1999. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valentini V, Van Stiphout RG, Lammering G,
Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko
K, Cionini L, et al: Nomograms for predicting local recurrence,
distant metastases, and overall survival for patients with locally
advanced rectal cancer on the basis of European randomized clinical
trials. J Clin Oncol. 29:3163–3172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SM, Yoon G and Seo AN: What are the
most important prognostic factors in patients with residual rectal
cancer after preoperative chemoradiotherapy? Yeungnam Univ J Med.
36:124–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhadda A, Dickinson P, Zaitoun A, Gandhi N
and Bessell E: Prognostic importance of Mandard tumour regression
grade following pre-operative chemo/radiotherapy for locally
advanced rectal cancer. Eur J Cancer. 47:1138–1145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valentini V, Gambacorta MA, Barbaro B,
Chiloiro G, Coco C, Das P, Fanfani F, Joye I, Kachnic L, Maingon P,
et al: International consensus guidelines on clinical target volume
delineation in rectal cancer. Radiother Oncol. 120:195–201. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hodapp N: The ICRU report 83: Prescribing,
recording and reporting photon-beam intensity-modulated radiation
therapy (IMRT). Strahlenther Onkol. 188:97–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morini A, Annicchiarico A, Romboli A,
Ricco M, Crafa P, Montali F, Dell'Abate P and Costi R:
Retrospective survival analysis of stage II–III rectal cancer:
tumour regression grade, grading and lymphovascular invasion are
the only predictors. ANZ J Surg. 91:E112–E118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiser MR: AJCC 8th edition: Colorectal
cancer. Ann Surg Oncol. 25:1454–1455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HG, Kim HS, Yang SY, Han YD, Cho MS,
Hur H, Min BS, Lee KY and Kim NK: Early recurrence after
neoadjuvant chemoradiation therapy for locally advanced rectal
cancer: Characteristics and risk factors. Asian J Surg. 44:298–302.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dinaux AM, Leijssen L, Bordeianou LG,
Kunitake H, Amri R and Berger DL: Outcomes of persistent lymph node
involvement after neoadjuvant therapy for stage III rectal cancer.
Surgery. 163:784–788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen K, Collins G, Wang H and Toh JWT:
Pathological features and prognostication in colorectal cancer.
Curr Oncol. 28:5356–5383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Zhao S and Wang X: A prognostic
nomogram for T3N0 rectal cancer after total mesorectal excision to
help select patients for adjuvant therapy. Front Oncol.
11:6988662021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weiser MR, Chou JF, Keshinro A, Chapman
WC, Bauer PS, Mutch MG, Parikh PJ, Cercek A, Saltz LB, Gollub MJ,
et al: Development and assessment of a clinical calculator for
estimating the likelihood of recurrence and survival among patients
with locally advanced rectal cancer treated with chemotherapy,
radiotherapy, and surgery. JAMA Netw Open. 4:e21334572021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HY, Feng LL, Li M, Ju HQ, Ding Y, Lan
M, Song SM, Han WD, Yu L, Wei MB, et al: College of American
pathologists tumor regression grading system for long-term outcome
in patients with locally advanced rectal cancer. Oncologist.
26:e780–e793. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P and Samama
G: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laohawiriyakamol S, Chaochankit W,
Wanichsuwan W, Kanjanapradit K and Laohawiriyakamol T: An
investigation into tumor regression grade as a parameter for
locally advanced rectal cancer and 5-year overall survival rate.
Ann Coloproctol. 39:59–70. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Capirci C, Valentini V, Cionini L, De
Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G,
Palazzi S, et al: Prognostic value of pathologic complete response
after neoadjuvant therapy in locally advanced rectal cancer:
Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol
Phys. 72:99–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liebig C, Ayala G, Wilks JA, Berger DH and
Albo D: Perineural invasion in cancer: A review of the literature.
Cancer. 115:3379–3391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knijn N, Mogk SC, Teerenstra S, Simmer F
and Nagtegaal ID: Perineural invasion is a strong prognostic factor
in colorectal cancer. Am J Surg Pathol. 40:103–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Y: A prognostic nomogram for stage
II/III rectal cancer patients treated with neoadjuvant
chemoradiotherapy followed by surgical resection. BMC Surg.
22:2562022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng H, Lyu Z, Zheng J, Zheng C, Wu DQ,
Liang W and Li Y: Association of tumor size with prognosis in colon
cancer: A surveillance, epidemiology, and end results (SEER)
database analysis. Surgery. 169:1116–1123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Q, Zhang K, Guo K, Liu S, Wasan HS,
Jin H, Yuan L, Feng G, Shen F, Shen M, et al: Value of tumor size
as a prognostic factor in metastatic colorectal cancer patients
after chemotherapy: A population-based study. Future Oncol.
15:1745–1758. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai W, Li Y, Meng X, Cai S, Li Q and Cai
G: Does tumor size have its prognostic role in colorectal cancer?
Re-evaluating its value in colorectal adenocarcinoma with different
macroscopic growth pattern. Int J Surg. 45:105–112. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang Y, You K, Qiu X, Bi Z, Mo H, Li L
and Liu Y: Tumor volume predicts local recurrence in early rectal
cancer treated with radical resection: A retrospective
observational study of 270 patients. Int J Surg. 49:68–73. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tayyab M, Razack A, Sharma A, Gunn J and
Hartley JE: Correlation of rectal tumor volumes with oncological
outcomes for low rectal cancers: Does tumor size matter? Surg
Today. 45:826–833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yeo SG, Kim DY, Park JW, Oh JH, Kim SY,
Chang HJ, Kim TH, Kim BC, Sohn DK and Kim MJ: Tumor volume
reduction rate after preoperative chemoradiotherapy as a prognostic
factor in locally advanced rectal cancer. Int J Radiat Oncol Biol
Phys. 82:e193–e199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCarthy K, Pearson K, Fulton R and Hewitt
J: Pre-operative chemoradiation for non-metastatic locally advanced
rectal cancer. Cochrane Database Syst Rev.
12:CD0083682012.PubMed/NCBI
|
|
37
|
Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li
N, Liu WY, Chen SL, Li S, Lu NN, et al: Multicenter, randomized,
phase III trial of short-term radiotherapy plus chemotherapy versus
long-term chemoradiotherapy in locally advanced rectal cancer
(STELLAR). J Clin Oncol. 40:1681–1692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bahadoer RR, Dijkstra EA, van Etten B,
Marijnen CA, Putter H, Kranenbarg EMK, Roodvoets AGH, Nagtegaal ID,
Beets-Tan RGH, Blomqvist LK, et al: Short-course radiotherapy
followed by chemotherapy before total mesorectal excision (TME)
versus preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:29–42.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dijkstra EA, Nilsson PJ, Hospers GA,
Bahadoer RR, Kranenbarg EM-K, Roodvoets AG, Putter H, Berglund Å,
Cervantes A, Crolla RMPH, et al: Locoregional failure during and
after short-course radiotherapy followed by chemotherapy and
surgery compared to long-course chemoradiotherapy and surgery-a
five-year follow-up of the RAPIDO trial. Ann Surg. 278:e766–e772.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Conroy T, Bosset JF, Etienne PL, Rio E,
François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O,
Gargot D, et al: Neoadjuvant chemotherapy with FOLFIRINOX and
preoperative chemoradiotherapy for patients with locally advanced
rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:702–715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diao JD, Wu CJ, Cui HX, Bu MW, Yue D, Wang
X, Liu YL and Yang YJ: Nomogram predicting overall survival of
rectal squamous cell carcinomas patients based on the SEER
database: A population-based STROBE cohort study. Medicine
(Baltimore). 98:e179162019. View Article : Google Scholar : PubMed/NCBI
|