Introduction
Soft tissue sarcoma (STS) is a category of rare
malignant tumors that can arise in various locations within the
body, with ~50% occurring in the extremities, 40% in the trunk and
retroperitoneum, and 10% in the head and neck (1). Its incidence is <6 cases per
100,000 individuals, which represents 1–2% of all adult cancers
(2). The etiology of the majority
of STS is unknown; however, in most cases, the risk of developing
sporadic STS is increased in patients with a history of previous
radiotherapy and certain genetic mutations (1). Surgery is a mainstay of sarcoma
treatment, and radiation is used for unresectable tumors and as a
neoadjuvant or an adjuvant to resection (3). Common soft-tissue sarcomas include
malignant fibrous histiocytoma, liposarcoma (LPS), leiomyosarcoma
and synovial sarcoma (4). LPS, a
malignant neoplasm differentiated by adipocytes, represents one of
the most prevalent subtypes of STS, accounting for 15–20% of all
STS cases (5). According to the
characteristics of tumor cells, LPS can be classified into four
types: i) Atypical lipomatous/well-differentiated LPS (WDLPS); ii)
dedifferentiated LPS (DDLPS); iii) myxoid/round cell LPS (MLPS);
and iv) pleomorphic LPS (PLPS) (6).
WDLPS and DDLPS constitute the largest subgroup of
LPS, with WDLPS accounting for 40–45% of cases (7). Notedly, up to 10% of WDLPS cases may
be dedifferentiated to DDLPS (7).
DDLPS is characterized by the presence of both WDLPS components and
non-lipogenic components (8). WDLPS
and DDLPS share common genetic aberrations involving high-level
amplifications of murine double minute 2 (MDM2) and CDK4 in the
chromosomal region 12q14-15 (7).
Immunohistochemistry analysis frequently demonstrates positive
expression of MDM2 and CDK4, which may be accompanied by p16
positivity (9).
The most frequent site of DDLPS is the
retroperitoneum. Primary LPS of the pancreas remains exceptionally
rare (10). Only 9 cases have been
reported in the English literature since 1979, Among these cases, 4
were classified as DDLPS (10–13), 3
as WDLPS (14–16), 1 as MLPS (17) and 1 as PLPS (18). Additionally, only 1 case (13) of pancreatic DDLPS lacking a fat
component has been reported. The present study reports another case
of primary pancreatic DDLPS without fat components, with CT
features described in detail.
Case report
In May 2023, a 40-year-old female patient presented
with a fever that had persisted for several days without
accompanying symptoms to Sichuan Provincial People's Hospital
(Chengdu, China) for treatment. The medical and family history of
the patient were unremarkable, and the patient was in normal
physical condition. All laboratory data, including tumor markers
such as carcinoembryonic antigen (<1.73 ng/ml; normal range, ≤5
ng/ml) and carbohydrate antigen 19-9 (10.97 U/ml; normal range, ≤43
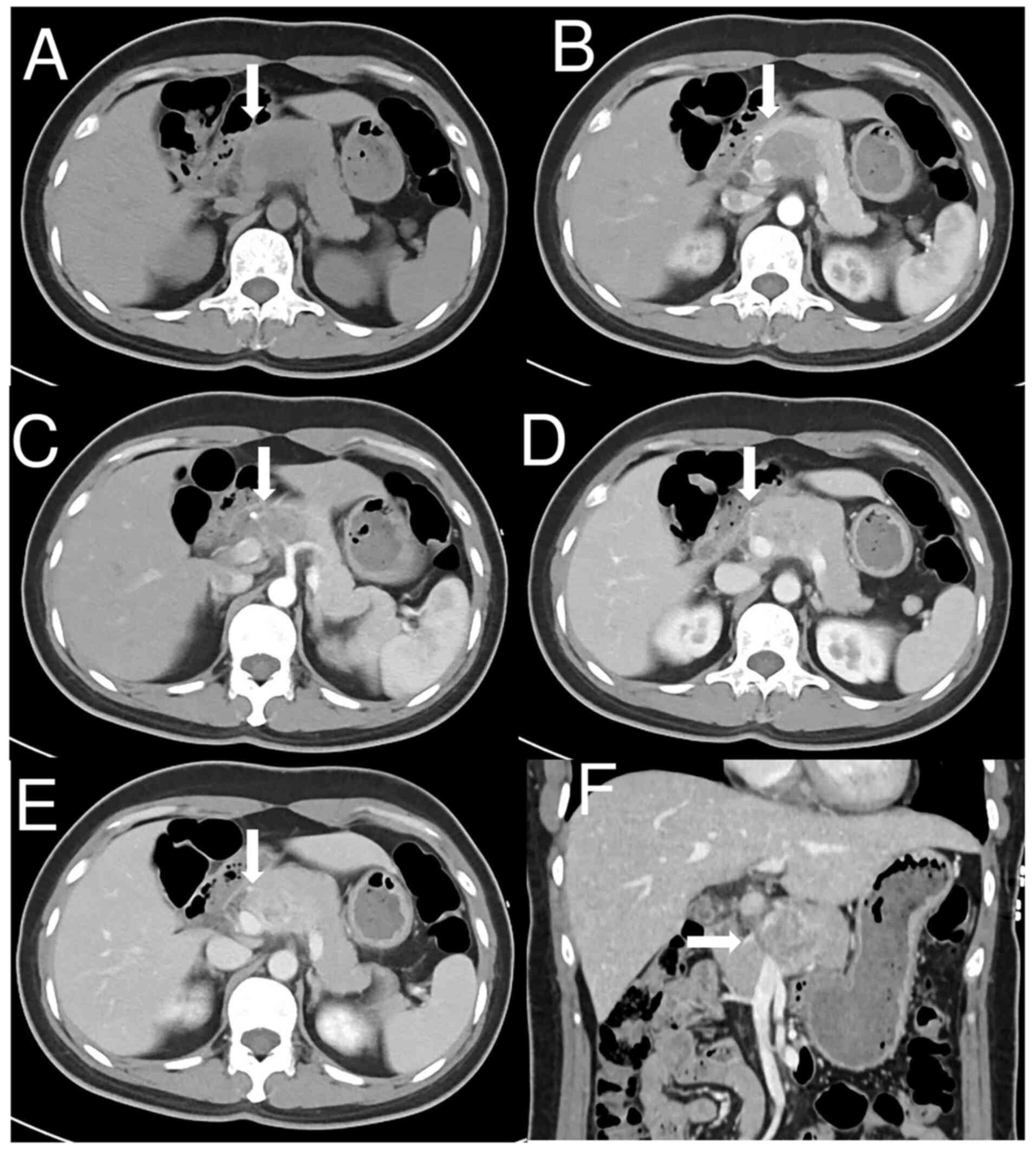
U/ml) were within normal limits. Abdominal CT (Fig. 1A) demonstrated a solid mass
measuring 2.4×4.5 cm located in the pancreatic head and body. The
tumor was heterogeneous and lacked a fat component. Dynamic
contrast-enhanced CT demonstrated slightly heterogeneous
enhancement in the arterial phase (Fig.
1B), moderate enhancement in the venous phase (Fig. 1D) and persistent enhancement in the
delayed phase (Fig. 1E), with
encapsulation of the common hepatic artery, splenic vein and portal
vein (Fig. 1C and F). No
lymphadenopathy was observed.
The patient underwent distal pancreatectomy and
splenectomy in May 2023. Laparotomy determined that the tumor
measured 4×4×3 cm in the head and body of the pancreas. The tumor
firmly adhered to the splenic vein, inferior mesenteric vein and
common hepatic artery, and showed invasion into the duodenum.
Grossly, the tumor was elastic and firm, centered with fish
flesh-like texture. Histologically, the tumor predominantly
consisted of medium and highly atypical spindle cells alongside
multivacuolated lipoblasts (Fig.
2A). H&E staining was performed as follows: The tumor
tissues were fixed in 10% formalin for >24 h at 25°C (Beijing
BioDee Biotechnology Co., Ltd.), followed by gradient alcohol
dehydration and embedding in paraffin. Subsequently, 3-µm thick
sections were sectioned and stained with H&E. Sections were
stained with hematoxylin for 5 min at room temperature, rinsed with
running water for 3 min, incubated with 1% HCl/ethyl for 3 sec,
rinsed with running water for 1 sec, stained with bluing solution
for 10 sec at room temperature, rinsed with running water for 5
sec, stained with eosin Y stain (water soluble; 0.5%) for 3 min at
room temperature, incubated with 95% ethyl alcohol for 2 sec,
incubated with 100% ethyl alcohol for 2 sec twice and incubated
with 100% xylene three times, followed by neutral balsam mounting.
The images were acquired using an Olympus BX53 light microscope
(Olympus Corporation).
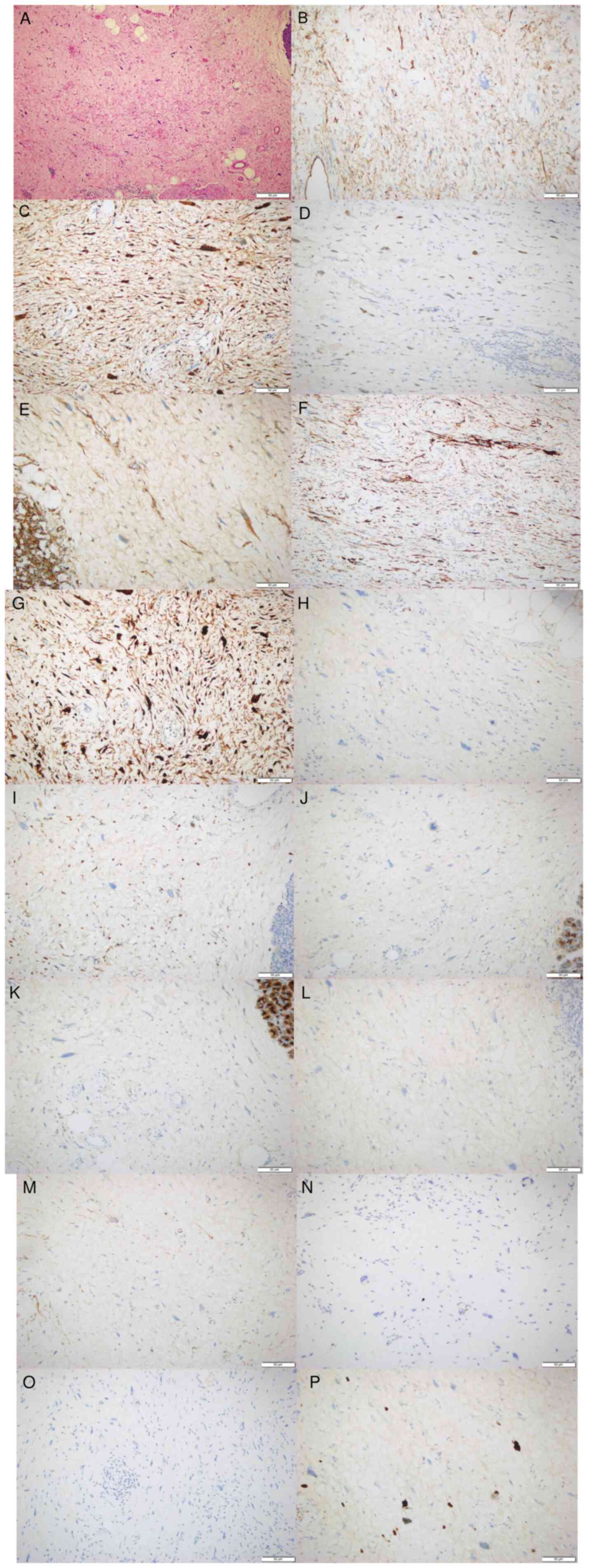 | Figure 2.Representative images of pathological
findings. (A) H&E staining of the specimen demonstrated that
the tumor cells predominantly consisted of medium and highly
atypical spindle cells with the presence of multivacuolated
lipoblasts (scale bar, 50 µm). Positive nuclear immunostaining in
neoplastic cells of (B) CD34, (C) CDK4, (D) murine double minute 2,
(E) catenin, (F) desmin and (G) P16 (scale bar, 50 µm). (H) S-100,
(I) CD68, (J) creatine kinase, (K) epithelial membrane antigen, (L)
HMB-45, (M) smooth muscle actin, (N) STAT6 and (O) mucin 4 negative
nuclear immunostaining in neoplastic cells (scale bar, 50 µm). (P)
The positive index of Ki-67 in tumor cells was ~20% (scale bar, 50
µm). |
Immunohistochemistry analysis of the tissue sample
revealed positive expression of CD34 (Fig. 2B), CDK4 (Fig. 2C), MDM2 (Fig. 2D), catenin (Fig. 2E), desmin (Fig. 2F) and p16 (Fig. 2G), and negative expression of S-100
(Fig. 2H), CD68 (Fig. 2I), creatine kinase (Fig. 2J), epithelial membrane antigen
(Fig. 2K), HMB-45 (Fig. 2L), smooth muscle actin (Fig. 2M), STAT6 (Fig. 2N) and mucin 4 (Fig. 2O). The Ki-67 (Fig. 2P) index was 20%. IHC staining was
conducted according to the manufacturer's protocol (KIT-9701/9706;
Fuzhou Maixin Biotechnology Development Co., Ltd.). H&E
staining was performed as follows: The tumor tissues were fixed in
10% formalin for >24 h at 25°C (Beijing BioDee Biotechnology
Co., Ltd.), followed by gradient alcohol dehydration and embedding
in paraffin. Subsequently, 3-µm thick sections were sectioned and
stained with IHC. Tissue sections were dewaxed in xylene and
rehydrated in graded alcohols (descending series). Endogenous
peroxidase activity was blocked with methanol + 0.3% peroxide (SP
KIT-A1) for 10 min at room temperature. Tissue sections were
pretreated with heat-induced antigen retrieval using citrate buffer
(pH 6.0; MVS-0100) for 10 min at 98°C. Non-immune serum of animals
(SP KIT-B1; 10% goat serum; Fuzhou Maixin Biotechnology Development
Co., Ltd.) was applied for protein blocking at room temperature for
20 min prior to incubation with the first primary antibody.
Sections were incubated with a primary antibody overnight at 4°C,
incubated with 3% H2O2 for 10 min, incubated
with 10% goat serum (Fuzhou Maixin Biotechnology Development Co.,
Ltd.) at room temperature for 20 min and finally incubated with
primary antibody at 4°C overnight. The primary antibodies included
CD34 (1:100; Kit-0004; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), CDK4 (1:100; RMA-0771; Fuzhou Maixin Biotechnology
Development Co., Ltd.), catenin (1:100; RMA-1054; Fuzhou Maixin
Biotechnology Development Co., Ltd.), desmin (1:100; MAB-0766;
Fuzhou Maixin Biotechnology Development Co., Ltd.), Ki-67 (1:100;
RMA-0542; Fuzhou Maixin Biotechnology Development Co., Ltd.), MDM2
(1:100; MAB-0774; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), p16 (1:100; MAB-0673; Fuzhou Maixin Biotechnology
Development Co., Ltd.), CD68 (1:100; MAB-0687; Fuzhou Maixin
Biotechnology Development Co., Ltd.), EMA (1:100; Kit-0011; Fuzhou
Maixin Biotechnology Development Co., Ltd.), HMB-45 (1:100;
MAB-0098; Fuzhou Maixin Biotechnology Development Co., Ltd.), SMA
(1:100; MAB-0890; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), STAT6 (1:100; RMA-0845; Fuzhou Maixin Biotechnology
Development Co., Ltd.), mucin 4 (1:100; MAB-0749; Fuzhou Maixin
Biotechnology Development Co., Ltd.), Ki-67 (1:100; MAB-0672;
Fuzhou Maixin Biotechnology Development Co., Ltd.), CK (1:100;
Kit-0004; Fuzhou Maixin Biotechnology Development Co., Ltd.) and
s-100 (1:100; Kit-0007; Fuzhou Maixin Biotechnology Development
Co., Ltd.) antibodies. Subsequently, the slides were incubated with
secondary antibodies (1:100; KIT-9701/9706; Fuzhou Maixin
Biotechnology Development Co., Ltd.; peroxidase/DAB+) for 10 min at
room temperature, and then stained with DAB and hematoxylin for 1
min at 25°C. Finally, images were captured using a laboratory
microscopy (Olympus BX53 light microscope; Olympus Corporation),
and the integrated optical density of the
immunohistochemistry-positive areas was analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.).
Next-generation sequencing (NGS) demonstrated
amplifications of the CDK4 and MDM2 genes. Briefly, genomic DNA was
extracted from formalin-fixed paraffin-embedded sections using the
QIAamp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen GmbH), and
quantified using a Qubit® 3.0 Fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc.) using the dsDNA HS Assay Kit (cat.
no. Q32854; Thermo Fisher Scientific, Inc.). Libraries were
prepared using 2,420 ng genomic DNA using a Geneseeq Prime 425-gene
panel (cat. no. 20233401452; Nanjing Geneseeq Technology, Inc.)
according to the manufacturer's protocol. Libraries were quantified
by quantitative polymerase chain reaction (qPCR) using Illumina p5
(5′-AATGATACGGCGACCACCGA-3′) and p7
(5′-CAAGCAGAAGACGGCATACGAGAT-3′) primers in the KAPA Library
Quantification kit (cat. no. KK4824; Kapa Biosystems; Roche
Diagnostics). qPCR was run using the KAPA Library Quantification
kit on the QuantStudio™ 5 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). qPCR cycling conditions were as follows: Initial
denaturation at 95°C for 5 min, followed by 35 cycles of
denaturation at 95°C for 30 sec and annealing/extension/data
acquisition at 60°C for 30 sec. The standard curve was generated
using QuantStudio 5 Real-Time PCR System v1.6.0 software (Thermo
Fisher Scientific, Inc.). The standard curve was used to calculate
the concentration of the library according to the manufacturer's
protocol for the KAPA Library Quantification kit. The library
fragment size was determined using a Bioanalyzer 2100 (Agilent
Technologies, Inc.). Different libraries with unique indices were
pooled together in desirable ratios for up to 4,030 pmol/µl of
total library input. The library sequencing (loading concentration
of the final library, 100–200 pM) was performed on Illumina NovaSeq
platforms (Illumina, Inc.) using 150-bp paired-end sequencing. The
average coverage depth was 1,939.24X. For mutation calling,
Trimmomatic (version 0.38; http://www.usadellab.org/cms/index.php?page=trimmomatic)
was used for FASTQ file quality control. Qualified reads were then
mapped to the reference human genome (hg19) using Burrows-Wheeler
Aligner (version 0.7.17.tar.bz2; http://bio-bwa.sourceforge.net/). Somatic mutations
were detected using VarScan2 (version 2.4.4, http://dkoboldt.github.io/varscan) with default
parameters. Annotation was performed using ANNOVAR (version
2019-10-24 00:05:27-0400; http://annovar.openbioinformatics.org/) using the
reference human genome (GRCh37/hg19). Finally, the tumor was
diagnosed as DDLPS based on histological, immunohistochemical and
NGS tests. The postoperative recovery was uneventful, and the
patient was under surveillance without additional treatment. The
abdominal ultrasound exhibited no signs of recurrence during the
follow-up between May 2023 and June 2024.
Discussion
LPS is the most common type of STS and typically
occurs in the extremities or retroperitoneum (10). The occurrence of LPS in visceral
organs, such as the pancreas, is extremely rare, with only
individual cases reported, which leads to the uncertainty regarding
the exact incidence. The peak incidence of pancreatic LPS occurs in
the sixth and seventh decades of life without obvious sex
predilection (10). In the reported
cases thus far, including the present study, the patients included
7 female and 3 male patients, indicating a slight female
predominance (Table I). Patient age
ranged between 24 and 81 years, with a mean age of 51.6 years. This
in contrast to retroperitoneum LPS, where patients are generally
older without a noted sex preference (19).
 | Table I.Clinical characteristics of patients
with pancreatic LPS. |
Table I.
Clinical characteristics of patients
with pancreatic LPS.
| First author/s,
year | Age, years | Sex | Symptoms | Surgery | LPS subtype |
Immunohistochemistry | Outcome (follow-up
duration) | (Refs.) |
|---|
| Tanabe et
al, 2022 | 81 | F | None | DPS | DDLPS | CDK4(+) and
MDM2(−) | Recurrence (7
months) | (10) |
| Cao et al,
2019 | 72 | F | None | DPSA | WDLPS | N/A | N/A | (16) |
| Liu et al,
2019 | 28 | F | Abdominal pain | DPS | DDLPS | MDM2(+) | No recurrence (26
months) | (11) |
| Han et al,
2017 | 29 | F | Abdominal pain | DPS | DDLPS | MDM2(−) | Recurrence (1
year) | (12) |
| Machado et
al, 2016 | 42 | M | Abdominal pain | DPS | DDLPS with high
grade components | MDM2(+) | No recurrence (5
years) | (13) |
| Matthews et
al, 2016 | 65 | F | None | DP | WDLPS | MDM2(+) | N/A | (14) |
| Kuramoto et
al, 2013 | 24 | M | Abdominal
distension | CP | Myxoid | N/A | Recurrence (44
months) | (17) |
| Dodo, 2005 | 76 | M | Abdominal pain | DPS | WDLPS with area of
DDLPS | N/A | No recurrence (26
months) | (15) |
| Elliott et
al, 1980 | 59 | F | Abdominal
distension | DPS | Pleomorphic | N/A | No recurrence (6
years) | (18) |
| Present study | 40 | F | Fever | DPS | DDLPS | CDK4(+) and
MDM2(+) | No recurrence (12
months) | - |
Clinical presentations of pancreatic LPS can be
asymptomatic or nonspecific, including abdominal pain, distension
and diarrhea (13). In the present
case, the patient attended a hospital because of a fever. The
pancreatic tumor was identified incidentally, without
gastrointestinal symptoms. The literature indicates that the
average tumor diameter is 17.5 cm in symptomatic patients and 5.2
cm in asymptomatic patients. Symptomatic patients tend to have
larger tumors (10–18). The tumor reported in the present
case measured 4 cm, which was in accordance with the absence of
symptoms due to tumor compression.
Pancreatic LPS has been identified in the pancreatic
head and neck in 1 case, pancreatic body in 2 cases, pancreatic
body and tail in 2 cases and pancreatic tail in 4 cases (Table II). In the present study, the tumor
was situated in the head and body of the pancreas, which was
relatively rare. In half of the reported 10 cases of pancreatic
LPS, the tumor margins were unclear. As the tumor grows, it can
invade surrounding organs, including the duodenum, spleen and left
adrenal gland (10–18).
 | Table II.Imaging features of patients with
pancreatic liposarcoma. |
Table II.
Imaging features of patients with
pancreatic liposarcoma.
| First author/s,
year | Location | Size, cm | Vascular
invasion | Abdominal
lymphade-nopathy | Fat component | Imaging
modality | Precontrast | Arterial phase | Venous phase | Delayed phase | (Refs.) |
|---|
| Tanabe et
al, 2022 | Tail | 2.6 | N/A | No | Yes |
CT/18F-FDG PET | Heterogeneous | Early
enhancement | N/A | Delayed
enhancement | (10) |
| Cao et al,
2019 | Body and tail | 9.7 | N/A | N/A | Yes | CT | Heterogeneous | N/A | N/A | N/A | (16) |
| Liu et al,
2019 | Tail | 18.0 | No | Yes | N/A | CT | Heterogenous | N/A | N/A | N/A | (11) |
| Han et al,
2017 | Tail | 20.0 | N/A | N/A | N/A | CT | Heterogenous | N/A | N/A | N/A | (12) |
| Machado et
al, 2016 | Head and neck | 6.8 | N/A | No | No | MRI | Heterogenous | N/A | N/A | N/A | (13) |
| Matthews et
al, 2016 | Tail | 4.0 | N/A | N/A | N/A | CT/PET | Hypointensity | N/A | N/A | N/A | (14) |
| Kuramoto et
al, 2013 | Body | 25.0 | N/A | N/A | Yes | CT | Heterogenous | N/A | N/A | N/A | (17) |
| Dodo, 2005 | Body and tail | 9.0 | N/A | N/A | Yes | CT | Heterogenous | N/A | N/A | N/A | (15) |
| Elliott et
al, 1980 | Body | 16.0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (18) |
| Present study | Head and body | 4.0 | Yes | No | No | CT | Heterogenous | Slight
heterogenous | Moderate
heterogenous | Persistent
enhancement | - |
DDLPS is a malignant adipocytic neoplasm defined as
the transition from WDLPS to a non-lipogenic sarcoma with
morphologically distinct components, a mature adipocytic component
(atypical lipomatous tumor; WDLPS) and a higher-grade non-lipogenic
(dedifferentiated) component (8).
Histologically, DDLPS typically manifests as undifferentiated
pleomorphic or spindle cell sarcomas, usually displaying moderate
or high cellularity with moderate to significant pleomorphism
(7).
Both WDLPS and DDLPS exhibit a simple genomic
profile characterized by the amplification of the 12q14-15 region,
while the increased expression of MDM2 and CDK4 is consistent with
this amplification (7,20). Immunohistochemical analyses of a
range of sarcomas have shown sensitivities of 95% for MDM2 and 92%
for CDK4, with specificities of 81 and 95%, respectively (21). According to the relevant literature,
the combination of p16 with MDM2 and CDK4 is insufficient to fully
meet the needs of distinguishing between WDLPS and DDLPS
occasionally, with 100% of WDLPS and 93% of DDLPS expressing at
least two of the three markers, and 68% of WDLPS and 72% of DDLPS
expressing all three markers (9,22). LPS
harbors characteristic genetic abnormalities, including
amplification of the MDM2 and CDK4 region (7). Consequently, molecular genetics and
cytogenetic analyses (such as NGS) can serve as valuable ancillary
diagnostic tools when significant histological features are absent
(23).
In the present study, H&E staining demonstrated
that the tumor cells predominantly consisted of medium and highly
atypical spindle cells alongside multivacuolated lipoblasts.
Immunohistochemistry showed positive results for MDM2, CDK4 and
p16. NGS confirmed amplifications of MDM2 and CDK4. Thus, the final
pathology-based diagnosis was established to be DDLPS (24,25).
Besides the present study, seven reports described
the CT findings of pancreatic LPS, and one report detailed the MRI
findings (Table II). CT showed the
tumor was heterogeneous, with low-density areas indicating a fat
component in 4 cases (10,15–17).
Notably, only one report demonstrated a case of pancreatic DDLPS
without fat components (13). A
previous report described the MRI finding of a heterogeneous mass
with a thick wall and septations (13). To the best of our knowledge, the
present report describes the second instance of pancreatic LPS
devoid of fat and is the second to illustrate its CT enhancement
pattern, characterized by slightly heterogeneous enhancement in the
arterial phase, moderate enhancement in the venous phase and
persistent enhancement in the delayed phase. The deficiency of fat
in this tumor may be attributed to the pathological features of
DDLPS, which often predominantly contains non-lipogenic components,
complicating the diagnostic process (8). It has been reported that the early
enhancement in the arterial phase on CT may reflect vascular growth
and the delayed enhancement denotes fibrous components within the
tumor (26). Although 2 cases of
LPS were located in the pancreatic head, dilation of bile and
pancreatic ducts was absent, likely because the tumor did not
originate from the epithelial cells of pancreatic ducts (27). To the best of our knowledge, the
present study is the first to demonstrate vascular invasion
involving the common hepatic artery, splenic vein and portal vein
via CT, underscoring the high malignancy of this tumor. Therefore,
CT served a pivotal role in characterizing the tumor and its
surrounding structures.
Pancreatic LPS needs to be differentiated from
several other pancreatic lesions, including solid pseudopapillary
tumor, neuroendocrine tumor and pancreatic ductal adenocarcinoma.
Solid pseudopapillary tumors usually occur in women aged 20–30
years (28). Typically, these
tumors present as encapsulated solid masses with well-defined
borders and varying degrees of internal cystic and hemorrhagic
degeneration on CT (29).
Contrast-enhanced CT images demonstrate progressive delayed
enhancement for the solid component, and no enhancement for cystic
portions of tumors, but the cyst walls enhance similarly to solid
components (29). However, in the
present study, the patient was a middle-aged woman, and the tumor
was solid without hemorrhage or cystic changes, distinguishing it
from solid pseudopapillary tumors.
Pancreatic neuroendocrine tumors (pNETs) can be
classified as functional pNETs (F-pNETs) or non-functional pNETs
(NF-pNETs) (30). A large
proportion of F-pNETs are characterized by well-circumscribed
hypervascular lesions, and are typically hyper-enhanced in the
arterial phase and wash out in the delayed venous phase (31). Additionally, F-pNETs often present
with specific clinical syndromes; insulinomas typically manifest as
Whipple's triad (hypoglycemic syndromes, low plasma glucose
measured at the time of the symptoms and signs, and relief of
symptoms and signs when the glucose is raised to normal) and
gastrinoma leading to Zollinger-Ellison syndrome (abdominal pain,
gastroesophageal reflux, diarrhea and duodenal ulcers) (32). These clinical syndromes can aid the
differentiation of DDLPS from F-PNETs. By contrast, NF-pNETs
generally remain asymptomatic before significant tumor compression.
Then, NF-pNETs may present with nonspecific abdominal pain, early
satiety or weight loss (32).
NF-pNETs are usually larger with less intense but more
heterogeneous enhancement (31).
However, these manifestations are not specific. The present study
described a heterogeneous tumor with progressive enhancement, which
was challenging to differentiate from NF-pNETs.
Pancreatic ductal adenocarcinoma is typically viewed
as a hypo-enhancing mass and the auxiliary signs include dilatation
of the biliary and pancreatic duct (double-duct sign),
peripancreatic vascular invasion and upstream parenchymal atrophy
(33). In the present study, the
contrast-enhanced CT showed progressive enhancement of the tumor
without dilation of biliary and pancreatic ducts or distal
parenchymal atrophy, further differentiating it from pancreatic
ductal adenocarcinoma.
Surgery is the gold standard for treatment of LPS
(12). All 10 patients reported in
the literature underwent surgical intervention, with the specific
surgical approach tailored to the tumor location. A key factor
influencing prognosis is the adequacy of surgical resection at the
time of primary presentation. Although chemotherapy is generally
considered to be resisted by WDLPS and DDLPS, it still serves an
essential role in treatment (34).
First-line therapies for advanced or unresectable DDLPS may include
single-agent anthracycline or anthracycline combined with
ifosfamide (35). Among the 10
patients, 3 (10,12,17)
experienced recurrence post-surgery, although 1 (12) had already received chemotherapy.
Regarding the present case, aggressive surgical resection was
considered to offer the best chance of cure. The patient showed no
recurrence post-surgery with no signs of relapse during the
12-month follow-up.
Regular follow-up is recommended for patients who
have undergone complete excision of pancreatic LPS, as local
recurrence of LPSs in other organs is common (34). According to previous studies, the
local recurrence rate of DDLPS is ~40%, with a metastatic rate of
15–30% (7) and the 5-year survival
rate is 49.4% (36).
The rarity of the present case lies in the
identification of pancreatic LPS without fat components.
Furthermore, the present study demonstrated the enhancement pattern
of this fat-deficient pancreatic LPS, which can serve as a
reference for the diagnosis of this uncommon tumor. However, a
limitation of the present study is the absence of an MRI
examination, which typically provides a clearer visualization of
lipomatous components. When lipomatous components are diminished in
DDLPS, MRI is superior to CT in detecting any residual fat within
the tumor (1,13).
In conclusion, pancreatic LPS is extremely rare. It
possesses a wide range of onset ages and a female predilection.
Imaging is pivotal in detecting and characterizing the tumor and
its surrounding structures. Pancreatic LPS may lack fat components
and exhibit progressive enhancement, which complicates the
differentiation from non-functional neuroendocrine tumors from
imaging. The tumor can invade adjacent vascular structures,
including the common hepatic artery, splenic vein and portal vein,
but excluding the bile and pancreatic ducts, likely because the
tumor did not originate from the epithelial cells of pancreatic
ducts. In brief, as both the clinical and imaging manifestations of
pancreatic liposarcoma are nonspecific, it is difficult to make a
definite diagnosis when fat components are lacking, and final
diagnosis hinges upon histological, immunohistochemistry and NGS
tests.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to the PACS system regulated by Sichuan
Provincial People's Hospital but may be requested from the
corresponding author.
Authors' contributions
TL conceived and designed the study, and contributed
to manuscript drafting. YZ and SH obtained medical images and
confirmed the authenticity of all the raw data. JY carried out the
high-throughput sequencing experiments and performed the
bioinformatics analysis. HS performed the histological examination
of the tumor, and contributed to writing the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Institutional Review Board of the Department of Radiology, Sichuan
Provincial People's Hospital, and the University of Electronic
Science and Technology of China (approval no. 2024-709; Chengdu,
China).
Patient consent for publication
Written and verbal consent was obtained from the
patient for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STS
|
soft tissue sarcoma
|
|
LPS
|
liposarcoma
|
|
DDLPS
|
dedifferentiated LPS
|
|
WDLPS
|
well-differentiated LPS
|
|
MLPS
|
myxoid/round cell LPS
|
|
PLPS
|
pleomorphic LPS
|
|
MDM2
|
murine double minute 2
|
|
NGS
|
next-generation sequencing
|
|
pNET
|
pancreatic neuroendocrine tumor
|
|
F-pNET
|
functional pNET
|
|
NF-pNET
|
non-functional pNET
|
References
|
1
|
Ardakani AHG, Woollard A, Ware H and Gikas
P: Soft tissue sarcoma: Recognizing a rare disease. Cleve Clin J
Med. 89:73–80. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bourcier K, Le Cesne A, Tselikas L, Adam
J, Mir O, Honore C and de Baere T: Basic knowledge in soft tissue
sarcoma. Cardiovasc Intervent Radiol. 42:1255–1261. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gamboa AC, Gronchi A and Cardona K:
Soft-tissue sarcoma in adults: An update on the current state of
histiotype-specific management in an era of personalized medicine.
CA Cancer J Clin. 70:200–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilbert NF, Cannon CP, Lin PP and Lewis
VO: Soft-tissue sarcoma. J Am Acad Orthop Surg. 17:40–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee ATJ, Thway K, Huang PH and Jones RL:
Clinical and molecular spectrum of liposarcoma. J Clin Oncol.
36:151–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sbaraglia M, Bellan E and Dei Tos AP: The
2020 WHO classification of soft tissue tumours: News and
perspectives. Pathologica. 113:70–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thway K: Well-differentiated liposarcoma
and dedifferentiated liposarcoma: An updated review. Semin Diagn
Pathol. 36:112–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dehner CA, Hagemann IS and Chrisinger JSA:
Retroperitoneal dedifferentiated liposarcoma. Am J Clin Pathol.
156:920–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kammerer-Jacquet SF, Thierry S, Cabillic
F, Lannes M, Burtin F, Henno S, Dugay F, Bouzillé G, Rioux-Leclercq
N, Belaud-Rotureau MA and Stock N: Differential diagnosis of
atypical lipomatous tumor/well-differentiated liposarcoma and
dedifferentiated liposarcoma: Utility of p16 in combination with
MDM2 and CDK4 immunohistochemistry. Hum Pathol. 59:34–40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanabe M, Matsui H, Higashi M, Tokumitsu
Y, Nagano H and Ito K: Pancreatic liposarcoma: A case report. Abdom
Radiol (NY). 47:1912–1916. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Fan WF, Li GC, Long J, Xu YH and Ma
G: Huge primary dedifferentiated pancreatic liposarcoma mimicking
carcinosarcoma in a young female: A case report. World J Clin
Cases. 7:1344–1350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han T, Luan Y, Xu Y, Yang X, Li J, Liu R,
Li Q and Zheng Z: Successful treatment of advanced pancreatic
liposarcoma with apatinib: A case report and literature review.
Cancer Biol Ther. 18:635–639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machado MCC, Fonseca GM, de Meirelles LR,
Zacchi FFS and Bezerra ROF: Primary liposarcoma of the pancreas: A
review illustrated by findings from a recent case. Pancreatology.
16:715–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matthews M, Nelson S, Hari D and French S:
Well differentiated liposarcoma, sclerosing type, of the pancreas a
case report. Exp Mol Pathol. 101:320–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dodo IM, Adamthwaite JA, Jain P, Roy A,
Guillou PJ and Menon KV: Successful outcome following resection of
a pancreatic liposarcoma with solitary metastasis. World J
Gastroenterol. 11:7684–7685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao D, Wang J and Guo L: Pancreatic
liposarcoma: A rare cause of pancreatic mass in adult. J
Gastroenterol Hepatol. 34:12752019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuramoto K, Hashimoto D, Abe S, Chikamoto
A, Beppu T, Iyama K and Baba H: Hepatobiliary and pancreatic: Large
pancreatic liposarcoma. J Gastroenterol Hepatol. 28:18002013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elliott TE, Albertazzi VJ and Danto LA:
Pancreatic liposarcoma. Case report with review of retroperitoneal
liposarcomas. Cancer. 45:1720–1723. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Improta L, Pasquali S, Iadecola S,
Barisella M, Fiore M, Radaelli S, Colombo C, Alloni R, Callegaro D,
Valeri S, et al: Organ infiltration and patient risk after
multivisceral surgery for primary retroperitoneal liposarcomas. Ann
Surg Oncol. 30:4500–4510. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tyler R, Wanigasooriya K, Taniere P,
Almond M, Ford S, Desai A and Beggs A: A review of retroperitoneal
liposarcoma genomics. Cancer Treat Rev. 86:1020132020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coindre JM, Pédeutour F and Aurias A:
Well-differentiated and dedifferentiated liposarcomas. Virchows
Arch. 456:167–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thway K, Flora R, Shah C, Olmos D and
Fisher C: Diagnostic utility of p16, CDK4, and MDM2 as an
immunohistochemical panel in distinguishing well-differentiated and
dedifferentiated liposarcomas from other adipocytic tumors. Am J
Surg Pathol. 36:462–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thway K, Wang J, Swansbury J, Min T and
Fisher C: Fluorescence in situ hybridization for MDM2 amplification
as a routine ancillary diagnostic tool for suspected
well-differentiated and dedifferentiated liposarcomas: Experience
at a tertiary center. Sarcoma. 2015:1–10. 2015. View Article : Google Scholar
|
|
24
|
Sirvent N, Coindre JM, Maire G, Hostein I,
Keslair F, Guillou L, Ranchere-Vince D, Terrier P and Pedeutour F:
Detection of MDM2-CDK4 amplification by fluorescence in situ
hybridization in 200 paraffin-embedded tumor samples: Utility in
diagnosing adipocytic lesions and comparison with
immunohistochemistry and real-time PCR. Am J Surg Pathol.
31:1476–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu L, Xie X, Shi X, Zhang P, Liu A, Wang J
and Zhang B: Potential application of genomic profiling for the
diagnosis and treatment of patients with sarcoma. Oncol Lett.
21:3532021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Win TT, Jaafar H and Yusuf Y: Relationship
of angiogenic and apoptotic activities in soft-tissue sarcoma.
South Asian J Cancer. 3:171–174. 2020.PubMed/NCBI
|
|
27
|
Park BK, Koh HD, Won SY, Cho YS, Seo JH,
An C and Park S: Suspicious findings observed retrospectively on CT
imaging performed before the diagnosis of pancreatic cancer. J
Gastrointest Oncol. 14:1008–1018. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He C, Zhu L, Wang X, Dai M, Wu H, Xu Q,
Sun Z, Liu J, Xue H and Jin Z: Presumed radiological diagnosis of
solid pseudopapillary tumors: Do we really know what we are
watching? Pancreatology. 23:120–128. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li DL, Li HS, Xu YK, Wang QS, Chen RY and
Zhou F: Solid pseudopapillary tumor of the pancreas: Clinical
features and imaging findings. Clin Imaging. 48:113–121. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo GC and Kambadakone A: MR imaging of
pancreatic neuroendocrine tumors. Magn Reson Imaging Clin N Am.
26:391–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wan Y, Hao H, Meng S, Li Z, Yu F, Chi M,
Chao Q and Gao J: Application of low dose pancreas perfusion CT
combined with enhancement scanning in diagnosis of pancreatic
neuroendocrine tumors. Pancreatology. 21:240–245. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perri G, Prakash LR and Katz MHG:
Pancreatic neuroendocrine tumors. Curr Opin Gastroenterol.
35:468–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Srisajjakul S, Prapaisilp P and
Bangchokdee S: CT and MR features that can help to differentiate
between focal chronic pancreatitis and pancreatic cancer. Radiol
Med. 125:356–364. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crago AM and Dickson MA: Liposarcoma:
Multimodality management and future targeted therapies. Surg Oncol
Clin N Am. 25:761–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gahvari Z and Parkes A: Dedifferentiated
liposarcoma: Systemic therapy options. Curr Treat Options Oncol.
21:152020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amer KM, Congiusta DV, Thomson JE, Elsamna
S, Chaudhry I, Bozzo A, Amer R, Siracuse B, Ghert M and Beebe KS:
Epidemiology and survival of liposarcoma and its subtypes: A dual
database analysis. J Clin Orthop Trauma. 11:S479–S484. 2020.
View Article : Google Scholar : PubMed/NCBI
|