Introduction
The incidence of thyroid cancer has increased
worldwide over the past several decades. In 2020, the
age-standardized incidence rate of thyroid cancer was 10–1 per
100,000 women and 3–1 per 100,000 men, and the age-standardized
mortality rate was 0–5 per 100,000 women and 0–3 per 100,000 men
(1). Advancements in the accuracy
of ultrasound equipment and the widespread use of fine-needle
aspiration cytology have led to the increased detection of
early-stage cancers. Despite this trend, the mortality rate for
thyroid cancer has remained stable. A global assessment of thyroid
cancer in 2020 showed a rise in incidence rates across many
countries, whereas the mortality rate remained low (1,2). Some
reports attribute this phenomenon to overdiagnosis (3). The early detection of papillary
thyroid carcinoma (PTC) could benefit patients considered for
surgery by correctly identifying tumor grade, for instance, if it
were feasible to identify an indicator of malignancy in
differentiated thyroid cancer, such as the expression of cancer
driver genes or the impact of late-stage genetic mutations prior to
treatment. If patients are willing to accept a diagnosis of PTC
with slow disease progression, the need for aggressive therapeutic
intervention may be eliminated.
In the field of cervical cancer, persistent
infection with the human papillomavirus (HPV) is a driver of
cervical carcinogenesis, with the HPV type playing a key role in
determining the grade and prognosis of cervical lesions (4). Therefore, genetic testing for HPV is
recommended for all patients with cervical cancer (5). In thyroid cancer, various genetic
mutations have been implicated in carcinogenesis and described in
the fifth edition of the World Health Organization (WHO)
classification (6). As the
preoperative diagnosis of thyroid cancer is based on cytology,
determining the nature of the tumor by genetic testing on
cytological specimens is in the best interest of the patient.
PTC is pathologically diagnosed based on cytological
nuclear findings. Advances in ultrasound-guided puncture aspiration
cytology have led to the early detection and increased treatment of
the disease. In Japan, PTC is defined as low-risk thyroid carcinoma
without lymph node or distant metastasis, invasion of the recurrent
nerve or trachea, or high-grade histological findings (tall cell
variant or poorly differentiated components). To minimize
overdiagnosis, various studies recommend the treatment of PTC <1
cm as micropapillary carcinoma without the need for surgery
(7,8). Although PTC has a better prognosis
than other types of cancer, 5–10% of PTC have a high proliferative
tendency and recur after surgery, with some cases being difficult
to treat and resulting in death (9).
Various genetic abnormalities are involved in the
development of thyroid tumors, which are expected to be clinically
applicable. These include mutually exclusive driver mutations of
PTC, such as the BRAFV600E mutation and
RET/PTC translocation. Theoretically, the activation of
telomerase reverse transcriptase (TERT), which elongates
telomere DNA, is believed to be involved in the infinite
proliferative capacity of various tumors. Recently, mutations in
the human TERT promoter (TERTp) have been reported as
attractive prognostic factors for thyroid carcinoma. Point
mutations of TERTp in thyroid carcinoma were first reported
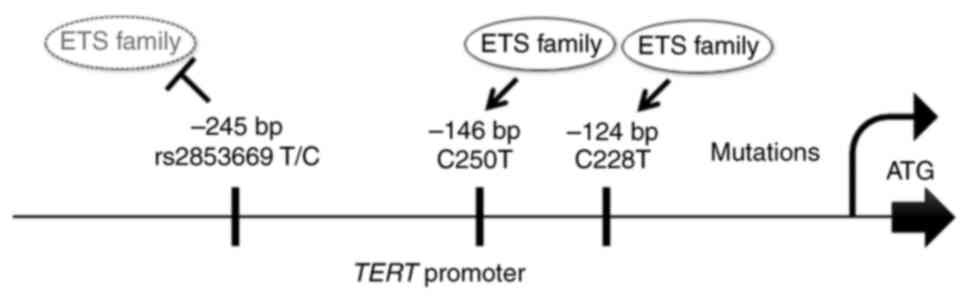
in 2013, with −124 C >T (Chr5:1295228; C228T) and −146 C >T
(Chr5:1295250; C250T) mutations located 124 and 146 bp upstream of
the translational initiation site, respectively (10,11)
(Fig. 1). A few studies have
demonstrated that these two mutations are molecular markers
associated with thyroid tumor grade. These mutations are strongly
correlated with high-risk clinicopathologic features, including
age, tumor size, extraglandular invasion and distant metastasis.
The frequencies of mutations in PTC, follicular thyroid carcinoma,
and follicular adenoma are 5–24%, 10–35%, and 2–8%, respectively
(12,13). The fifth edition of the WHO
classification, published in 2023 describes C228T and C250T as late
mutations in thyroid carcinoma that are added to early mutations in
driver genes, such as BRAF and NRAS (6). Additionally, TERT mutations are
synergistic with the BRAFV600E mutation. The
coexistence of TERTp point and BRAFV600E
mutations worsens the prognosis of PTC (14–19).
A recent study revealed that mutations in the core
TERTp create a new binding site for the E-twenty-six
transcription factor, which is believed to be the cancer-specific
TERTp reactivation mechanism (20). Two-step mechanisms have been
proposed for the involvement of TERTp mutations in
tumorigenesis based on in vitro experiments. These
experiments suggest that TERTp mutations contribute to
tumorigenesis through two steps: immortalization and promotion of
genomic instability. TERTp mutations elevate telomerase
activity but do not prevent the wear and tear of telomers, and
instead repair short telomeres to extend cell life. When telomeres
are critically short and increase in number, the genome becomes
unstable, prompting further upregulation in TERT expression
to maintain cell proliferation (21).
A regulatory single nucleotide polymorphism (SNP) of
the TERTp, C allele of rs2853669 (Chr5:1295234), −245 T>C
(TrSNP), manifests an allelic change from thymine (T) to cytosine
(C). It has been identified in the proximity of the two
TERTp mutations.
The C allele of rs2853669 (TrSNP) is not an acquired
mutation, such as C228T or C250T; however, it is a familial
pleomorphism in the germ cell lineage. It is present in all cells
of the body; therefore, it can be easily detected preoperatively
using a blood sample. TrSNP alters the expression levels of
wild-type and mutant (C228T and C250T) TERT in vitro,
potentially impacting the cancer phenotype (22). TrSNP is associated with malignancy
in glioblastoma, lung carcinoma, acute leukemia, and hepatocellular
carcinoma (22–28). Moreover, the homozygous TrSNP (CC)
genotype affects the prognosis of neuroglioma. Notably, the overall
survival periods of patients who additionally carry the C228T or
C250T mutations are markedly short. Notably, the homozygous TrSNP
genotype was significantly higher (41%) in South Indian women with
cervical cancer (29). In a further
report on 144 cases of oral squamous cell carcinoma, it has been
demonstrated that the C228T mutation and the TrSNP wild-type TrSNP
genotype are independent prognostic biomarkers (30).
Our previous study using cultured thyroid cells
demonstrated that TrSNP and C228T mutations enhance the
transcriptional activity of TERTp in thyroid tumors.
Specifically, our TERTp luciferase assay revealed that TrSNP
increased the promoter activity, with the coexistence of TrSNP and
C228T further enhancing TERTp activity (31). In our previous study, we also showed
that TrSNP was associated with tumor size in PTC and follicular
neoplasm surgical specimens. In particular, thyroid tumors with the
C228T mutation and TrSNP were found to be larger in size compared
to tumors with either variant alone (32,33).
The C allele of the TrSNP of TERTp showed a statistically
significant correlation with thyroid tumor size, similar to C228T,
indicating that it influences thyroid tumor growth via regulating
the expression of TERT. However, no statistical association
with tumor grade, such as invasion or lymph node metastasis, was
observed, probably owing to the small number of cases included in
the previous study. Notably, these analyses were performed using
surgical specimens and not blood samples, which are easily obtained
clinically. Additionally, the small number of cases hindered
comprehensive analysis of the detailed association between TrSNP
and clinical outcomes or the effect of the coexistence of the C
allele of TrSNP with TERTp point mutations on tumor
progression or exacerbation.
The BRAFV600E mutation, a known
driver gene for papillary thyroid carcinoma, is a common mutation
in the MAPK pathway that results in uncontrolled cell growth and
proliferation. According to the AACR's Precision Medicine GENIE
tumor prognostic database (version 13.0), the most common
concomitant BRAF V600E mutations and the most frequent
concomitant mutations were TERT and TP53 (34). The objective of this study was to
test the hypothesis that the coexistence of BRAF mutations
in driver genes upstream of the MAPK pathway and late mutations
unrelated to signal transduction, such as point mutations in the
TERT p, would result in increased tumor virulence. In this
study, the allelic genotype of TERTp rs2853669 was analyzed
in detail in patient blood samples with the aim of evaluating the
potential utility of TERTp rs2853669 in the preoperative
evaluation of PTC. The objective of this study is to elucidate
whether point mutations and single nucleotide polymorphisms (SNPs)
within TERTp can serve as molecular markers to determine
preoperative indicators of PTC malignancy.
Materials and methods
Patients
We selected 144 patients with PTC who had undergone
surgery at Kyorin University Hospital between January 2014 and
November 2021 and who provided written consent to participate in
the study. Blood samples were used for TrSNP analysis; therefore,
we excluded patients whose blood samples could not be collected
owing to the difficulty of visiting the hospital postoperatively or
resulted in inadequate DNA extraction owing to demineralization
during sample preparation. The final number of examined patients
was 133.
Patient backgrounds are presented in Table I. The men/women ratio was 35/98,
with a median age of 54 (18–90)
years. The patients were categorized by clinicopathological factors
according to the Japanese thyroid cancer handling, and their
distributions were statistically evaluated (35). The mean tumor diameter was 21.1
(2–115) mm.
 | Table I.Characteristics of patients with
papillary thyroid carcinoma (n=133). |
Table I.
Characteristics of patients with
papillary thyroid carcinoma (n=133).
| Clinicopathological
features | Value |
|---|
| Sex, n (%) |
|
|
Male | 35 (26.3) |
|
Female | 98 (73.7) |
| Age, years |
|
|
Median | 54 |
|
Range | 18-90 |
| Tumor size, mm |
|
| Mean ±
SD | 21.86±17.38 |
|
Range | 2-115 |
| Age, n (%) |
|
| <55
years | 66 (49.6) |
| ≥55
years | 67 (50.4) |
| Size, n (%) |
|
| <2.0
cm | 78 (58.6) |
| ≥2.0
cm | 55 (41.4) |
| <4.0
cm | 117 (88.0) |
| ≥4.0
cm | 16 (12.0) |
| Pathological T
factor, n (%) |
|
|
≤pT2 | 81 (61.0) |
|
≥pT3 | 52 (39.1) |
| Lymph node
metastasis, n (%) |
|
| 0 | 79 (59.4) |
| ≥1 | 54 (40.6) |
| Intrathyroidal
spread, n (%) |
|
|
Presence | 60 (45.1) |
|
Absence | 73 (54.9) |
| Extraglandular
invasion n (%) |
|
|
Presence | 70 (52.6) |
|
Absence | 63 (47.4) |
Methods
To examine the C allele of rs2853669 and C228T and
C250T mutations in TERTp, we extracted DNA from
formalin-fixed paraffin-embedded (FFPE) surgical tumor tissue
specimens (5–10 slices of 5 µm thickness) and blood samples (1 cc)
collected in blood collection tubes (PAXgeneR,
PreAnalytiX, Hombrechtikon, Switzerland). Following
deparaffinization with xylene and alcohol and protease treatment,
DNA was extracted from FFPE samples using the QIAamp DNA FFPE
Tissue Kit (Qiagen) with the spin column method. DNA extraction
from whole blood samples was performed using the QIAamp DNA Mini
Kit (Qiagen). After quantifying the amount of extracted DNA using
NanoDrop (Thermo Fisher Scientific, Wilmington, DE, USA), 200 ng
DNA was amplified using Taq polymerase (GoTaq Green Master Mix,
Promega Corporation, Madison, WI, USA) under the following PCR
(36) cycling conditions: 95°C for
10 min, followed by 40 cycles of 95°C for 30 sec, 68°C for 30 sec,
and 72°C for 30 sec, with a final extension at 72°C for 4 min
(31). DNA extracted from blood
samples was also subjected to PCR to confirm that C228T, C250T, and
BRAFV600E were somatic mutations. Following
confirmation of specific amplification using gel agarose
electrophoresis, the PCR products were purified using the FastGene
Gel/PCR Extraction Kit (FastGene, Tokyo, Japan). Finally, the
nucleotide sequence of the PCR products was determined using Sanger
sequencing analysis (Macrogen, Tokyo, Japan) with reverse primers.
The primers used are listed in Table
II.
 | Table II.Primers used in the present
study. |
Table II.
Primers used in the present
study.
| Target | Primer sequences
(5′-3′) | Ta, °C | Amplicon, bp |
|---|
| TERT
promoter | F:
ACGCCCAGGACCGCGCT | 68 | 236 |
|
| R:
CCCACGTGCGCAGCAGG |
|
|
| BRAF | F:
GCTTGCTCTGATAGGAAAATGAG | 58 | 237 |
|
| R:
GTAACTCAGCAGCATCTCAGG |
|
|
Statistical analysis
The cases in which the height of peaks in the
sequence waveform reached 25% of the normal peak were considered
point-mutation or heterozygous TrSNP variants. For the C allele of
rs2853669, cases with 100% overlapping peaks were considered
homozygous variants. Sanger sequencing images are shown in Fig. S1. The results were categorized by
clinicopathological parameters, including sex, age, tumor diameter
(size), pT classification, lymph node metastasis, extraglandular
infiltration, and intrathyroidal spread (as per the WHO and
American Joint Committee on Cancer guidelines), and statistically
analyzed. Tumors located at a distance of at least 5 mm from the
primary tumor were defined as intrathyroidal spread. In the
comparative analysis of the abnormality of each gene and
clinicopathological parameters, the Mann-Whitney test was used for
continuous data (age and tumor diameter), whereas the χ2
and Fisher's exact tests were used for categorical data. The
absorbance ratios A260/A280 and A260/A230 (a measure of DNA purity)
of blood and FFPE samples obtained from the same patients were
compared using the paired sample t-test. Furthermore, multiple
comparisons of combinations of genetic mutations were performed for
each group using the Fisher's exact test followed by Bonferroni's
post hoc test and the Kruskal-Wallis test followed by Bonferroni's
post hoc test. All analyses were performed using IBM SPSS software
ver. 28 (IBM, Chicago, IL, USA). Statistical significance was set
at P≤0.05.
Results
Concordance between FFPE and blood
samples
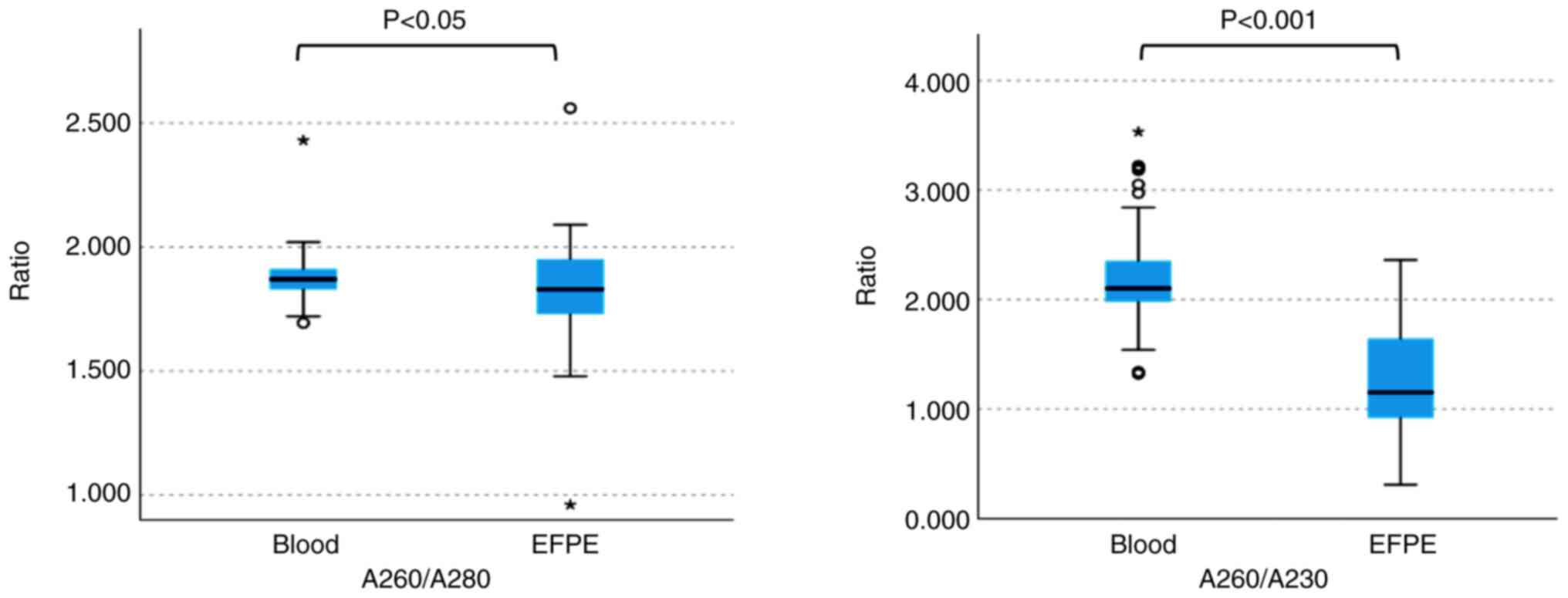
In a quality assurance, the concordance rate between
FFPE and blood samples for TrSNP polymorphisms was an impressive
97.7%. In order to evaluate DNA purity, we compared the A260/A280
and A260/A230 absorbance ratios of samples. The average A260/A280
ratio was 1.88±0.08 for blood samples and 1.82±0.18 for FFPE
samples, whereas the A260/A230 ratio was 2.17±0.39 for blood
samples and 1.27±0.49 for FFPE samples. Hence, blood samples
demonstrated better stability and less variability than FFPE
samples (P<0.05, P<0.001) (Fig.
2).
Genetic analysis of TrSNP (C allele of
rs2853669) in PTC patients
Table III shows
the results of the genetic analysis of the C allele of rs2853669
(TrSNP), TERTp point mutations C228T and C250T, and
BRAFV600E in 133 cases of PTC and their
relationship with clinicopathological parameters. TrSNP was
detected in 70 (52.6%) out of 133 patients. We found that 51 women
(52.0%) and 19 men (54.3%) carried TrSNP. Among them, 31 (47.0%)
patients with PTC were aged ≤55 years, whereas 39 (58.2%) were ≥55
years of age. We did not detect any significant difference in the
presence or absence of TrSNP by sex or age.
 | Table III.Clinicopathological parameters and
frequency of TrSNP, C228T, C250T and BRAFV600E in 133
patients with papillary thyroid carcinoma. |
Table III.
Clinicopathological parameters and
frequency of TrSNP, C228T, C250T and BRAFV600E in 133
patients with papillary thyroid carcinoma.
|
| TERT promoter |
|---|
|
|
|
|---|
| Parameters | TrSNP, % (n) | C228T, % (n) | C250T, % (n) |
BRAFV600E, % (n) |
|---|
| Positive cases
(n=133) | 52.6 (70) | 17.3 (23) | 8.3 (11) | 61.7 (82) |
| Sex |
|
|
|
|
| Female
(n=98) | 52.0 (51) | 17.3 (17) | 9.2 (9) | 60.2 (59) |
| Male
(n=35) | 54.3 (19) | 17.1 (6) | 5.7 (2) | 65.7 (23) |
| Age, years |
|
|
|
|
| <55
(n=66) | 47.0 (31) | 13.6 (9) | 13.6 (9)d | 65.2 (43) |
| ≥55
(n=67) | 58.2 (39) | 20.9 (14) | 3.0 (2)d | 58.2 (39) |
| Size, cm |
|
|
|
|
| ≤2.0
(n=78) | 44.9 (35) | 11.5 (9)a | 6.4 (5) | 64.1 (50) |
| >2.0
(n=55) | 63.6 (35) | 25.5 (14)a | 10.9 (6) | 58.2 (32) |
| ≤4.0
(n=117) | 51.3 (60) | 14.5 (17)b | 7.7 (9) | 60.7 (71) |
| >4.0
(n=16) | 62.5 (10) | 37.5 (6)b | 1.3 (2) | 68.8 (11) |
| Pathological T
factor |
|
|
|
|
| ≤pT2
(n=81) | 49.4 (40) | 12.3 (10) | 7.4 (6) | 55.6 (45) |
| ≥pT3
(n=52) | 57.7 (30) | 25.0 (13) | 9.6 (5) | 71.2 (37) |
| Lymph node
metastasis |
|
|
|
|
| 0
(n=79) | 46.8 (37) | 12.7 (10) | 3.8 (3)e | 59.5 (47) |
| ≥1
(n=54) | 61.1 (33) | 24.1 (13) | 14.8 (8)e | 64.8 (35) |
| Extraglandular
invasion |
|
|
|
|
| 0
(n=70) | 45.7 (32) | 8.6 (6)c | 7.1 (5) | 58.6 (41) |
| ≥1
(n=63) | 60.3 (38) | 27.0 (17)c | 9.5 (6) | 65.1 (41) |
| Intrathyroidal
spread |
|
|
|
|
|
Presence (n=60) | 55.0 (33) | 21.7 (13) | 13.3 (8) | 65.0 (39) |
| Absence
(n=73) | 50.7 (37) | 13.7 (10) | 4.1 (3) | 58.9 (43) |
Clinicopathological analysis showed a significant
association between TrSNP and PTC size (P<0.05), with larger
tumor diameters observed in patients with TrSNP. When categorized
by tumor diameter, 35 (44.9%) of the 78 cases of PTC tumors <2.0
cm and 35 (63.6%) of the 55 cases of tumors >2.0 cm had TrSNP,
indicating a significant association of TrSNP with PTC tumors
>2.0 cm (P<0.05). However, when we used a cutoff of 4.0 cm,
we did not observe any significant difference between the
occurrence of TrSNP and PTC tumor size.
Interestingly, we did not detect any significant
association between the presence or absence of TrSNP and other
clinicopathological parameters, which was consistent with the
results of previous studies.
Genotype distribution and tumor
size
We detected the homozygous genotype (CC) of TrSNP in
14 cases (10.5%), the heterozygous genotype (CT) in 56 cases
(42.1%), and the wild type (TT) in 63 cases (47.4%). The frequency
of the C allele was 31.6% (84/266 genes), with the minor allele
frequency (MAF) being 0.316. The goodness-of-fit test verified that
the P-value of the Hardy-Weinberg equilibrium was 0.95.
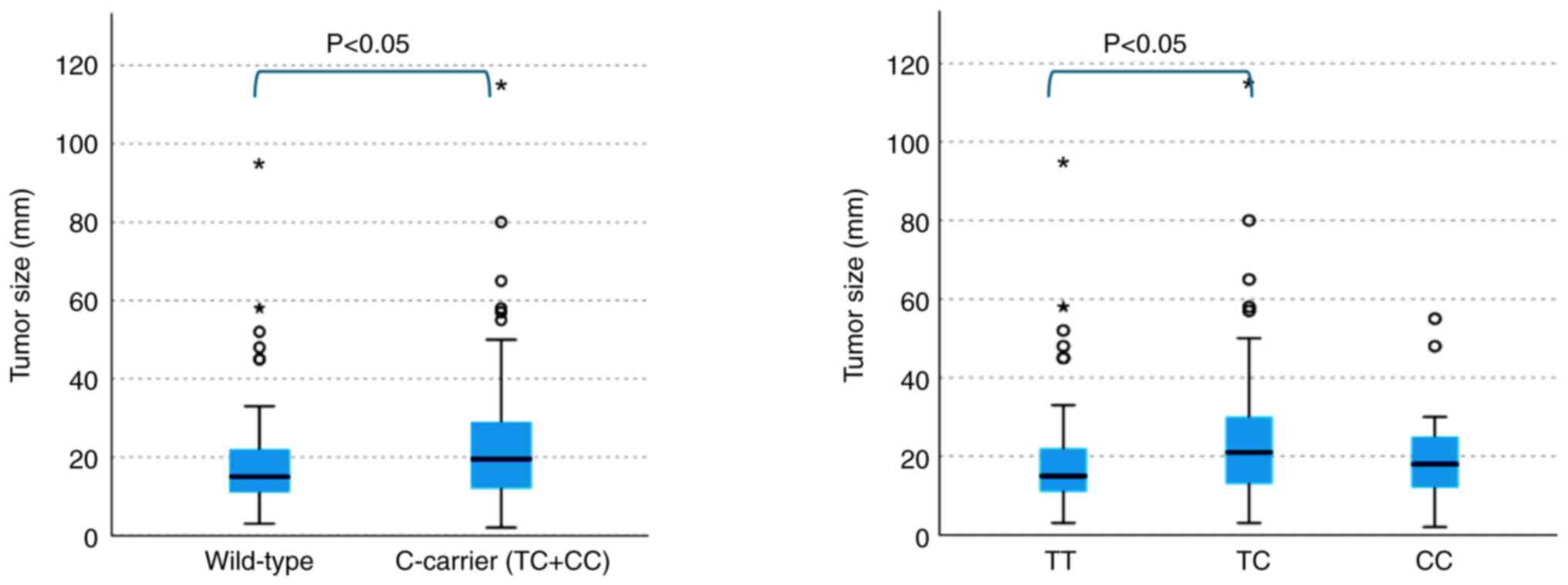
The tumor diameter was significantly greater in
cases with the C allele than in those carrying the wild type
(P<0.05). The tumor size was significantly larger in the
heterozygous group than in the wild-type group (P<0.05)
(Fig. 3). However, no significant
difference was detected in tumor diameter between the wild-type and
homozygous group.
Clinicopathological implications of
the TERT promoter mutations (C228T and C250T)
We found TERTp mutations in 29 of 133
patients (21.8%): more specifically, 23 (17.3%) and 11 (8.3%) had
C228T and C250T point mutations, respectively, whereas five (3.8%)
had both. However, no somatic C228T or C250T mutations were
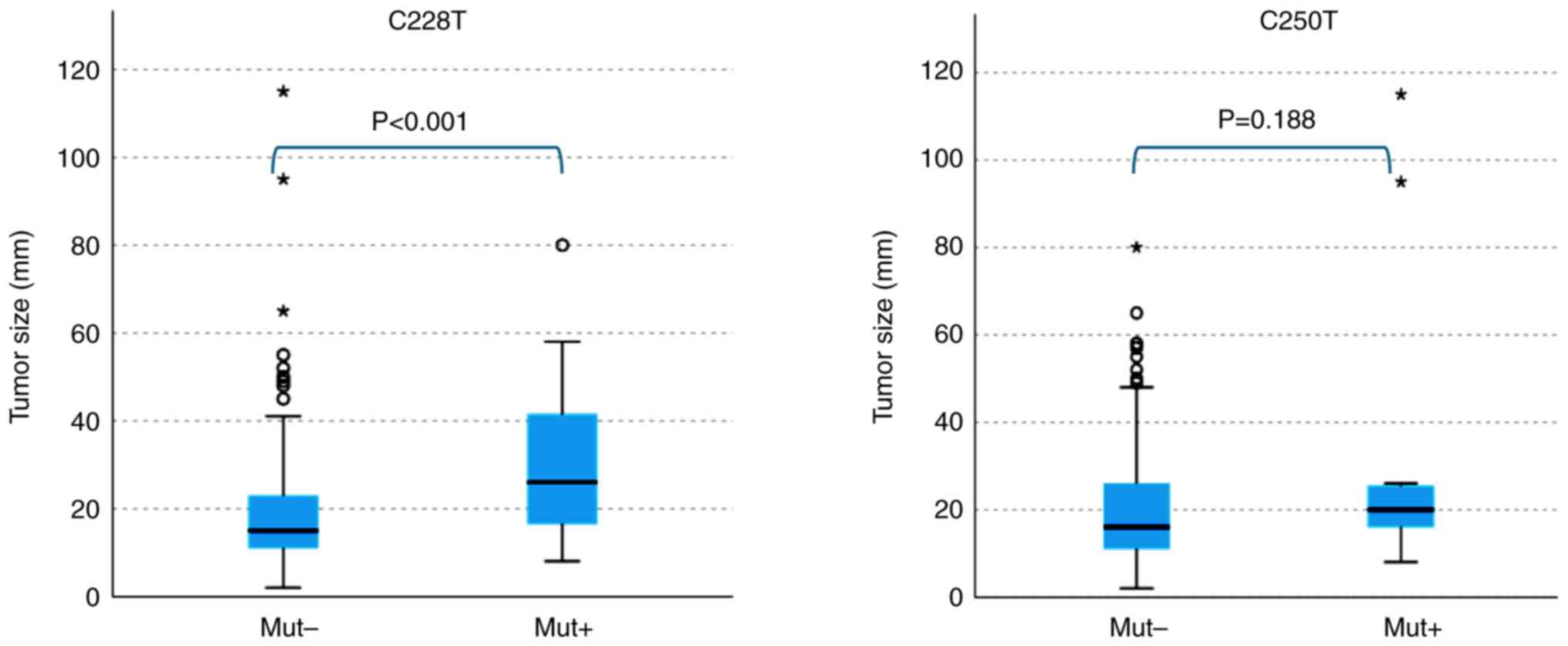
detected in the blood samples. In addition, the C228T mutation was
significantly correlated with tumor size (mm) (P<0.001),
indicating a higher detection rate of C228T in larger tumors,
whereas no correlation was observed between the C250T mutation and
tumor size (Fig. 4).
In categorical analysis, we detected the C228T
mutation in 9 (11.5%) of 78 PTC tumors measuring ≤2.0 cm and in 14
(25.5%) of 55 tumors measuring >2.0 cm, indicating that C228T
significantly correlated (P<0.05) with PTC tumors >2.0 cm in
size. When a cutoff of 4.0 cm was used, the C228T mutation was
detected in 17 (14.5%) of 117 patients with tumors ≤4.0 cm and in 6
(37.5%) of 16 patients with tumors >4.0 cm, further confirming
the significant correlation of C228T with PTC tumor size
(P<0.05). Regarding other clinicopathological parameters, we
found the C228T mutation in 17 (27.0%) of 63 patients with
extraglandular invasion and in 6 (8.6%) of 70 patients without
invasion, indicating a significant association of C228T with the
presence of extraglandular invasion (P<0.05). Additionally, 13
of the 54 patients (24.1%) with lymph node metastasis and 10 of the
79 patients (12.7%) without metastasis had the C228T mutation;
however, the difference was not statistically significant. We did
not observe any significant association of the C228T mutation with
other factors. In contrast to C228T, we observed that the C250T
mutation was not associated with tumor size; however, we noticed
that C250T was positively correlated with a younger age
(P<0.05). Categorical analysis showed that the C250T mutation
tended to be more frequent in patients younger than 55 years
(P<0.05). Regarding the other clinicopathological parameters, we
found that eight (14.8%) of 54 patients with lymph node metastasis
and three (3.8%) of 79 patients without lymph node metastasis had
the C250T mutation, indicating that C250T was significantly
associated with lymph node metastasis (P<0.05) (Table III).
Co-occurrence of TrSNP and TERT
mutations
We detected TrSNP in 56.5% (13/23) of the cases with
the C228T mutation and in 45.5% (5/11) of the cases with the C250T
mutation. In particular, three patients had TrSNP and both
mutations (C228T and C250T). Subsequently, we divided the patients
into four groups according to TrSNP and the C228T point mutation
and analyzed the relationship between TrSNP and C228T with tumor
size and grade. The group with both C228T and TrSNP had larger
tumors than those in the group with only the wild-type and TrSNP
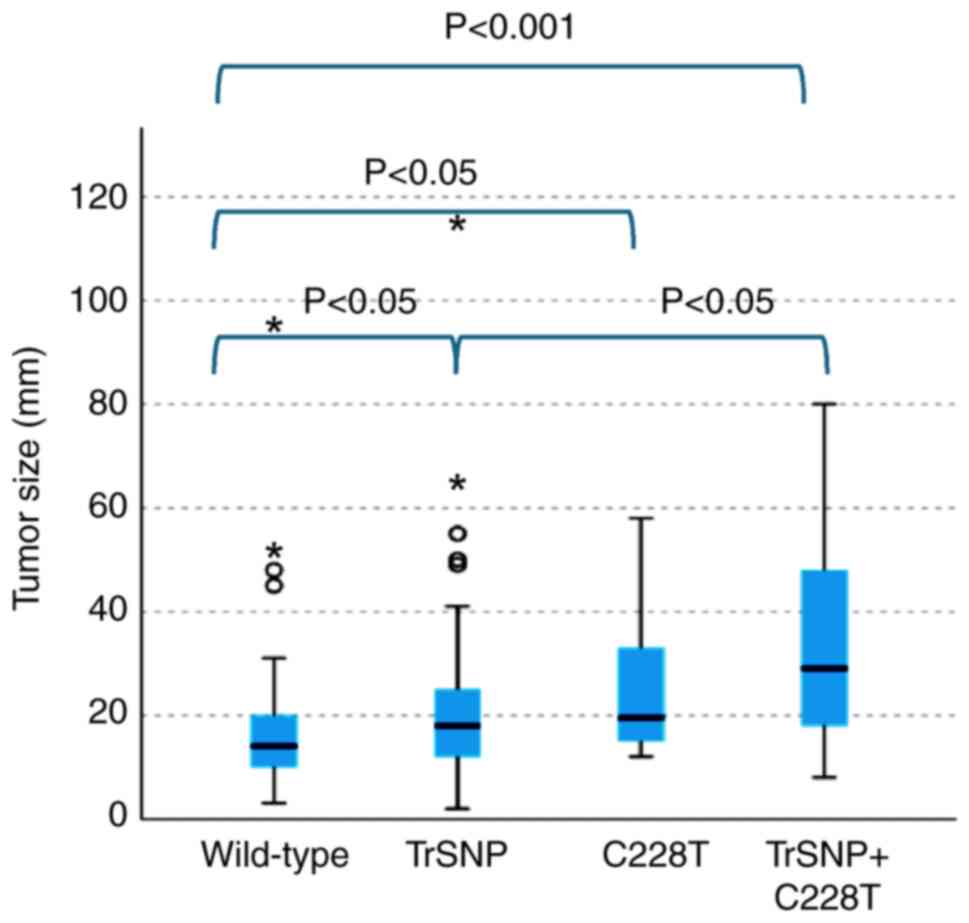
(Fig. 5).
In categorical analysis, we observed significant
associations between the variants and tumor diameter (2.0 cm, 4.0
cm), extraglandular invasion, and lymph node metastasis, with
increased numbers of higher grade and larger tumors in the group
with the C228T and TrSNP (P<0.05, P<0.05; P<0.05;
P<0.05). Additionally, the incidence of extraglandular invasion
was higher in the group with both C228T and TrSNP than in the group
with TrSNP alone (P<0.05) (Table
IV).
 | Table IV.Relationship between telomerase
reverse transcriptase: TrSNP (rs2853669) and C228T point mutation
in papillary carcinoma and tumor size based on category and the
presence of extraglandular invasion and lymph node metastasis. |
Table IV.
Relationship between telomerase
reverse transcriptase: TrSNP (rs2853669) and C228T point mutation
in papillary carcinoma and tumor size based on category and the
presence of extraglandular invasion and lymph node metastasis.
|
|
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | TrSNP/C228T | -/- | -/+ | +/- | +/+ | -/- vs. -/+ | -/- vs. +/- | -/- vs. +/+ | -/+ vs. +/- | -/+ vs. +/+ | +/- vs. +/+ |
|---|
| Tumor size | <2.0 cm, n | 29 | 4 | 33 | 0 | 0.271 | 0.780 | 0.021 | 0.835 | 0.666 | 0.340 |
|
| ≥2.0 cm, n | 5 | 2 | 6 | 6 |
|
|
|
|
|
|
|
| <4.0 cm, n | 49 | 2 | 27 | 4 | 0.228 | 0.527 | 0.029 | 0.596 | 0.646 | 0.067 |
|
| ≥4.0 cm, n | 4 | 8 | 41 | 9 |
|
|
|
|
|
|
| Extraglandular
invasion | EX0, n | 34 | 4 | 30 | 2 | 0.297 | 0.444 | 0.009 | 0.505 | 0.348 | 0.026 |
|
| EX1,2, n | 19 | 6 | 27 | 11 |
|
|
|
|
|
|
| Lymph node
metastasis | N0, n | 36 | 6 | 33 | 4 | 0.733 | 0.558 | 0.054 | 0.949 | 0.391 | 0.124 |
|
| >N1a, n | 17 | 4 | 24 | 9 |
|
|
|
|
|
|
Clinicopathological implications of
the BRAFV600E mutation
We also detected the BRAFV600E
mutation in 82 (61.7%) patients. The BRAFV600E
mutation alone was not significantly associated with any
clinicopathologic parameters. Of the 82 patients, 43 (52.4%)
carried TrSNP. Additionally, 15 (18.2%) had the C228T mutation, and
5 (6.0%) had the C250T mutation. Eight patients had TrSNP and C228T
mutations along with the BRAFV600E mutation. An
analysis of the association among the BRAFV600E
mutation, TrSNP and the C228T TERT mutation revealed no
significant difference in tumor size between the wild-type group
and the group with only the BRAFV600E mutation.
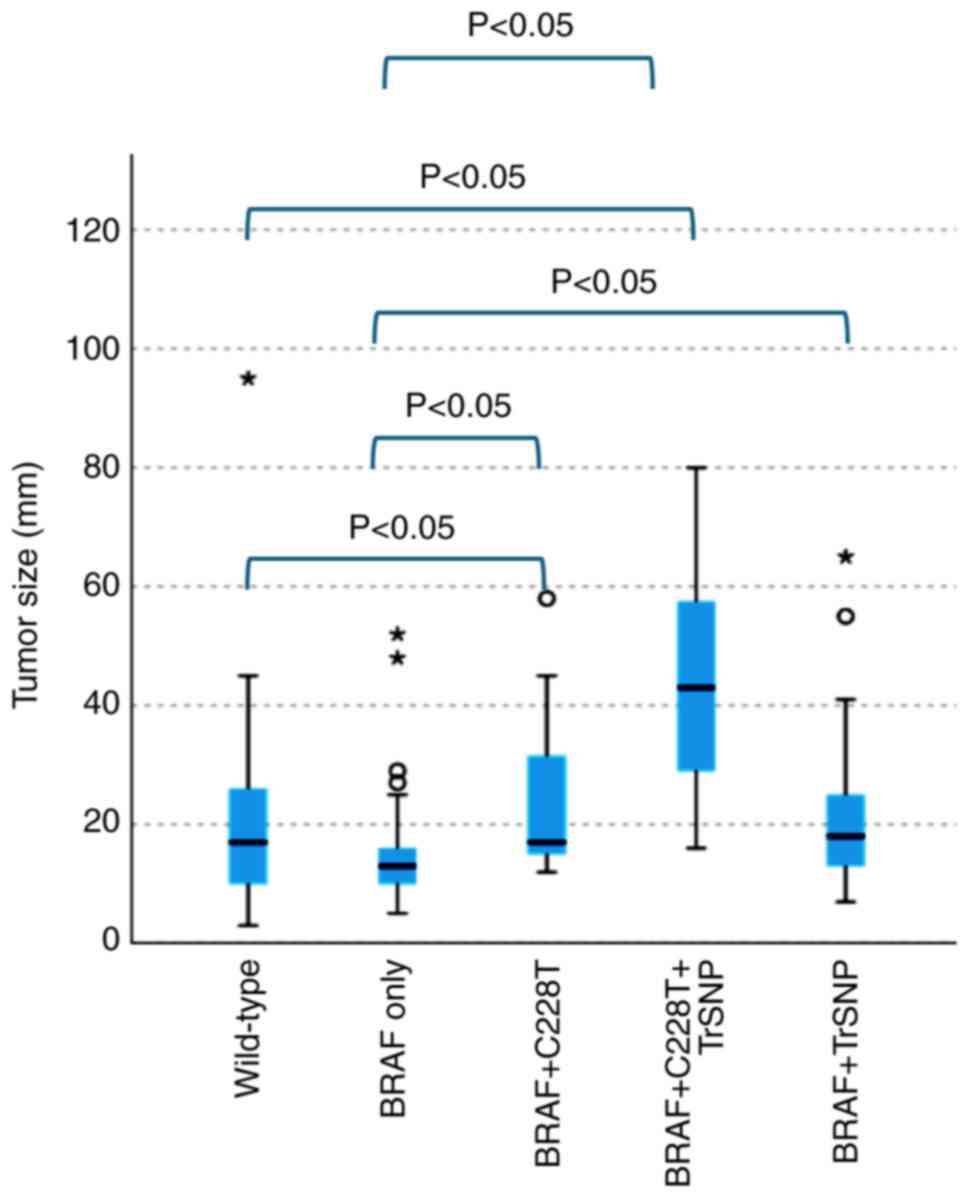
However, when the groups with wild-type and two mutations
(BRAFV600E and C228T) and the groups with
wild-type and three mutations (BRAFV600E, C228T
and TrSNP) were compared, a significant trend towards larger tumors
was observed (P<0.05; Fig. 6).
Furthermore, a notable increase in tumor size was observed in the
groups with two mutations (BRAFV600E and C228T,
and BRAFV600E and TrSNP) and the group with three
mutations (BRAFV600E, C228T and TrSNP) compared
with that in the group with only the BRAFV600E
mutation (P<0.05; Fig. 6).
Moreover, the group with three mutations
(BRAFV600E, C228T and TrSNP) exhibited
significantly increased tumor progression, including extraglandular
invasion, compared with the group with only the
BRAFV600E mutation (P<0.05; Table V).
 | Table V.Association of C228T, TrSNP and BRAF
with grade in papillary thyroid cancer. |
Table V.
Association of C228T, TrSNP and BRAF
with grade in papillary thyroid cancer.
| Variable | BRAF(+) only, n (%)
(n=33) | BRAF(+) C228T(+)
TrSNP(+), n (%) (n=8) | P-value |
|---|
| Extraglandular
invasion |
|
| 0.015 |
|
Absent | 19 (57.6) | 1 (12.5) |
|
|
Present | 14 (42.4) | 7 (87.5) |
|
| Lymph node
metastasis |
|
| 0.070 |
|
Absent | 20 (60.6) | 2 (25.0) |
|
|
Present | 13 (39.4) | 6 (75.0) |
|
Discussion
In recent years, the discovery of oncogenes has
prompted a cross-organ approach through the use of molecular
targeting therapies. Immune checkpoint inhibitors have emerged as a
pioneering approach in this field. For tumors with high PD-1 or
PD-L1 expression determined by immunostaining, immune checkpoint
inhibitors such as pembrolizumab have been used to suppress tumor
growth (37). Response to
pembrolizumab has been documented in solid tumors exhibiting high
microsatellite instability (MSI) and a high tumor mutation burden
(TMB) (38,39). However, in thyroid cancer, the
prevalence of high MSI and high TMB is approximately 2% each,
thereby restricting the potential for utilizing immune inhibitors
in treatment (40).
Thyroid cancer is associated with a small number of
actionable genetic abnormalities that have implications for
clinical decision-making. The BRAFV600E gene has
a mutation upstream of the MAPK pathway and the TERTp
mutations that are unrelated to signaling, C228T and C250T, are of
particular interest in PTC, due to their potential correlation with
tumor progression. C228T and C250T have been implicated in several
multi-organ cancers, including other thyroid carcinomas,
hepatocellular carcinoma, bladder cancer, renal pelvic cancer, and
glioblastoma. In general, cases with C228T and C250T show higher
clinicopathologic malignancy regardless of the organ. As mentioned
previously, rs2853669 functions as a regulatory SNP of the
TERTp located in the proximity of C228T and C250T. It
manifests an allelic change from thymine (T) to cytosine (C). Our
previous in vitro study demonstrated that the C allele of
rs2853669 (TrSNP) increases the activity of TERTp in thyroid
cancer cells and C228T and C250T (31). Some reports have also indicated that
TrSNP may interact with C228T and C250T (41). We attempted to detect TrSNP in a
simple and accurate blood sample for clinical application due to
its status as a germline polymorphism.
The use of blood samples has technical advantages in
the extraction of nucleic acids. It increases the accuracy of the
genetic analysis and enables preoperative assessment for
therapeutic decision-making. In the genetic analysis of blood and
FFPE samples, 2.3% of the results were discordant. A lower protein
content is indicated by an A260/280 absorbance ratio closer to
1.8–2.0. A lower A260/A230 ratio indicates contamination by organic
compounds, such as chaotropic agents that absorb light at 230
nm.
A previous study of 58 cases reported that the TrSNP
was found in 58.6% of patients and was significantly associated
with the size of PTC tumors (33).
In the present study, we examined more cases and found that TrSNP
was found in 52.6% of 133 patients with PTC and was significantly
associated with tumor size (P<0.05), similar to the findings of
previous studies. The frequency of TrSNP as a genetic polymorphism
was previously reported by Muzza et al (42) in an Italian cohort of differentiated
thyroid cancer. In the present study, TrSNP was found in 44.4% of
254 patients; a higher rate than that previously detected. This
discrepancy suggested that such polymorphisms may differ among
ethnic groups (43). Although we
did not detect any homozygous forms of TrSNP in patients with PTC
in our previous studies, in the present study, we clearly showed
that the rate of the homozygous genotype of the C allele (CC) in
PTC cases was 10.5%, that of the heterozygous genotype (CT) was
42.1%, whereas that of the wild type (TT) was 47.4%. The frequency
of the C allele was 31.6% (84/266 genes), which we believe was
slightly higher than the 29.3% reported in a previous study owing
to the increased number of cases, increased accuracy of the
analysis, and improved waveform definitions. A significant
difference in tumor size was observed between wild-type and
heterozygous samples; however, no significant correlation was
observed for homozygous samples owing to the small number of cases.
Hence, this required further evaluation in future studies using
larger sample sizes. The allele C frequency of rs2853669 in the
population of Tokyo is 24.5% (44).
A meta-analysis to determine the association between TrSNP and lung
cancer risk shows that the frequency of the wild type in healthy
Asians was 10.4%, that of heterozygous was 43.1%, and that of
homozygous 46.5%, with the C allele frequency being 32.0%
(1237/3984 genes) (22). The
authors reported that the homozygous form of TrSNP was
significantly associated with lung cancer risk (22). In the present study, the frequency
of the C allele in PTC cases was 31.6%, similar to the reported
statistics for healthy Asians. Additionally, homozygosity for TrSNP
alone did not confer a significant risk for PTC. This suggested
that TrSNP is not involved in the development of PTC. In a 2015
study, TrSNP was associated with malignancy but not with the risk
of developing glioblastoma (45).
Despite the differences in the effects of TrSNP depending on the
type of cancer, the individual effect of TrSNP on cancer
development has not been determined (23). The correlation of TrSNP with tumor
size shown in previous studies of PTC was more clearly confirmed in
the present study with a larger number of cases (P<0.05).
Furthermore, the coexistence of TrSNP with the C228T point mutation
in the TERTp was associated with tumor size and cancer
progression. The frequencies of both variants in previous studies
ranged from 4.7 to 25.5%, and the frequencies in the present study
did not differ from those previously reported (14,46,47).
Additionally, in the present study, both C228T and C250T mutations
were found in 3.8% of cases, which is a rare finding. Notably,
these were cases of patients with advanced cancer with lymph node
metastasis. This was consistent with the fact that point mutations
in TERTp (in addition to tumor size) are associated with PTC
grade, including extraglandular invasion, lymph node metastasis,
and recurrence. This study also showed that C228T was significantly
associated with tumor size and tumor grade, such as extraglandular
invasion. Additionally, C250T was associated with lymph node
metastasis, although the number of cases was small. Moreover, the
coexistence of TrSNP with the C228T mutation was more strongly
associated with cancer progression, including extraglandular
invasion and lymph node metastasis, as well as with tumor size
compared with the single presence of the C228T mutation. Overall,
TrSNP in TERTp may increase the risk of tumor growth and
progression associated with the C228T point mutation.
In this study, the frequency of the
BRAFV600E mutation in PTC was 61.7%, which was at
the upper limit of the 38–62% range previously reported (48,49).
There is a divergence of opinions regarding the impact of the
BRAFV600E mutation alone on the grade and
prognosis of PTC. Some claim that there is no association, whereas
others have reported that BRAFV600E in PTC is
associated with extraglandular invasion (50) and lymph node metastasis (51). No significant association with
clinicopathologic parameters was observed in the present study in
cases with BRAFV600E mutation only. However,
cases with BRAFV600E accompanied by TERTp
mutations were correlated with tumor size and extraglandular
invasion, similar to C228T mutations. The prognosis cannot be
discussed in this study due to the short observation period. Muzza
et al (42) reported that
TERTp mutations (but not BRAFV600E) affect
the prognosis of thyroid cancer. Liu et al (14) also observed that the hazard ratio
(HR) for PTC-specific mortality in patients with BRAF
V600E mutations was 3.08 (95% CI, 0.87–10.84) compared
to patients without mutations, while the HR for BRAF
V600E alone was 8.18 (95% CI, 2.04–32.5). The specific
mortality for cases with both mutations remained significant after
adjustment for clinicopathologic factors (HR, 9.34; 95% CI,
2.53–34.48), indicating that the coexistence of the TERT and
BRAF genes is more strongly associated with mortality than
either mutation alone (14).
Similarly, Moon et al (15)
reported that mortality associated with PTC was significantly
higher in patients with coexisting mutations than in those with
BRAFV600E alone. Chung (17) also concluded that the coexistence of
BRAF and TERTp mutations was associated with
increased relapse and mortality and worse survival. Another
meta-analysis of 3911 patients with PTC showed that tumors with
concurrent BRAFV600E and TERTp mutations
were more aggressive than those with BRAFV600E or
TERTp mutations alone (48).
Another study of 653 patients with PTC also reported that the
coexistence of BRAFV600E and TERTp
mutations was associated with extraglandular invasion, and it was
concluded that certain clinicopathologic features increased the
risk of extraglandular invasion compared with that in the group
without either mutation (52). As
shown in the present study, extraglandular invasion was
significantly more common when the BRAFV600E
mutation coexisted with a TERT point mutation. In addition,
when TrSNP was present, the tumor was larger and there was a higher
incidence of extraglandular invasion. These findings suggested that
the coexistence of BRAFV600E, TERT point
mutations, and TrSNP may increase the risk of PTC growth and
progression. Therefore, careful follow-up is required for carriers
of TrSNP regardless of their tumor size and grade. Furthermore, the
presence or absence of C228T and BRAFV600E
mutations in cytology specimens can be utilized to infer the grade
of malignancy exhibited by individual papillary carcinomas. This
information is particularly valuable in the context of patient
treatment planning. Nevertheless, inconsistencies have been noted
between the genetic variants identified in cytology and FFPE
specimens (53). This discrepancy
must be validated in a subsequent step.
Molecularly targeted therapies are being developed
based on the specific gene expression profile of the patient. The
ATA guidelines recommend using the BRAF inhibitor dabrafenib
for patients carrying BRAF V600E mutations
(54,55). Further research can validate
TERTp mutations as a novel therapeutic target. Although
treatment with eribulin is an option for malignant meningiomas and
soft tissue sarcomas, it is still in the early stages of
development (56,57).
This study has some limitations. It was a
retrospective study conducted at a single institution. Prospective
studies conducted at multiple institutions should be considered for
further validation with a larger number of cases. The prognosis of
thyroid cancer is favorable; therefore, a prolonged period of
observation is necessary to evaluate the risk of recurrence and the
overall prognosis.
In conclusion, based on the analysis of 133 patients
with PTC, TrSNP in the TERTp region and the C228T mutation
were significantly correlated with the PTC tumor size and grade. In
particular, the TrSNP and TERTp mutations, particularly
C228T increased the TERTp activity. In this study, we
elucidated the involvement of TrSNP and C228T in the adverse
biology of PTC. The coexistence of the BRAFV600E
mutation with TERTp mutations and TrSNP was significantly
associated with tumor size as well as extraglandular invasion
indicating a higher malignant potential. Therefore, the TrSNP
polymorphism accompanied by the C228T point mutation and the
BRAFV600E mutation could serve as potential
molecular markers for tumor growth or exacerbation of PTC. As the
diagnosis of thyroid cancer is based on cytologic analysis, it is
in the patient's best interest to confirm the nature of the tumor
by oncogene testing of a preoperative cytologic specimen.
Preoperative genetic testing of the tumor and assessing the risk of
progression can assist in decision-making, including the decision
to proceed with surgery. Therefore, it is recommended that TrSNP
carriers undergo careful follow-up regardless of tumor size or
grade. It would be beneficial to consider the possibility of
testing cytology specimens for the C228T and
BRAFV600E mutations.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Ayumi Sumiishi,
Ms. Namiko Kondo, Ms. Kaoruko Kojima (Department of Pathology,
Kyorin University School of Medicine, Mitaka, Japan) and Ms. Miyuki
Murayama (Department of General Thoracic and Thyroid Surgery,
Kyorin University, School of Medicine, Mitaka, Japan) for their
technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to the institution's policy but may be
requested from the corresponding author.
Authors' contributions
YN and HKa designed the study, wrote the first
draft of the manuscript and confirm the authenticity of all the raw
data. KH and RT contributed to data analysis and interpretation and
assisted in drafting the manuscript. TC and MF contributed to data
analysis. YA, TH, TM and HKo contributed to data collection and
interpretation, and critically reviewed the manuscript. All authors
agree to take responsibility for all aspects of the work to ensure
that questions about the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of the Faculty of Medicine of Kyorin University on April
28, 2023 (No. R01-002_759-02; Mitaka, Tokyo, Japan). Written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
Glossary
Abbreviations
Abbreviations:
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
PTC
|
papillary thyroid carcinoma
|
|
SNP
|
single nucleotide polymorphism
|
|
TERTp
|
telomerase reverse transcriptase gene
promoter
|
|
TrSNP
|
C allele of rs2853669
|
|
WHO
|
World Health Organization
|
References
|
1
|
Pizzato M, Li M, Vignat J, Laversanne M,
Singh D, La Vecchia C and Vaccarella S: The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN estimates for
incidence and mortality rates in 2020. Lancet Diabetes Endocrinol.
10:264–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seib CD and Sosa JA: Evolving
understanding of the epidemiology of thyroid cancer. Endocrinol
Metab Clin North Am. 48:23–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruiz FJ, Inkman M, Rashmi R, Muhammad N,
Gabriel N, Miller CA, McLellan MD, Goldstein M, Markovina S,
Grigsby PW, et al: HPV transcript expression affects cervical
cancer response to chemoradiation. JCI Insight. 6:e1387342021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruiz FJ, Sundaresan A, Zhang J, Pedamallu
CS, Halle MK, Srinivasasainagendra V, Zhang J, Muhammad N, Stanley
J, Markovina S, et al: Genomic characterization and therapeutic
targeting of HPV undetected cervical carcinomas. Cancers (Basel).
13:45512021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
WHO Classification of Tumors Editorial
Board, . Endocrine tumors. 5th edition. IARC; Lyon: 2022
|
|
7
|
Ito Y, Miyauchi A and Oda H: Low-risk
papillary microcarcinoma of the thyroid: A review of active
surveillance trials. Eur J Surg Oncol. 44:307–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugitani I, Ito Y, Miyauchi A, Imai T and
Suzuki S: Active surveillance versus immediate surgery:
Questionnaire survey on the current treatment strategy for adult
patients with low-risk papillary thyroid microcarcinoma in Japan.
Thyroid. 29:1563–1571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omry-Orbach G: Risk stratification in
differentiated thyroid cancer: An ongoing process. Rambam
Maimonides Med J. 7:e00032016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang N, Liu T, Sofiadis A, Juhlin CC,
Zedenius J, Höög A, Larsson C and Xu D: Tert promoter mutation as
an early genetic event activating telomerase in follicular thyroid
adenoma (FTA) and atypical FTA. Cancer. 120:2965–2979. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu R and Xing M: TERT promoter mutations
in thyroid cancer. Endocr Relat Cancer. 23:R143–R155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu R, Bishop J, Zhu G, Zhang T, Ladenson
PW and Xing M: Mortality risk stratification by combining BRAF
V600E and TERT promoter mutations in papillary thyroid cancer:
Genetic duet of BRAF and TERT promoter mutations in thyroid cancer
mortality. JAMA Oncol. 3:202–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moon S, Song YS, Kim YA, Lim JA, Cho SW,
Moon JH, Hahn S, Park DJ and Park YJ: Effects of coexistent
BRAFV600E and TERT promoter mutations on poor clinical
outcomes in papillary thyroid cancer: A meta-analysis. Thyroid.
27:651–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bullock M, Ren Y, O'Neill C, Gill A, Aniss
A, Sywak M, Sidhu S, Delbridge L, Learoyd D, de Vathaire F, et al:
TERT promoter mutations are a major indicator of recurrence and
death due to papillary thyroid carcinomas. Clin Endocrinol (Oxf).
13:e01915602016.
|
|
17
|
Chung JH: BRAF and TERT promoter
mutations: Clinical application in thyroid cancer. Endocr J.
67:577–584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G,
Murugan AK, Guan H, Yu H, Wang Y, et al: TERT promoter mutations
and their association with BRAF V600E mutation and aggressive
clinicopathological characteristics of thyroid cancer. J Clin
Endocrinol Metab. 99:E1130–E1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staubitz JI, Müller C, Heymans A, Merten
C, Roos B, Poplawski A, Ludt A, Strobl S, Springer E, Schad A, et
al: Approach to risk stratification for papillary thyroid carcinoma
based on molecular profiling: Institutional analysis. BJS Open.
7:zrad0292023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKelvey BA, Umbricht CB and Zeiger MA:
Telomerase reverse transcriptase (TERT) regulation in thyroid
cancer: A review. Front Endocrinol (Lausanne). 11:4852020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiba K, Lorbeer FK, Shain AH, McSwiggen
DT, Schruf E, Oh A, Ryu J, Darzacq X, Bastian BC and Hockemeyer D:
Mutations in the promoter of the telomerase gene TERT contribute to
tumorigenesis by a two-step mechanism. Science. 357:1416–1420.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Wang T, Wu Z, Zhang K, Li W, Yang
J, Chen C, Chen L and Xing J: Association between TERT rs2853669
polymorphism and cancer risk: A meta-analysis of 9,157 cases and
11,073 controls. PLoS One. 13:e01915602018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen N, Lu Y, Wang X, Peng J, Zhu Y and
Cheng L: Association between rs2853669 in TERT gene and the risk
and prognosis of human cancer: A systematic review and
meta-analysis. Oncotarget. 8:50864–50872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoo SS, Do SK, Choi JE, Lee SY, Lee J, Cha
SI, Kim CH and Park JY: TERT polymorphism rs2853669 influences on
lung cancer risk in the Korean population. J Korean Med Sci.
30:1423–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nencha U, Rahimian A, Giry M, Sechi A,
Mokhtari K, Polivka M, Schmitt Y, Di Stefano AL, Alentorn A,
Labussière M and Sanson M: TERT promoter mutations and rs2853669
polymorphism: Prognostic impact and interactions with common
alterations in glioblastomas. J Neurooncol. 126:441–446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mosrati MA, Willander K, Falk IJ,
Hermanson M, Höglund M, Stockelberg D, Wei Y, Lotfi K and
Söderkvist P: Association between TERT promoter polymorphisms and
acute myeloid leukemia risk and prognosis. Oncotarget.
6:25109–25120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko E, Seo HW, Jung ES, Kim BH and Jung G:
The TERT promoter SNP rs2853669 decreases E2F1 transcription factor
binding and increases mortality and recurrence risks in liver
cancer. Oncotarget. 7:684–699. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Powter B, Jeffreys SA, Sareen H, Cooper A,
Brungs D, Po J, Roberts T, Koh ES, Scott KF, Sajinovic M, et al:
Human tert promoter mutations as a prognostic biomarker in glioma.
J Cancer Res Clin Oncol. 147:1007–1017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vinothkumar V, Arun K, Arunkumar G,
Revathidevi S, Ramani R, Bhaskar LVKS, Murugan AK and Munirajan AK:
Association between functional tert promoter polymorphism rs2853669
and cervical cancer risk in south Indian women. Mol Clin Oncol.
12:485–494. 2020.PubMed/NCBI
|
|
30
|
Giunco S, Boscolo-Rizzo P, Rampazzo E,
Tirelli G, Alessandrini L, Di Carlo R, Rossi M, Nicolai P,
Menegaldo A, Carraro V, et al: Tert promoter mutations and
rs2853669 polymorphism: useful markers for clinical outcome
stratification of patients with oral cavity squamous cell
carcinoma. Front Oncol. 11:7826582021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirokawa T, Arimasu Y, Chiba T, Nakazato
Y, Fujiwara M and Kamma H: Regulatory single nucleotide
polymorphism increases TERT promoter activity in thyroid carcinoma
cells. Pathobiology. 87:338–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirokawa T, Arimasu Y, Chiba T, Fujiwara M
and Kamma H: Clinicopathological significance of the single
nucleotide polymorphism, rs2853669 within the TERT promoter in
papillary thyroid carcinoma. Pathol Int. 70:217–223. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirokawa T, Arimasu Y, Nakazato Y, Chiba
T, Fujiwara M and Kamma H: Effect of single-nucleotide polymorphism
in TERT promoter on follicular thyroid tumor development. Pathol
Int. 70:210–216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gouda MA, Nelson BE, Buschhorn L, Wahida A
and Subbiah V: Tumor-Agnostic precision medicine from the AACR
GENIE database: Clinical implications. Clin Cancer Res.
29:2753–2760. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamma H, Kameyama K, Kondo T, Imamura Y,
Nakashima M, Chiba T and Hirokawa M: Pathological diagnosis of
general rules for the description of thyroid cancer by Japanese
Society of Thyroid Pathology and Japan Association of Endocrine
Surgery. Endocr J. 69:139–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumagai S, Togashi Y, Kamada T, Sugiyama
E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y,
Matsui S, et al: The PD-1 expression balance between effector and
regulatory T cells predicts the clinical efficacy of PD-1 blockade
therapies. Nat Immunol. 21:1346–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akagi K, Oki E, Taniguchi H, Nakatani K,
Aoki D, Kuwata T and Yoshino T: Real-world data on microsatellite
instability status in various unresectable or metastatic solid
tumors. Cancer Sci. 112:1105–1113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrence MS, Stojanov P, Polak P, Kryukov
GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH,
Roberts SA, et al: Mutational heterogeneity in cancer and the
search for new cancer-associated genes. Nature. 499:214–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bertol BC, Massaro JD, Debortoli G, Santos
ALP, de Araújo JNG, Giorgenon TMV, Silva MC, de Figueiredo-Feitosa
NL, Collares CVA, de Freitas LCC, et al: BRAF, TERT and HLA-G
status in the papillary thyroid carcinoma: A clinicopathological
association study. Int J Mol Sci. 24:124592023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Muzza M, Colombo C, Rossi S, Tosi D,
Cirello V, Perrino M, De Leo S, Magnani E, Pignatti E, Vigo B, et
al: Telomerase in differentiated thyroid cancer: Promoter
mutations, expression, and localization. Mol Cell Endocrinol.
399:288–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: the NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
1000 Genomes Project Consortium, . Auton
A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini
JL, McCarthy S, McVean GA and Abecasis GR: A global reference for
human genetic variation. Nature. 526:68–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spiegl-Kreinecker S, Lötsch D, Ghanim B,
Pirker C, Mohr T, Laaber M, Weis S, Olschowski A, Webersinke G,
Pichler J and Berger W: Prognostic quality of activating TERT
promoter mutations in glioblastoma: Interaction with the rs2853669
polymorphism and patient age at diagnosis. Neuro Oncol.
17:1231–1240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Landa I, Ganly I, Chan TA, Mitsutake N,
Matsuse M, Ibrahimpasic T, Ghossein RA and Fagin JA: Frequent
somatic TERT promoter mutations in thyroid cancer: Higher
prevalence in advanced forms of the disease. J Clin Endocrinol
Metab. 98:E1562–E1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu T, Wang N, Cao J, Sofiadis A, Dinets
A, Zedenius J, Larsson C and Xu D: The age- and shorter
telomere-dependent TERT promoter mutation in follicular thyroid
cell-derived carcinomas. Oncogene. 33:4978–4984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vuong HG, Altibi AMA, Duong UNP and
Hassell L: Prognostic implication of BRAF and TERT promoter
mutation combination in papillary thyroid carcinoma-A
meta-analysis. Clin Endocrinol (Oxf). 87:411–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soares P, Celestino R, Melo M, Fonseca E
and Sobrinho-Simões M: Prognostic biomarkers in thyroid cancer.
Virchows Arch. 464:333–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
da Silva RC, de Paula HS, Leal CB, Cunha
BC, de Paula EC, Alencar RC, Meneghini AJ, Silva AM, Gontijo AP,
Wastowski IJ and Saddi VA: BRAF overexpression is associated with
BRAF V600E mutation in papillary thyroid carcinomas. Genet Mol Res.
14:5065–5075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng L, Li M, Zhang QP, Piao ZA, Wang ZH
and Lv S: Utility of BRAF protein overexpression in predicting the
metastasis potential of papillary thyroid carcinoma. Oncol Lett.
2:59–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin L, Chen E, Dong S, Cai Y, Zhang X,
Zhou Y, Zeng R, Yang F, Pan C, Liu Y, et al: BRAF and TERT promoter
mutations in the aggressiveness of papillary thyroid carcinoma: a
study of 653 patients. Oncotarget. 7:18346–18355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakao T, Matsuse M, Saenko V, Rogounovitch
T, Tanaka A, Suzuki K, Higuchi M, Sasai H, Sano T, Hirokawa M, et
al: Preoperative detection of the TERT promoter mutations in
papillary thyroid carcinomas. Clin Endocrinol (Oxf). 95:790–799.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American Thyroid Association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nakano T, Fujimoto K, Tomiyama A,
Takahashi M, Achiha T, Arita H, Kawauchi D, Yasukawa M, Masutomi K,
Kondo A, et al: Eribulin prolongs survival in an orthotopic
xenograft mouse model of malignant meningioma. Cancer Sci.
113:697–708. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mochizuki T, Ikegami M and Akiyama T:
Factors predictive of second-line chemotherapy in soft tissue
sarcoma: An analysis of the National Genomic Profiling Database.
Cancer Sci. 115:575–588. 2024. View Article : Google Scholar : PubMed/NCBI
|