Nifuroxazide (NFZ) is a gastrointestinal antibiotic
that was first patented by Laboratoires Robert & Carriere SA
(France) in 1961 (France) and 1966 (USA) (1). It was widely used and heavily promoted
in the 1970s. When taken orally, the drug can be absorbed and
metabolized in the liver (2). NFZ
was first defined as a broad-spectrum intestinal antibiotic for the
effective and safe treatment of bacterial vaginitis, chlamydia
trachomatis, mycoplasma and candida infections (3). NFZ has a long history of clinical
application. In 2008, NFZ was identified as a potent inhibitor of
signal transduction and transcriptional activation factor 3
(STAT3)-dependent gene expression by screening 1,200 bioactive
compounds in the Prestwick library (4). NFZ is defined as a STAT3 inhibitor. A
study has shown that this kinase inhibitory activity explains the
anti-proliferative activity of NFZ in myeloma cells, which performs
a constitutive activation of STAT3 with negligible effects on
normal cells (4). Recent studies
have shown that NFZ has obvious anti-tumor effects and numerous
studies have been conducted on its anticancer activity (5–7).
NFZ can downregulate cyclin D, resulting in the
failure of cells to enter the S phase from G1-G0 phase, and the
cell cycle stagnates at G1-G0 in a concentration-dependent manner.
It has anti-melanoma activity in vitro and in vivo,
has strong anti-proliferation activity against a variety of tumor
cells, can induce G2/M phase arrest and cell apoptosis, and inhibit
the migration and invasion of tumor cells (8). It can also significantly inhibit tumor
growth, reduce cell proliferation and metastasis, and induce cell
apoptosis in tumor-bearing mouse models (9).
NFZ is a multi-target drug that can fight tumors
through multiple target proteins and target pathways.
The JAK-STAT pathway has a variety of roles in
physiological processes such as cell growth, differentiation and
immune response. It plays a role in cell cycle regulation, cytokine
signaling and apoptosis (10).
STAT3 is present in numerous malignant tumor cells. STAT3 is
continuously activated in most human primary cancer sites and tumor
cell lines, such as human astrocytoma, multiple osteosarcoma,
prostate cancer, colon cancer, stomach cancer, liver cancer and
breast cancer (5,11–17).
Dysregulation of this pathway is closely related to carcinogenesis
and poor prognosis of various cancers, including kidney cancer
(18), lung cancer (19), cervical cancer (20) and bladder cancer (21). Immunohistochemical results of 149
patients with invasive bladder cancer showed that 51.3% of them had
high STAT3 expression (21).
The amide group of NFZ is located in the
para-position of the hydroxyphenyl part. This configuration
promotes conjugation between carboxyl oxygen atoms, phenyl radicals
and hydroxyl oxygen atoms. This structural arrangement makes the
hydrogen atoms in the hydroxyl part more fluid and is a classical
STAT3 inhibitor that can inhibit JAK/STAT3 phosphorylation
(3,5,9,12,22–25).
NFZ has antitumor effects in hematologic tumors and solid tumors by
inhibiting the STAT3 signaling pathway (4,11). NFZ
inhibits the constitutive phosphorylation of STAT3 by reducing the
self-phosphorylation of JAK and leads to the downregulation of the
target gene of STAT3, myeloid cell leukemia−1, thereby
reducing the survival activity of myeloma cells without affecting
normal peripheral blood mononuclear cells (4).
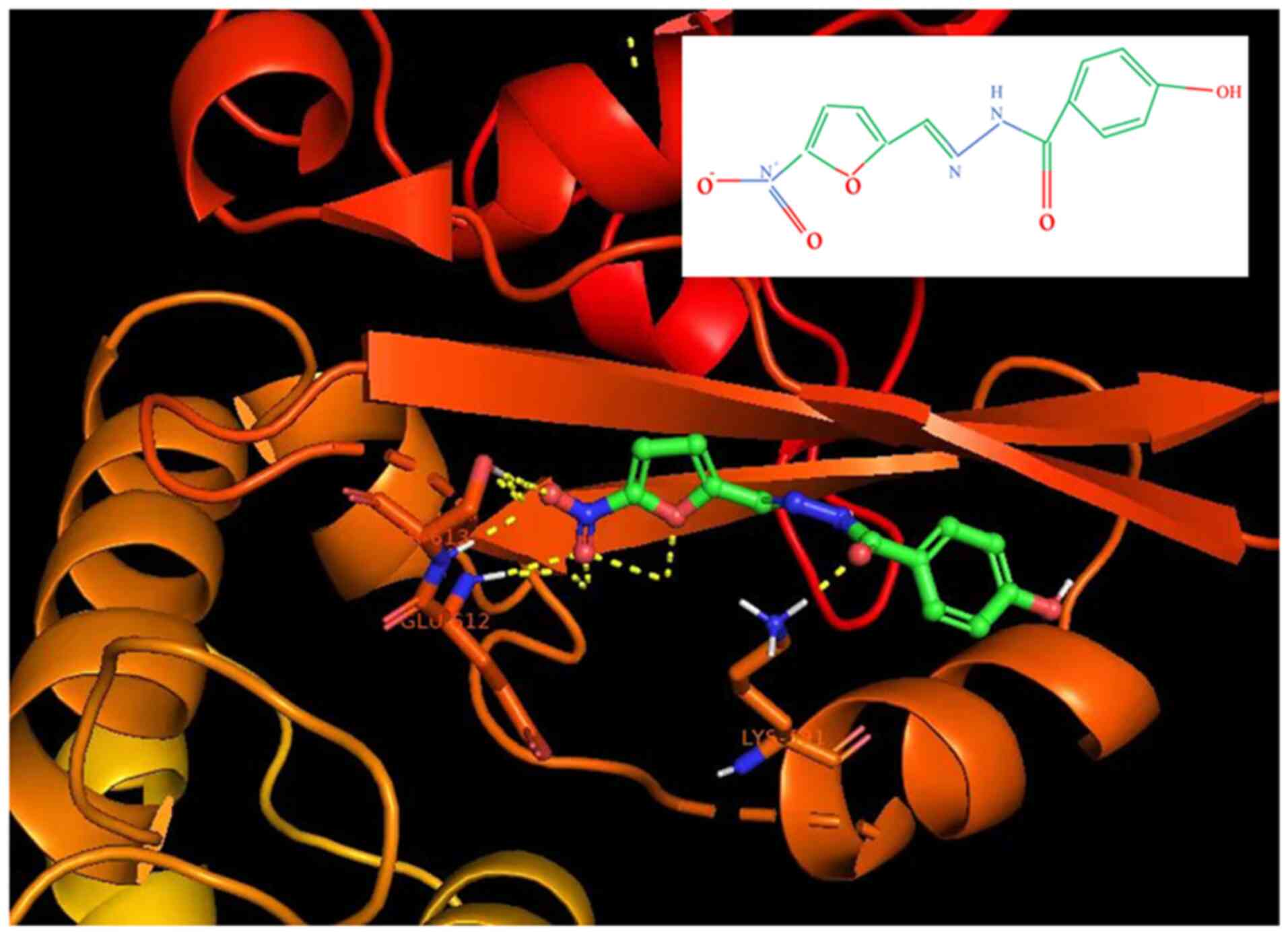
AutoDock Vina (version 1.1.2) was used for molecular
docking on STAT3. The mesh sizes of catalytic and allosteric sites
were 15, 15 and 22.5, respectively. The dimensions x, y and z were
11.25, 15 and 15, respectively. The Lamarque genetic algorithm was
used to perform 2,500 evaluations and 100 run docking simulations.
Bio via Discovery Studio Visualizer 2020 was used to show molecular
interactions between proteins and ligands. Molecular docking
techniques were used to dock the spatial structure of STAT3
(Fig. 1). When NFZ and Stat3 bind
(binding sites Lys591, Glu612 and SER613), it can inhibit STAT3
phosphorylation and thus inhibit transcriptional pathways related
to this pathway. Binding site residues, such as Lys591, are
consistent with previous structural studies of STAT3 (11).
In numerous cancers, inflammatory cytokines [e.g.
transcription factors (TFs)] are the most direct and promising
targets (11). IL-6, discovered in
1976 (26), is a glycosylated
protein composed of 184 amino acids (27) and a pleiotropic cytokine, which not
only participates in immune response, but also in the basic
processes such as inflammation, hematopoietic function, bone
metabolism and embryonic development. Physiological processes that
play important roles in a variety of diseases include cell
proliferation, immune surveillance, acute inflammation, metabolism
and bone remodeling (26–31). It was originally described as
stimulating B lymphocytes and hepatocytes (31). Numerous human malignancies are
caused by a variety of cell types, including tumor cells and
stromal cells (10,25,32,33).
IL-6 binds to the IL-6 receptor and then binds to
the glycoprotein 130 receptor to produce a signal transduction
hexamer receptor complex. Recruited and activated JAKs
phosphorylate STAT3 in turn, leading to gene regulation. Abnormal
expression of IL-6 occurs in various cancer types and is associated
with poor clinical prognosis and metastasis. In pathophysiological
states, IL-6 mediates inflammation and regulates the MAPK, PI3K and
JAK/STAT carcinogenic pathways (34). STAT3 is the main downstream
regulatory signal of IL-6 in regulating inflammation and tumor
transformation (28,35,36).
NFZ effectively inhibited STAT3 and cancer-related inflammatory
phenotypes NFZ effectively inhibits STAT3 and cancer-related
inflammatory phenotypes (overexpression of Bcl-2, transcriptional
activation of IL-6, and association of TNF-α, Bcl-2, EGFR, STAT3,
transcription factor p65 (RELA p65), and wingless-type MMTV
integration site family, member 5A (WNT5a) with cell survival); NFZ
alone or in combination is used to prevent or treat tumors
(37). Overexpression of IL-6,
IL-6Ra and gp130, the upstream effectors of the IL-6/JAK/STAT3
pathway, may lead to abnormal activation of STAT3 (38), and IL-6 is an upstream signaling
component of STAT3 (13,39,40).
Secretion of IL-6 induced the expression of STAT3 target genes,
such as Cyclin D1, Bcl-2, Bcl-xL, VEGF, VEGFR2 and matrix
metalloproteinases (MMPs) (41–44).
IL-6 and p-Stat3 were overexpressed in 83 breast cancer samples.
The IL-6/JAK/STAT3 signaling pathway can promote tumor cell
proliferation, angiogenesis, epithelial-to-mesenchymal transition
and cancer stem cell subpopulation growth, while inhibiting the
anti-tumor immune response (45–47).
In breast cancer, the IL-6 pathway is frequently
activated, simultaneously promoting breast cancer metastasis and
suppressing the anti-tumor immune response (47). NFZ decreased the viability of three
breast cancer cell lines, MCF-7, MDA-MB-231 and 4T1 cells, and
induced cancer cell apoptosis in a dose-dependent manner. Activated
cleavage of caspase-3 and Bax downregulated Bcl-2. It also
significantly blocked the migration of cancer cells and
phosphorylated STAT3 Tyr705 to reduce the expression of MMP-2 and
MMP-9. It inhibited tumor growth and blocked lung metastasis
formation in mice with no detectable toxicity. The number of
Ki-67-positive cells and MMP-9-positive cells can be downregulated,
cleaved caspase-3 positive cells can be upregulated and the number
of myelo-derived suppressor cells in the lung can be reduced by NFZ
(48–52).
Abnormal STAT3 has a positive effect on breast
cancer and induces G1 cell cycle progression, proliferation,
anti-apoptosis, angiogenesis and metastasis through transcriptional
regulation of target gene expression (39,53–57).
Specific expression of STAT3 activated immunosuppressive
tumor-infiltrating medullary suppressor cells (MDSCs),
tumor-associated macrophages (TAM) and T-regulatory cells. STAT3
further induces upstream expression of cytokines and growth factors
to produce a malignant autocrine paracrine positive feedback loop
(47,57–59).
STAT3 and IL-6 can further activate nuclear factor κB (NF-κB)
signaling through nuclear factors in breast cancer. IL-6 is
targeted to the expression of let-7 miRNAIL-6 mRNA in the
3′-untranslated region. Activation of NF-κB inhibits let-7 and
leads to IL-6 hyperactivation and subsequent activation of STAT3
(60). Studies have shown that
oncostatin M (OSM) can further activate the IL-6/JAK/STAT3
signaling pathway and promote the progression of breast cancer both
in vivo and in vitro. OSM and IL-1β synergistically
induce further activation of STAT3 secreted by IL-6 in estrogen
receptor + and triple-negative breast cancer cells (54,61–63).
Studies have shown that NFZ targets STAT3 and
inactivates ALDH1 to inhibit multiple myeloma and melanoma cells,
respectively (10,75,76).
NFZ can be biologically activated by ALDH, which is highly
expressed in certain cancer-initiating cells (ALDH-high stem
cells). Although ALDH2 is a direct target of NFZ (75), the biological activity of NFZ
against ALDH1 is superior to that of ALDH2 (76). In vivo, the drug binds a
substrate bag suitable for the ald1a 1/A3 subtype to two cysteine
residues at the active site of the enzyme, resulting in oxidation
and inactivation of the enzyme. NFX selectively kills ALDH1-high
cancer-initiating cells, which correspond to a high tumorigenic
subgroup. In stark contrast, Odero cells were found to be resistant
to NFZ. In vivo experiments using mice transplanted with
A375-L2T melanoma cells showed that the ALDH1-high cancer
subpopulation was highly sensitive to NFZ, which completely
eradicated the population in vivo (77).
NFZ can activate different regulated cell death
pathways (parthanatos and apoptosis) (74–80).
NFZ affects multiple transcription factors through the
ERG-associated protein-protein interaction network in the MAPK
signaling pathway. NFZ can downregulate IL-1, IL-6 and C-C motif
chemokine ligand-2 (81). ERG and
other transcription factors containing ETS domains work with
multiple transcription factors (activator protein-1, nuclear factor
of activated T cells, NF-κB) or proteins to regulate downstream
gene expression (5,82,83).
After NFZ binds to ERG, the spatial conformation of ERG is changed,
which makes ERG unable to combine with poly(ADP-ribose) polymerase
1 (PARP1) and other proteins smoothly. Researchers such as Hossain
and Bostwick (84) through knocked
down ERG (83) confirmed that NFZ
blocked the interaction between ERG and PARP1 (84,85).
NFZ inhibited the proliferation of transmembrane protease
serine:ERG-positive cells by interfering with ERG or ERG-associated
TFs. NFZ has a stronger affinity for ERG than STAT3 and ALDH1
(75). NFZ showed a strong
inhibitory effect on the growth of ERG-positive prostate cancer
cell lines (VCaP, DU145-ERG), but did not inhibit the growth of
ERG-negative cell lines (LNCaP, DU145, WPMY) (84). NFZ had stronger inhibitory effects
on ERG-overexpressing cell lines. The IC50 difference
between the DU145-ERG and DU145 vectors was at least 7-fold, and
the addition of olapalil reduced the inhibition of DU145-ERG by NFZ
by at least 10-fold (82). NFZ
upregulated genes associated with DNA repair in prostate cancer
VCaP cells (BRCA1, FA complementation group and PARP1),
particularly PARP1. PARP1 inhibitors (olapalil) blocked
NFZ-mediated growth inhibition of VCaP cells in a dose-dependent
manner. An increase in intracellular allograft inflammatory factor
occurred, which eventually produced a large number of DNA fragments
to induce cell necrosis (86,87).
USP is an enzyme that catalyzes protein
deubiquitination and is involved in biological processes related to
metabolic disorders and cancer proliferation. Abnormal USP function
has been associated with a variety of diseases, including metabolic
dysfunction associated with liver disease and cancer (85). Certain USPs regulate oncogene
activity and/or tumor suppressor function, while others influence
pathways associated with tumor progression (88). In HepG2 cells, NFZ increased
miR-4458 levels and not only inhibited USP-21 and its substrate ATP
citrate lyase, but also increased p-AMP kinase α (the downstream
functional target of USP-21). Thus, NFZ may be characterized by the
fact that in the chemical structure of nifuramide, the oxygen atoms
of the nitro group and the oxygen atoms of the carbonyl group
exhibit higher electron densities due to their binding within the
conjugated system. As a result, these oxygen atoms have the ability
to form strong bonds with amino acids with opposite charges.
Previous studies have also revealed the anti-proliferative
properties of furan-containing compounds (85), while nitrofuran derivatives have
shown tumor growth inhibition through p53-dependent mechanisms
(89), and the anticancer potential
of amide compounds has been similarly confirmed. For instance,
decibuprofen and amide derivatives inhibit MCF-7 cell proliferation
(90), and these derivatives
inhibit the growth of multiple cancer cell lines by interacting
with the tyrosine kinase domain of human epidermal growth factor
receptor 2 (91). The association
of USP-21 (92,93) extends to the stem cell regulation of
cancer cells, which activates the Wnt pathway to enhance the stem
cell properties of pancreatic cancer cells (94). In addition, USP21 is involved in the
deubiquitination of K48-linked ubiquitin chains, stabilizing Nanog,
and maintaining the dryness of mouse embryonic stem cells in both
in vivo and in vitro environments (95). In bladder cancer, USP21 expression
is elevated and associated with poor prognosis. Notably, it blocks
ubiquitination of EZH2, thereby promoting the proliferation and
metastasis of bladder cancer cells (96). In non-small cell lung cancer, USP21
promotes tumor cell proliferation, invasion and migration through
the YY1/small nucleolar RNA host gene 16 pathway (97). The interaction of USP21 with MAPK
kinase 2 stabilizes the latter, ultimately leading to the
activation of ERK1/2 and the propagation of carcinogenic signals in
liver cancer (98,99).
Cell necrosis and downregulation of these
inflammatory factors caused by NFZ activation of parthanatos can
alter the immune microenvironment and enhance the immune response
(81). NFZ can increase the
infiltration of CD8+ T cells and decrease the number of M2
macrophages in colorectal cancer; the percentage of M2 macrophages
(CD11b+F4/80+CD206+) blank group was 10.2%, the percentage in the
25 mg/kg group (CD11b+F4/80+CD206+) was 8.6% and the percentage in
the 50 mg/kg group (CD11b+F4/80+CD206+) was 3.6% (11). NFZ reduced the number of bone
marrow-derived suppressor cells in breast cancer cells and
colorectal cancer cells, and increased intratumoral CD8+ T-cell
infiltration. Importantly, a significant decrease and changes in
the number of M2-type macrophages in the tumor were observed in a
model of abdominal metastasis (78).
NFZ has been successfully tested for safety and
efficacy in solid tumors in Phase I clinical trials (106), where, after oral administration,
the drug can be absorbed and metabolized in the liver, and very low
concentrations can be detected in blood and urine. The drug shows
almost unique enteral sterility and no systemic antibacterial
activity (4). NFZ is well
tolerated, but occasionally, side effects occur, manifested by
digestive process disturbances and eventual allergic reactions,
such as rashes, hives and vascular inflammation (and, in rare
cases, severe immune anaphylaxis and anaphylactic shock) (107). There is no carcinogenicity in
transgenic mouse models of NFZ mutagenicity (108). Mice treated with NFZ lost
significantly less weight than those treated with the carrier,
meaning that NFZ reduced weight loss in mice and that NFZ did not
show significant toxicity. In the NFZ treatment group, alanine and
aspartate aminotransferase values fluctuated within the normal
range (109), and NFZ could exert
its antitumor effects without significant toxicity. Toxicity
evaluation was performed on A375 and B16-F10 model mice to confirm
the safety of NFZ after drug therapy, including serological
analysis, blood analysis and H&E staining. Serological and
hematological analysis did not show any pathological changes and
there was no significant change in body weight compared to the
control group. In addition, no pathological changes were observed
in the heart, liver, spleen and kidney after NFZ treatment
(110). NFZ has been shown to
inhibit STAT3 phosphorylation by inhibiting JAK2 and Tyk2 family
kinases, leading to a decline in myeloma cell viability. However,
there was no cytotoxic effect on normal peripheral blood
mononuclear cells (8,12,111),
which indicates safety to a certain extent in clinical
practice.
Currently, a novel lipoprotein unstable S-2
phosphate, NFZ, has been synthesized, which, when assembled into
nanoparticles, significantly increases the local concentration of
the drug (240-fold), enhancing the local effect of the drug and
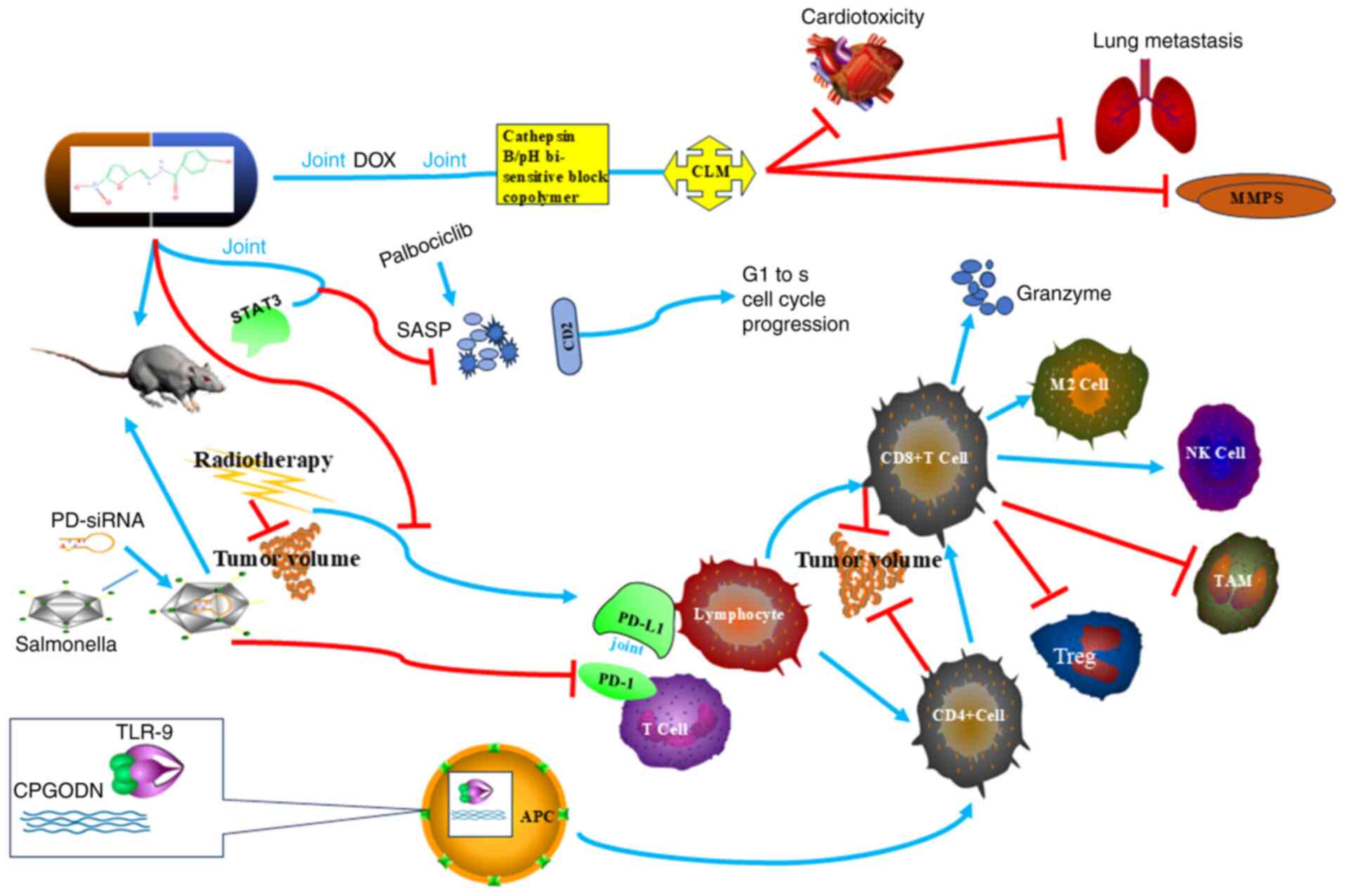
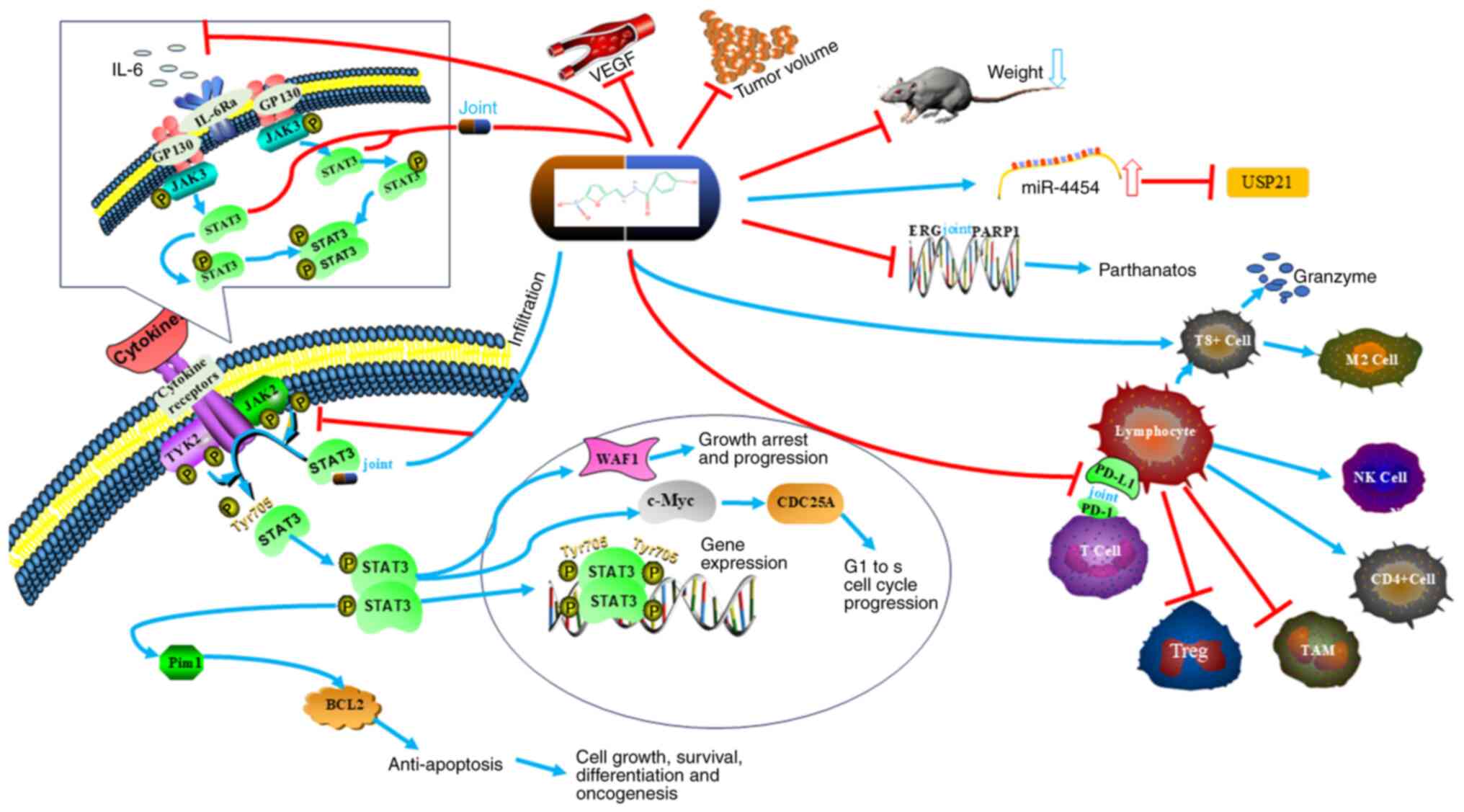
enhancing the antitumor effect (108). NFZ is a multi-target anti-tumor
drug (Table I), which inhibits cell
proliferation, promotes apoptosis and programmed necrosis of tumor
cells through the IL-6, STAT3, ALDH1, ERG and UPS-21 pathways, acts
on the tumor microenvironment and enhances the anti-tumor effects
of T lymphocytes and B lymphocytes (Fig. 3). As a combination drug, NFZ can
enhance the effect of radiation therapy, targeted therapy and
immunotherapy, and has shown obvious safety in in vivo and
in vitro experiments, thus making it a potential anti-tumor
drug (Fig. 2).
Not applicable.
Funding: No funding was received.
Not applicable.
LL wrote the manuscript, searched the literature and
prepared the figures. CM was involved in the design of the study
and revised the manuscript. DL provided article ideas, modified the
figures and revised the manuscript. JJ and RG performed the
literature search and critcally revised the manuscript for
important intellectual content. Data authentication is not
applicable. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Carron MCE: Antibacterial
nitrofurfuryldene derivatives and methods of using same. US Patent
US3290213, Filed July 9, 1962. issued December 6. 1966.
|
|
2
|
B Fernandes M, Gonçalves JE, C Tavares L
and Storpirtis S: Caco-2 cells permeability evaluation of
nifuroxazide derivatives with potential activity against
methicillin-resistant Staphylococcus aureus (MRSA). Drug Dev Ind
Pharm. 41:1066–1072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bailly C: Toward a repositioning of the
antibacterial drug nifuroxazide for cancer treatment. Drug Discov
Today. 24:1930–1936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Liu L, Li F, Ma C and Ge K:
Nifuroxazide induces the apoptosis of human non-small cell lung
cancer cells through the endoplasmic reticulum stress PERK
signaling pathway. Oncol Lett. 25:2482023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao T, Wei P, Zhang C, Zhou S, Liang L,
Guo S, Yin Z, Cheng S, Gan Z, Xia Y, et al: Nifuroxazide suppresses
PD-L1 expression and enhances the efficacy of radiotherapy in
hepatocellular carcinoma. Elife. 12:RP909112024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amin FM, Sharawy MH, Amin MN, El-Sherbiny
M, Said E, Salem HA and Ibrahim TM: Nifuroxazide mitigates
doxorubicin-induced cardiovascular injury: Insight into
oxidative/NLRP3/GSDMD-mediated pyroptotic signaling modulation.
Life Sci. 314:1213112023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Y, Zeng A, Fang A, Song L, Fan C, Zeng
C, Ye T, Chen H, Tu C and Xie Y: Nifuroxazide induces apoptosis,
inhibits cell migration and invasion in osteosarcoma. Invest New
Drugs. 37:1006–1013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hindupur SV, Schmid SC, Koch JA, Youssef
A, Baur EM, Wang D, Horn T, Slotta-Huspenina J, Gschwend JE, Holm
PS and Nawroth R: STAT3/5 inhibitors suppress proliferation in
bladder cancer and enhance oncolytic adenovirus therapy. Int J Mol
Sci. 21:11062020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Shi W, Wang X, Lu JJ, He P, Zhang
H and Chen X: Nifuroxazide boosts the anticancer efficacy of
palbociclib-induced senescence by dual inhibition of STAT3 and CDK2
in triple-negative breast cancer. Cell Death Discov. 9:3552023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Sherbiny M, El-Sayed RM, Helal MA,
Ibrahiem AT, Elmahdi HS, Eladl MA, Bilay SE, Alshahrani AM, Tawfik
MK, Hamed ZE, et al: Nifuroxazide mitigates angiogenesis in
ehlrich's solid carcinoma: molecular docking, bioinformatic and
experimental studies on inhibition of Il-6/Jak2/Stat3 signaling.
Molecules. 26:68582021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao T, Jia H, Cheng Q, Xiao Y, Li M, Ren
W, Li C, Feng Y, Feng Z, Wang H and Zheng J: Nifuroxazide prompts
antitumor immune response of TCL-loaded DC in mice with
orthotopically-implanted hepatocarcinoma. Oncol Rep. 37:3405–3414.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye TH, Yang FF, Zhu YX, Li YL, Lei Q, Song
XJ, Xia Y, Xiong Y, Zhang LD, Wang NY, et al: Inhibition of Stat3
signaling pathway by nifuroxazide improves antitumor immunity and
impairs colorectal carcinoma metastasis. Cell Death Dis.
8:e25342017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X,
Li Y, Jie H, Liu C, Xiong Y, et al: Nifuroxazide induces apoptosis
and impairs pulmonary metastasis in breast cancer model. Cell Death
Dis. 6:e17012015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huynh J, Etemadi N, Hollande F, Ernst M
and Buchert M: The JAK/STAT3 axis: A comprehensive drug target for
solid malignancies. Semin Cancer Biol. 45:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung KH, Yoo W, Stevenson HL, Deshpande D,
Shen H, Gagea M, Yoo SY, Wang J, Eckols TK, Bharadwaj U, et al:
Multi-functional effects of a small-molecule STAT3 inhibitor on
NASH and HCC in mice. Clin Cancer Res. 23:5537–5546. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo C, Yang G, Khun K, Kong X, Levy D, Lee
P and Melamed J: Activation of Stat3 in renal tumors. Am J Transl
Res. 1:283–290. 2009.PubMed/NCBI
|
|
19
|
Tong M, Wang J, Jiang N, Pan H and Li D:
Correlation between p-STAT3 overexpression and prognosis in lung
cancer: A systematic review and meta-analysis. PLoS One.
12:e01822822017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takemoto S, Ushijima K, Kawano K,
Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M,
Kakuma T, et al: Expression of activated signal transducer and
activator of transcription-3 predicts poor prognosis in cervical
squamous-cell carcinoma. Br J Cancer. 101:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CL, Cen L, Kohout J, Hutzen B, Chan
C, Hsieh FC, Loy A, Huang V, Cheng G and Lin J: Signal transducer
and activator of transcription 3 activation is associated with
bladder cancer cell growth and survival. Mol Cancer. 7:782008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hammarén HM, Virtanen AT, Raivola J and
Silvennoinen O: The regulation of JAKs in cytokine signaling and
its breakdown in disease. Cytokine. 118:48–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia H, Cui J, Jia X, Zhao J, Feng Y, Zhao
P, Zang D, Yu J, Zhao T, Wang H and Xu K: Therapeutic effects of
STAT3 inhibition by nifuroxazide on murine acute graft
graft-vs.-host disease: Old drug, new use. Mol Med Rep.
16:9480–9486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Althagafy HS, El-Aziz MKA, Ibrahim IM,
Abd-Alhameed EK and Hassanein EHM: Pharmacological updates of
nifuroxazide: Promising preclinical effects and the underlying
molecular mechanisms. Eur J Pharmacol. 951:1757762023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasson TS, Said E and Helal MG:
Nifuroxazide modulates hepatic expression of LXRs/SR-BI/CES1/CYP7A1
and LDL-R and attenuates experimentally-induced
hypercholesterolemia and the associated cardiovascular
complications. Life Sci. 306:1207902022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kishimoto T and Ishizaka K: Regulation of
antibody response in vitro. X. Biphasic effect of cyclic AMP on the
secondary anti-hapten antibody response to anti-immunoglobulin and
enhancing soluble factor. J Immunol. 116:534–541. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirano T: Interleukin 6 and its receptor:
ten years later. Int Rev Immunol. 16:249–284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brumfftt W, Reynolds AV and
Hamilton-Miller JM: Letter: Activity of nitrofurantoin and
nifuratel against anaerobic gram-negative bacilli. Lancet.
1:4601975. View Article : Google Scholar
|
|
30
|
Kang S, Narazaki M, Metwally H and
Kishimoto T: Historical overview of the interleukin-6 family
cytokine. J Exp Med. 217:e201903472020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirano T and Kishimoto T: Interleukin-6.
Peptide Growth Factors and Their Receptors I. Sporn MB and Roberts
AB: Springer; Berlin: pp. p6331990, View Article : Google Scholar
|
|
32
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kamimura D, Ishihara K and Hirano T: IL-6
signal transduction and its physiological roles: the signal
orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hasegawa H, Mizoguchi I, Chiba Y, Ohashi
M, Xu M and Yoshimoto T: Expanding diversity in molecular
structures and functions of the IL-6/IL-12 heterodimeric cytokine
family. Front Immunol. 7:4792016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Polatti F: Bacterial vaginosis, Atopobium
vaginae and nifuratel. Curr Clin Pharmacol. 7:36–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Wang L, Lin HK, Kan PY, Xie S,
Tsai MY, Wang PH, Chen YT and Chang C: Interleukin-6 differentially
regulates androgen receptor transactivation via PI3K-Akt, STAT3,
and MAPK, three distinct signal pathways in prostate cancer cells.
Biochem Biophys Res Commun. 305:462–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu H, Pardoll D and Jove R: STATs in
cancer Inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L,
Fernández-Luna JL, Nuñez G, et al: Constitutive activation of Stat3
signaling confers resistance to apoptosis in human U266 myeloma
cells. Immunity. 10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zilberstein A, Ruggieri R, Korn JH and
Revel M: Structure and expression of cDNA and genes for human
interferon-beta-2, a distinct species inducible by
growth-stimulatory cytokines. EMBO J. 5:2529–2537. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haegeman G, Content J, Volckaert G,
Derynck R, Tavernier J and Fier W: Structural analysis of the
sequence coding for an inducible 26-kDa protein in human
fibroblasts. Eur J Biochem. 159:625–632. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kubo M, Hanada T and Yoshimura A:
Suppressors of cytokine signaling and immunity. Nat Immunol.
4:1169–1176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: a
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lieblein JC, Ball S, Hutzen B, Sasser AK,
Lin HJ, Huang TH, Hall BM and Lin J: STAT3 can be activated through
paracrine signaling in breast epithelial cells. BMC Cancer.
8:3022008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fukada T, Hibi M, Yamanaka Y,
Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K and Hirano
T: Two signals are necessary for cell proliferation induced by a
cytokine receptorGp130: Involvementof STAT3 inAnti-apoptosis.
Immunity. 5:449–460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leslie K, Lang C, Devgan G, Azare J,
Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, et al:
Cyclin D1 is transcriptionally regulated by and required for
transformation by activated signal transducer and activator of
transcription 3. Cancer Res. 66:2544–2552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Burke WM, Jin X, Lin HJ, Huang M, Liu R,
Reynolds RK and Lin J: Inhibition of constitutively active stat3
suppresses growth of human ovarian and breast cancer cells.
Oncogene. 20:7925–7934. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee HT, Xue J, Chou PC, Zhou A, Yang P,
Conrad CA, Aldape KD, Priebe W, Patterson C, Sawaya R, et al: Stat3
orchestrates interaction between endothelial and tumor cells and
inhibition of stat3 suppresses brain metastasis of breast cancer
cells. Oncotarget. 6:10016–10029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vageli DP, Doukas PG, Siametis A and
Judson BL: Targeting STAT3 prevents bile reflux-induced oncogenic
molecular events linked to hypopharyngeal carcinogenesis. J Cell
Mol Med. 26:75–87. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Manore SG, Doheny DL, Wong GL and Lo HW:
IL-6/JAK/STAT3 signaling in breast cancer metastasis: Biology and
treatment. Front Oncol. 12:8660142022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hirano T, Yasukawa K, Harada H, Taga T,
Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K,
Iwamatsu A, et al: Complementary DNA for a novel human interleukin
(BSF-2) that induces B lymphocytes to produce immunoglobulin.
Nature. 324:73–76. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gauldie J, Richards C, Harnish D, Lansdorp
P and Baumann H: Interferon beta 2/B-Cell stimulatory factor type 2
shares identity with monocyte-derived hepatocyte-stimulating factor
and regulates the major acute phase protein response in liver
cells. Proc Natl Acad Sci USA. 84:7251–7255. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brakenhoff JP, de Groot ER, Evers RF,
Pannekoek H and Aarden LA: molecular cloning and expression of
hybridoma growth factor in escherichia coli. J Immunol.
139:4116–4121. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei
Y, Abbruzzese JL, Hortobagyi GN and Hung MC: Epidermal growth
factor receptor cooperates with signal transducer and activator of
transcription 3 to induce epithelial-mesenchymal transition in
cancer cells via up-regulation of TWIST gene expression. Cancer
Res. 67:9066–9076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 Activity Up-Regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kortylewski M and Yu H: Role of stat3 in
suppressing anti-tumor immunity. Curr Opin Immunol. 20:228–233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, et al: Regulation of the innate and adaptive immune responses by
stat-3 signaling in tumor cells. Nat Med. 10:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alvarez JV, Febbo PG, Ramaswamy S, Loda M,
Richardson A and Frank DA: Identification of a genetic signature of
activated signal transducer and activator of transcription 3 in
human tumors. Cancer Res. 65:5054–5062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dechow TN, Pedranzini L, Leitch A, Leslie
K, Gerald WL, Linkov I and Bromberg JF: Requirement of matrix
metalloproteinase-9 for the transformation of human mammary
epithelial cells by stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kujawski M, Kortylewski M, Lee H, Herrmann
A, Kay H and Yu H: Stat3 mediates myeloid cell-dependent tumor
angiogenesis in mice. J Clin Invest. 118:3367–3377. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang M, Chen J, Zhang W, Zhang R, Ye Y,
Liu P, Yu W, Wei F, Ren X and Yu J: Interleukin-6 transsignaling
pathway promotes immunosuppressive myeloid-derived suppressor cells
via suppression of suppressor of cytokine signaling 3 in breast
cancer. Front Immunol. 8:18402017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun Z, Yao Z, Liu S, Tang H and Yan X: An
oligonucleotide decoy for stat3 activates the immune response of
macrophages to breast cancer. Immunobiology. 211:199–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jones LM, Broz ML, Ranger JJ, Ozcelik J,
Ahn R, Zuo D, Ursini-Siegel J, Hallett MT, Krummel M and Muller WJ:
STAT3 establishes an immunosuppressive microenvironment during the
early stages of breast carcinogenesis to promote tumor growth and
metastasis. Cancer Res. 76:1416–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Iliopoulos D, Hirsch HA and Struhl K: An
epigenetic switch involving NF-KappaB, Lin28, Let-7 MicroRNA, and
IL6 links Inflammation to cell transformation. Cell. 139:693–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Barbieri I, Pensa S, Pannellini T,
Quaglino E, Maritano D, Demaria M, Voster A, Turkson J, Cavallo F,
Watson CJ, et al: Constitutively active stat3 enhances neu-mediated
migration and metastasis in mammary tumors via upregulation of
Cten. Cancer Res. 70:2558–2567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese J and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen RY, Yen CJ, Liu YW, Guo CG, Weng CY,
Lai CH, Wang JM, Lin YJ and Hung LY: CPAP promotes angiogenesis and
metastasis by enhancing STAT3 activity. Cell Death Differ.
27:1259–1273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Khatib A, Solaimuthu B, Ben Yosef M, Abu
Rmaileh A, Tanna M, Oren G, Schlesinger Frisch M, Axelrod JH,
Lichtenstein M and Shaul YD: The glutathione peroxidase 8
(GPX8)/IL-6/STAT3 axis is essential in maintaining an aggressive
breast cancer phenotype. Proc Natl Acad Sci USA. 117:21420–21431.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Siersbæk R, Scabia V, Nagarajan S,
Chernukhin I, Papachristou EK, Broome R, Johnston SJ, Joosten SEP,
Green AR, Kumar S, et al: IL6/STAT3 signaling hijacks estrogen
receptor α enhancers to drive breast cancer metastasis. Cancer
Cell. 38:412–423.e9. 2020. View Article : Google Scholar
|
|
73
|
Liu JY, Zhang YC, Song LN, Zhang L, Yang
FY, Zhu XR, Cheng ZQ, Cao X and Yang JK: Nifuroxazide ameliorates
lipid and glucose metabolism in palmitate-induced HepG2 cells. RSC
Adv. 9:39394–39404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang T, Fahrmann JF, Lee H, Li YJ,
Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al:
JAK/STAT3-regulated fatty acid β-oxidation is critical for breast
cancer stem cell self-renewal and chemoresistance. Cell Metab.
27:136–150.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sarvi S, Crispin R, Lu Y, Zeng L, Hurley
TD, Houston DR, von Kriegsheim A, Chen CH, Mochly-Rosen D, Ranzani
M, et al: ALDH1 bioactivates nifuroxazide to eradicate
ALDHHigh melanoma-initiating cells. Cell Chem Biol.
25:1456–1469.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhou L, Ishizaki H, Spitzer M, Taylor KL,
Temperley ND, Johnson SL, Brear P, Gautier P, Zeng Z, Mitchell A,
et al: ALDH2 mediates 5-nitrofuran activity in multiple species.
Cell Chem Biol. 27:14522020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ismail IH and Hendzel MJ: The gamma-H2A.X:
Is it just a surrogate marker of double-strand breaks or much more?
Environ Mol Mutagen. 49:73–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sizemore GM, Pitarresi JR, Balakrishnan S
and Ostrowski MC: The ETS family of oncogenic transcription
factorsin solid tumors. Nat Rev Cancer. 17:337–351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Verger A, Buisine E, Carrere S, Wintjens
R, Flourens A, Coll J, Stéhelin D and Duterque-Coquillaud M:
Identification of amino acid residues in the ETS transcription
factor Erg that mediate Erg-Jun/Fos-DNA ternary complex formation.
J Biol Chem. 276:17181–17189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bassuk AG, Anandappa RT and Leiden JM:
Physical interactions between Ets and NF-kappaB/NFAT proteins play
an important role in their cooperative activation of the human
immunodeficiency virus enhancer in T cells. J Virol. 71:3563–3573.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li C, Zhang J, Wu Q, Kumar A, Pan G and
Kelvin DJ: Nifuroxazide activates the parthanatos to overcome
TMPRSS2: ERG fusion-positive prostate cancer. Mol Cancer Ther.
22:306–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cobrinik D: Pocket proteins and cell cycle
control. Oncogene. 24:2796–2809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kron KJ, Murison A, Zhou S, Huang V,
Yamaguchi TN, Shiah YJ, Fraser M, van der Kwast T, Boutros PC,
Bristow RG and Lupien M: TMPRSS2-ERG fusion co-opts master
transcription factors and activates NOTCH signaling in primary
prostate cancer. Nat Genet. 49:1336–1345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Y, An R, Umanah GK, Park H, Nambiar
K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al: A nuclease
that mediates cell death induced by DNA damage and poly(ADP-ribose)
polymerase-1. Science. 354:aad68722016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tak J, Nguyen TK, Lee K, Kim SG and Ahn
HC: Utilizing machine learning to identify nifuroxazide as an
inhibitor of ubiquitin-specific protease 21 in a drug repositioning
strategy. Biomed Pharmacother. 174:1164592024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Harrigan JA, Jacq X, Martin NM and Jackson
SP: Deubiquitylating enzymes and drug discovery: Emerging
opportunities. Nat Rev Drug Discov. 17:57–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kanan D, Kanan T, Dogan B, Orhan MD, Avsar
T and Durdagi S: An integrated in silico approach and in vitro
study for the discovery of small-molecule USP7 inhibitors as
potential cancer therapies. ChemMedChem. 16:555–567. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Saito Y, Kishimoto M, Yoshizawa Y and
Kawaii S: Synthesis and structure-activity relationship studies of
furan-ring fused chalcones as antiproliferative agents. Anticancer
Res. 35:811–817. 2015.PubMed/NCBI
|
|
90
|
Al Koussa HK, Abrahamian CF, Elzahhar PM,
Serie MA, Belal A and El-Yazbi AF: A novel series of nitrofuran
derivatives produces an anti-tumor effect via a p53-dependent
mechanism. FASEB J. 34:12020. View Article : Google Scholar
|
|
91
|
Ashraf Z, Mahmood T, Hassan M, Afzal S,
Rafique H, Afzal K and Latip J: Dexibuprofen amide derivatives as
potential anticancer agents: Synthesis, in silico docking,
bioevaluation, and molecular dynamic simulation. Drug Des Devel
Ther. 13:1643–1657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Guo Q, Shi D, Lin L, Li H, Wei Y, Li B and
Wu D: De-ubiquitinating enzymes USP21 regulate MAPK1 expression by
binding to transcription factor GATA3 to regulate tumor growth and
cell stemness of gastric cancer. Front Cell Dev Biol. 9:6419812021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang Q, Chen Z, Tang Q, Wang Z, Lu J, You
Y and Wang H: USP21 promotes self-renewal and tumorigenicity of
mesenchymal glioblastoma stem cells by deubiquitinating and
stabilizing FOXD1. Cell Death Dis. 13:7122022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ali SH, Osmaniye D, Sağlık BN, Levent S,
Özkay Y and Kaplancıklı ZA: Design, synthesis, and molecular
docking studies of novel quinoxaline derivatives as anticancer
agents. Chem Biol Drug Des. 102:303–315. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hou P, Ma X, Zhang Q, Wu CJ, Liao W, Li J,
Wang H, Zhao J, Zhou X, Guan C, et al: USP21 deubiquitinase
promotes pancreas cancer cell stemness via Wnt pathway activation.
Genes Dev. 33:1361–1366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu X, Yao Y, Ding H, Han C, Chen Y, Zhang
Y, Wang C, Zhang X, Zhang Y, Zhai Y, et al: USP21 deubiquitylates
Nanog to regulate protein stability and stem cell pluripotency.
Signal Transduct Target Ther. 1:160242016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen Y, Zhou B and Chen D: USP21 promotes
cell proliferation and metastasis through suppressing EZH2
ubiquitination in bladder carcinoma. Onco Targets Ther. 10:681–689.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xu P, Xiao H, Yang Q, Hu R, Jiang L, Bi R,
Jiang X, Wang L, Mei J, Ding F and Huang J: The USP21/YY1/SNHG16
axis contributes to tumor proliferation, migration, and invasion of
non-small-cell lung cancer. Exp Mol Med. 52:41–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li W, Cui K, Prochownik EV and Li Y: The
deubiquitinase USP21 stabilizes MEK2 to promote tumor growth. Cell
Death Dis. 9:4822018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hassanein EHM, Abdel-Reheim MA, Althagafy
HS, Hemeda MS, Gad RA and Abdel-Sattar AR: Nifuroxazide attenuates
indomethacin-induced renal injury by upregulating Nrf2/HO-1 and
cytoglobin and suppressing NADPH-oxidase, NF-κB, and JAK-1/STAT3
signals. Naunyn Schmiedebergs Arch Pharmacol. 397:3985–3994. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mcintosh MT, Koganti S, Boatwright JL, Li
X, Spadaro SV, Brantly AC, Ayers JB, Perez RD, Burton EM, Burgula
S, et al: STAT3 imparts BRCAness by impairing homologous
recombination repair in Epstein-Barr virus-transformed B
lymphocytes. PLoS Pathog. 16:e10088492020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ettner NM, Vijayaraghavan S, Durak MG, Bui
T, Kohansal M, Ha MJ, Liu B, Rao X, Wang J, Yi M, et al: Combined
inhibition of STAT3 and DNA repair in palbociclib-resistant
ER-positive breast cancer. Clin Cancer Res. 25:3996–4013. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang Y, Liu W, Liu M, Wang H, Zhou L, Chen
J, Sun H, Wei X, Fan M, Yang M, et al: Nifuroxazide in combination
with CpG ODN exerts greater efficacy against hepatocellular
carcinoma. Int Immunopharmacol. 108:1089112022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Misra SK, Wu Z, Ostadhossein F, Ye M,
Boateng K, Schulten K, Tajkhorshid E and Pan D: Pro-nifuroxazide
self-assembly leads to triggerable nanomedicine for anti-cancer
therapy. ACS Appl Mater Interfaces. 11:18074–18089. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Luo L, Xu F, Peng H, Luo Y, Tian X,
Battaglia G, Zhang H, Gong Q, Gu Z and Luo K: Stimuli-responsive
polymeric prodrug-based nanomedicine delivering nifuroxazide and
doxorubicin against primary breast cancer and pulmonary metastasis.
J Control Release. 318:124–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhao T, Feng Y, Guo M, Zhang C, Wu Q, Chen
J, Guo S, Liu S, Zhou Q, Wang Z, et al: Combination of attenuated
Salmonella carrying PD-1 siRNA with nifuroxazide for colon cancer
therapy. J Cell Biochem. 121:1973–1985. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wong ALA, Hirpara JL, Pervaiz S, Eu JQ,
Sethi G and Goh BC: Do STAT3 inhibitors have potential in the
future for cancer therapy? Expert Opin Investig Drugs. 26:883–887.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shindano A, Marot L and Geubel AP:
Nifuroxazide-induced acute pancreatitis: A new side-effect for an
old drug? Acta Gastroenterol Belg. 70:32–33. 2007.PubMed/NCBI
|
|
109
|
Quillardet P, Arrault X, Michel V and
Touati E: Organ-targeted mutagenicity of nitrofurantoin in Big Blue
transgenic mice. Mutagenesis. 21:305–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mazzaccara C, Labruna G, Cito G, Scarfò M,
De Felice M, Pastore L and Sacchetti L: Age-related reference
intervals of the main biochemical and hematological parameters in
C57BL/6J, 129SV/EV and C3H/HeJ mouse strains. PLoS One.
3:e37722008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cipolla BG, Havouis R and Moulinoux JP:
Polyamine contents in current foods: A basis for polyamine reduced
diet and a study of its long term observance and tolerance in
prostate carcinoma patients. Amino Acids. 33:203–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|