Introduction
Primary squamous cell carcinoma of the liver (PSCCL)
is an exceedingly rare malignancy, with only ~30 cases reported
worldwide according to the available literature (1). Unlike hepatocellular carcinoma (HCC),
PSCCL lacks specific laboratory tests and imaging characteristics,
making diagnosis challenging. It is important to exclude metastatic
SCC from other sites in the differential diagnosis, and
histopathological examination remains the gold standard for
diagnosis (2).
PSCCL occurs in the liver, which lacks squamous
epithelium, and its pathogenesis is not fully understood. Research
suggests that chronic inflammation and liver injury (e.g., chronic
cholangitis, congenital biliary cysts, hepatic cysts, infections
and stones) may be primary causes (3). Histopathologically, PSCCL features
keratin pearls, polygonal cancer cells with abundant cytoplasm and
prominent nucleoli, intercellular bridges, and positive cytokeratin
(CK)5/6 and tumor protein p63 (p63) staining. These features help
distinguish PSCCL from other liver tumors and exclude metastatic
SCCs. The present case is unique as the patient received an
innovative treatment combining envafolimab, albumin-paclitaxel and
cisplatin, achieving sustained remission over 18 months. This
provides new insights for PSCCL diagnosis and treatment.
Case report
A 72-year-old man was admitted to Shaanxi Provincial
Cancer Hospital (Xi'an, China) in July 2023 with a diagnosis of a
hepatic space-occupying lesion. The patient had previously
experienced pain in the liver region and underwent an upper
abdominal computed tomography (CT) scan at a local hospital. The CT
scan revealed an intrahepatic lamellar shadow of slightly reduced
density, indicative of a space-occupying lesion. Slight dilatation
of the common bile duct was also observed, along with a left renal
cyst. Tumor marker evaluations performed at Shaanxi Provincial
Cancer Hospital showed significantly elevated levels of
carcinoembryonic antigen (CEA) at 40.10 ng/ml (reference range,
0–5.5 ng/ml), carbohydrate antigen 199 (CA19-9) at 335.2 IU/ml
(reference range, 0–28 IU/ml) and ferritin at 1,184.10 ng/ml
(reference range, 25–350 ng/ml). The patient also reported recent
weight loss of ~5 kg over a period of ~10 days. The patient had a
history of gallstones diagnosed 10 years prior and underwent a
cholecystectomy in 2019.
On admission in July 2023, the Eastern Cooperative
Oncology Group (ECOG; http://ecog-acrin.org/resources/ecog-performance-status/)
performance status score was 1 and the Numerical Rating Scale (NRS;
with 0 indicating no pain and 10 indicating the most severe pain)
score for pain was 1. A physical examination revealed an enlarged
liver palpable in the right upper abdomen, with positive percussion
tenderness. Laboratory findings upon admission showed elevated
tumor markers, including CEA at 40.10 ng/ml, CA-50 at 223.5 IU/ml
(reference value, 0–20 IU/ml), CA19-9 at 335.2 IU/ml and CA24-2 at
>200 IU/ml (reference value, 0–20 IU/ml). Liver function tests
showed decreased albumin at 36.9 g/l (reference value, 40–55 g/l)
and elevated aspartate transferase (AST) at 73 U/l (reference
value, 7–50 U/l).
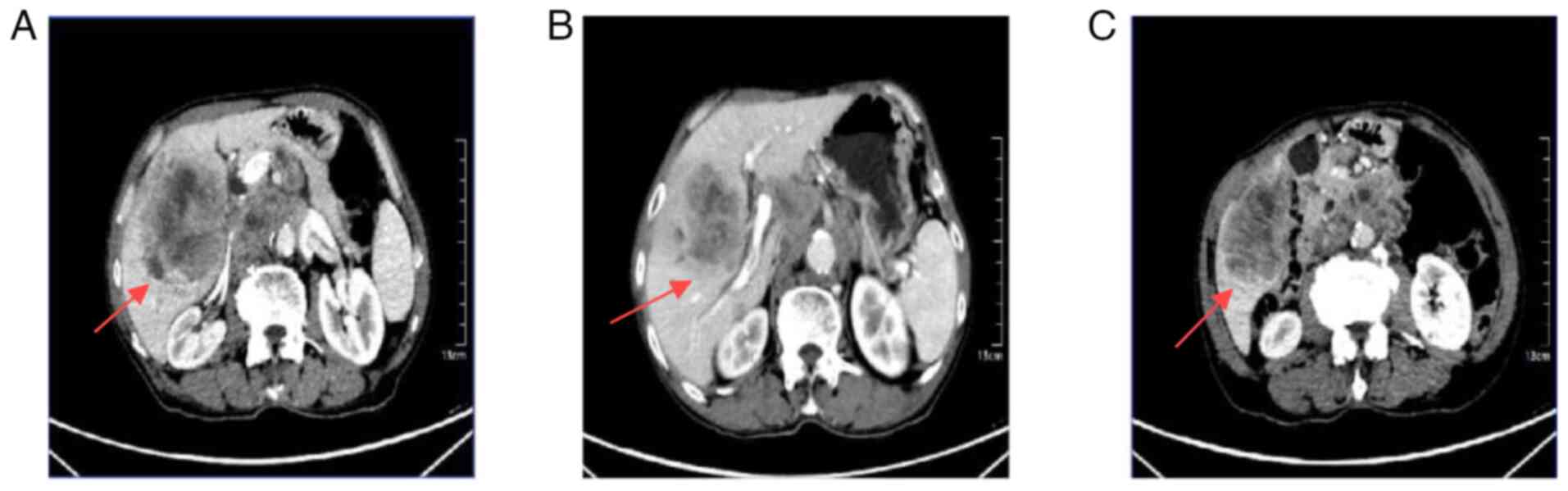
CT imaging showed multiple low-density nodules and
masses beneath the capsule of the right hepatic lobe and within the
right lobe of the liver, raising suspicion for potential
hepatocellular carcinoma or intrahepatic cholangiocarcinoma with
subperitoneal and intrahepatic multiple metastases (Fig. 1A). Thrombus formation was noted in
the lumen of the right branch of the portal vein (Fig. 1B). The gallbladder was not clearly
visualized. The kidneys showed multiple cysts. Multiple enlarged
lymph nodes were identified in the posterior mediastinal paraspinal
region, hepatic hilum and retroperitoneum, some of which were
partially fused, suggestive of metastatic disease (Fig. 1C).
A liver biopsy was performed 3 days after admission.
Histopathological examination revealed an invasive, poorly
differentiated carcinoma of the liver, with histopathological
features suggestive of a poorly differentiated SCC (Fig. 2A and B). Immunohistochemical results
showed Ki-67 positivity in 80% of cells (Fig. 2C), a lack of synaptophysin
expression (Fig. 2D), partial
positivity for p63 (Fig. 2E), p40
(Fig. 2F), partial positivity for
CK5/6 (Fig. 2G) and positivity for
pan cytokeratin (CKpan) (Fig. 2H).
Other positive immunohistochemical markers included CK7 (Fig. 2I), CK20 (Fig. 2J), villin (Fig. 2K), hepatocyte paraffin 1 (Fig. 2L), glypican-3 (Fig. 2M), CK19, GATA binding protein 3,
caudal type homeobox transcription factor 2, uroplakin III, nuclear
protein in testis, thyroid transcription factor 1, SWI/SNF related
matrix-associated actin dependent regulator of chromatin subfamily
a member 4/Brahma-related gene 1, and INI1 protein (data not
shown). Programmed death-ligand 1 (PD-L1) expression detected using
the 22C3 antibody showed a combined positive score (CPS) of 5
[(number of PD-L1-positive tumor cells + number of PD-L1-positive
immune cells)/total number of tumor cells ×100; Fig. 2N and O].
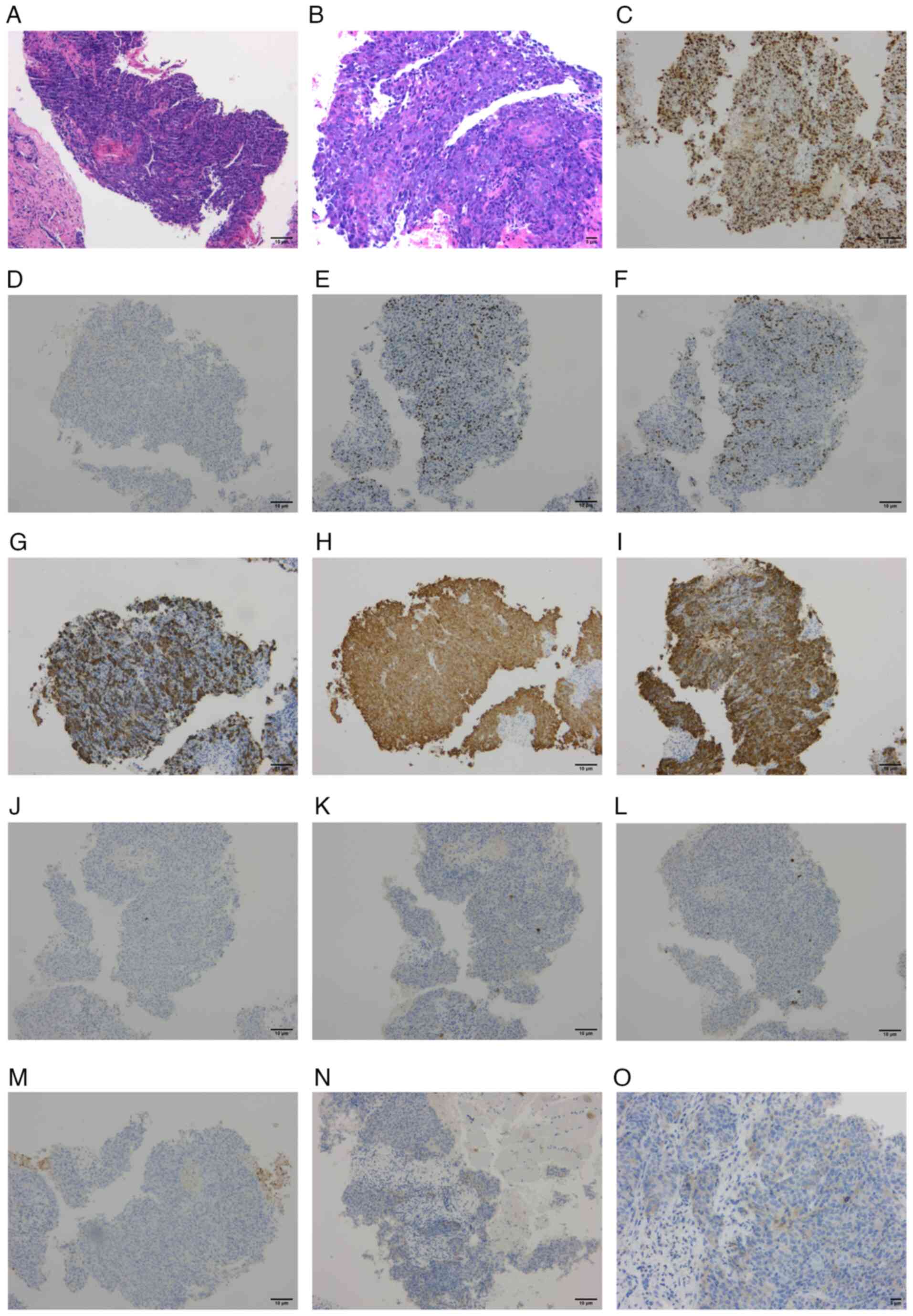 | Figure 2.Histopathological and
immunohistochemical results indicating squamous cell carcinoma. (A)
H&E staining, ×100 magnification. (B) H&E staining, ×200
magnification. (C-M) Immunohistochemical staining at ×100
magnification for (C) Ki-67, (D) synaptophysin, (E) tumor protein
p63, (F) tumor protein p40, (G) CK5/6, (H) pan CK, (I) CK7, (J)
CK20, (K) villin, (L) hepatocyte paraffin 1 and (M) glypican-3. (N
and O) Immunohistochemical staining for programmed death-ligand 1
at (N) ×100 magnification and (O) ×200 magnification. H&E,
hematoxylin and eosin; CK, cytokeratin. |
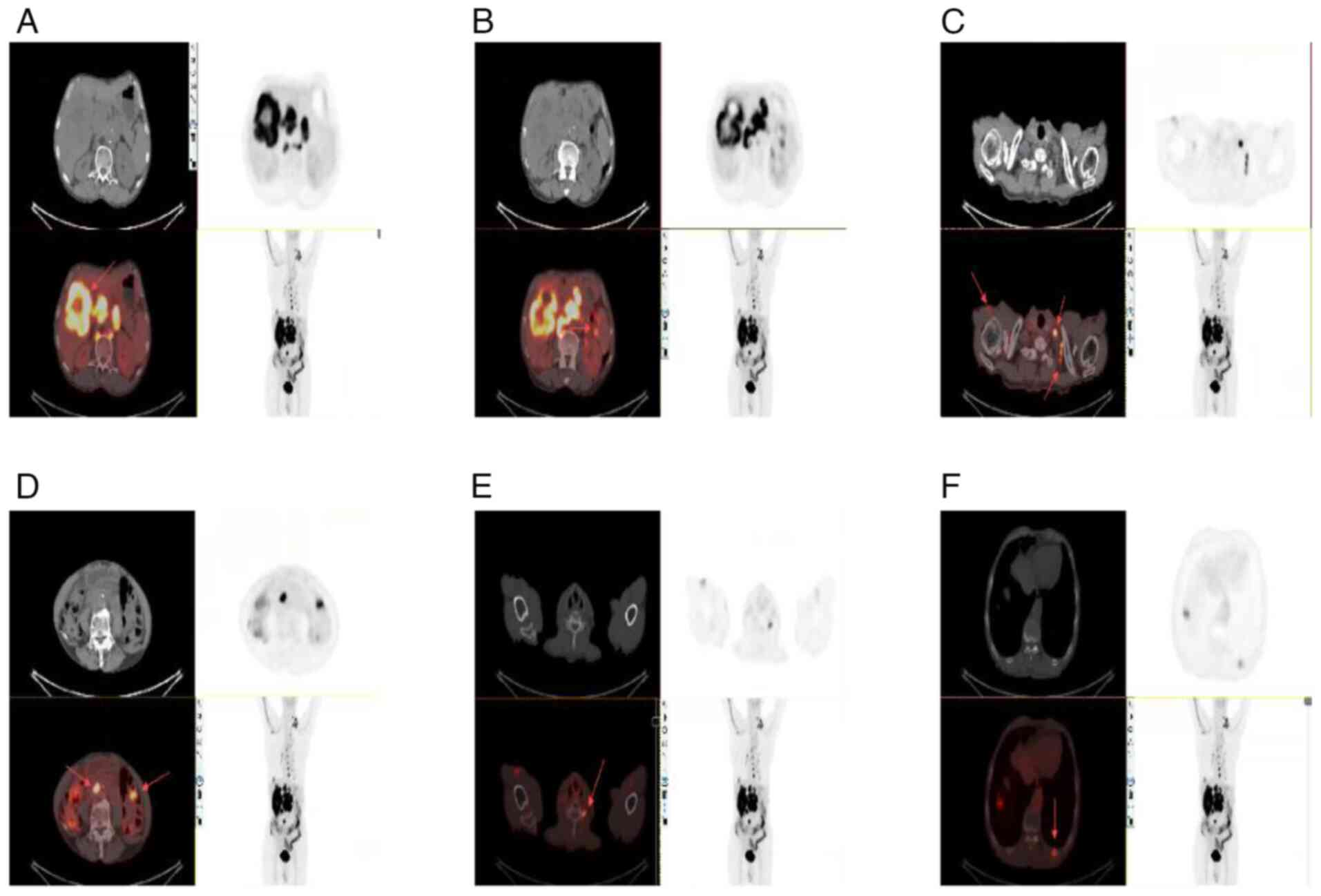
Given the rarity of hepatic SCC, a positron emission
tomography (PET)/CT scan was performed to exclude the possibility
of metastases from other primary sites. The PET/CT findings were as
follows: An irregular mass was observed in the right lobe of the
liver (Fig. 3A). Multiple
low-density nodules were identified within the liver, as well as
multiple enlarged lymph nodes in the hepatic hilum, posterior to
both diaphragmatic crura, around the abdominal aorta in the
retroperitoneum, and at the root of the mesentery (Fig. 3B). Additional enlarged lymph nodes
were noted in the upper abdominal peritoneal area, posterior
mediastinum, around the esophagus, the right axillary area
(Fig. 3C) and the left clavicular
area (Fig. 3D). Bone destruction
was observed in the left transverse process of the sixth cervical
vertebra (Fig. 3E), and in the left
10th and 11th posterior ribs (Fig.
3F), suggestive of metastases.
The increased glucose metabolism observed in these
regions suggested a primary malignant hepatic lesion, possibly
intrahepatic cholangiocarcinoma, with intrahepatic metastasis,
multiple lymph node metastases and bone metastases. No other
primary tumors were detected. Based on the pathology and imaging
findings, a final diagnosis of PSCCL was made.
After multidisciplinary discussion, the patient was
treated with envafolimab (200 mg subcutaneously once weekly) in
combination with albumin-paclitaxel (200 mg on day 1 and 100 mg on
day 5) plus cisplatin (30 mg on days 1–3). After two cycles (each
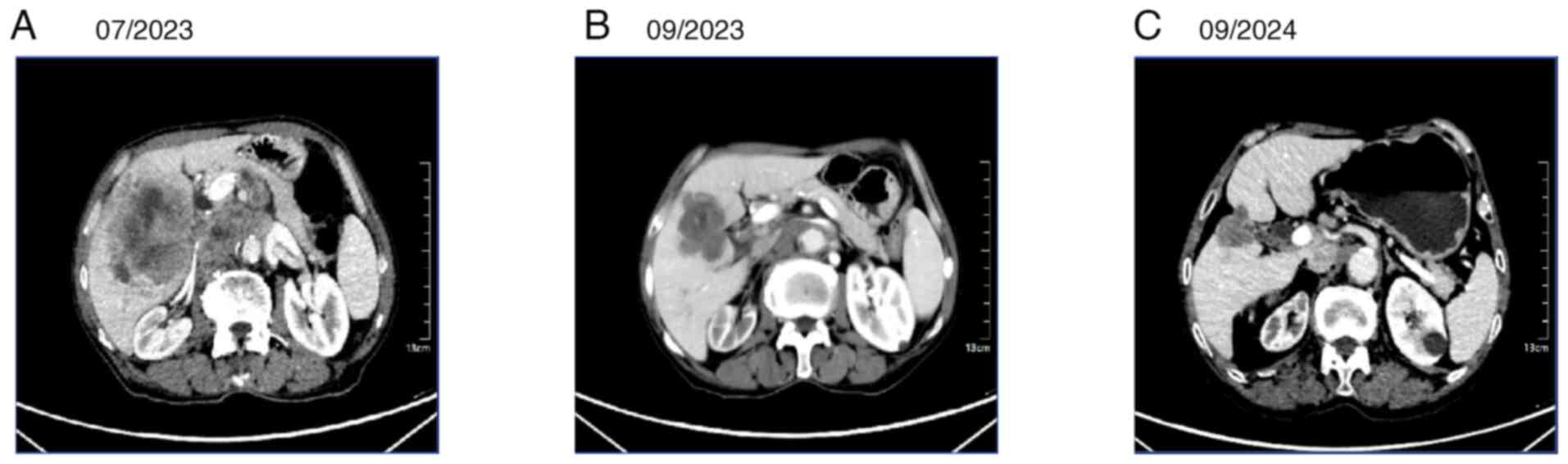
cycle lasting 21 days), the efficacy evaluation indicated a partial
response (PR) (Fig. 4A and B),
which was sustained on subsequent evaluation (Fig. 4C). The tumor, initially shown on CT
in July 2023 as multiple low-density nodules and a mass in the
subcapsular region of the right lobe of the liver, with the largest
measuring ~8.2×7.8 cm, had shrunk to 5.4×5.1 cm upon re-examination
in September 2023, and further reduced to 3.3×3.0 cm when reviewed
at Shaanxi Provincial Cancer Hospital in 2024. Following treatment,
the patient's pain was significantly alleviated, physical strength
was gradually recovered and mental status was improved. The various
symptoms subsided, indicating a marked treatment effect. The
multiple metastases were effectively controlled, the tumors
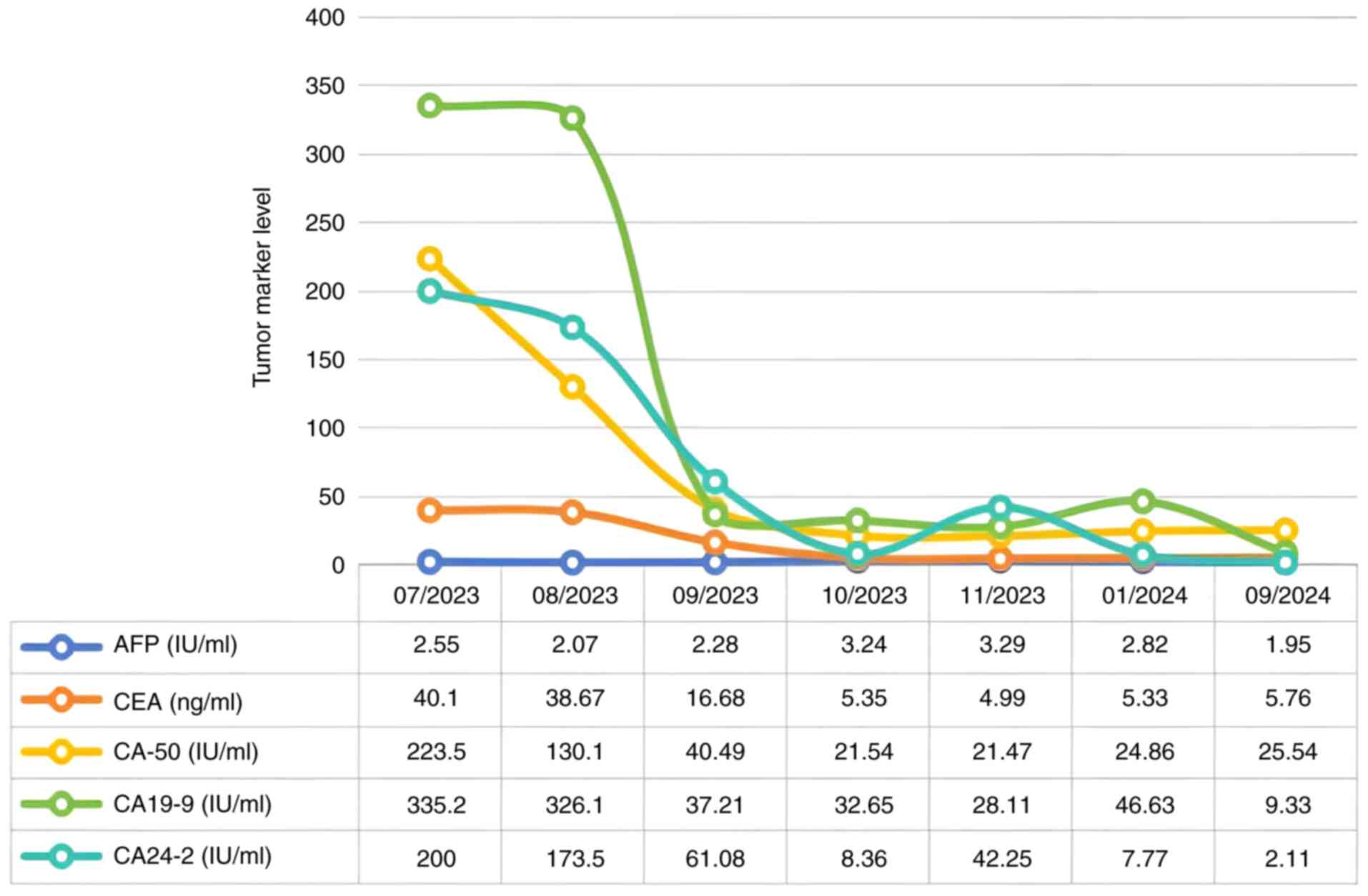
significantly reduced in size, the tumor marker levels continued to
decline (Fig. 5) and the patient's
condition tended to be stable. With the improvement of physical
condition, the patient's quality of life was also greatly enhanced,
with normal mobility and diet. Telephone follow-up continued until
December 2024 (a total of 18 months), during which time the patient
maintained a sustained PR. Tumor marker levels continue to
fall.
Tissue staining methods
Tissues were fixed in 10% neutral formalin for 6–24
h. The tissues were sectioned to a 3- to 4-µm thickness. For
hematoxylin and eosin staining, hematoxylin was added for 3–5 min,
followed by eosin for 2–10 sec, all at room temperature. Staining
was evaluated using a light microscope. For immunohistochemical
analysis, the EnVision two-step method was used. The primary and
secondary antibodies used were ready-to-use antibodies, all
purchased from Fuzhou Maixin Biotechnology Development Co., Ltd.
The PD-L1 (clone 22C3) antibody was also ready-to-use and purchase
from Dako; Agilent Technologies, Inc. All staining was performed
using an automated immunohistochemical staining machine and
performed according to the manufacturer's instructions.
Discussion
PSCCL is an extremely rare malignant tumor, with
only ~30 cases reported in the literature worldwide. Squamous
epithelial tissue is commonly found in areas such as the esophagus,
trachea, pharynx, skin and vulva. Since the liver lacks squamous
epithelial tissue, metastasis from other SCCs must be excluded when
diagnosing PSCCL. The etiology of PSCCL remains unclear, with
potential associations suggested with solitary non-parasitic liver
cysts, developmental liver cysts (4–6),
intrahepatic bile duct stones (7),
gallstones, chronic cholangitis, hepatic teratoma, liver cirrhosis
(8), and other related
diseases.
Several theories have been proposed regarding the
pathogenesis of PSCCL. Chronic inflammation from conditions such as
chronic cholangitis, congenital biliary cysts or hepatic cysts
combined with infections and stones are considered the main
etiological factors. Hepatic pluripotent stem cells can be
transformed into cancerous tissue containing squamous cells,
hepatocytes and biliary epithelial cells in response to various
oncogenic factors, eventually developing into SCC. The development
of most SCCs is considered to be associated with squamous
metaplasia and progressive carcinoma of the epithelial cells of the
biliary tract or cyst wall stimulated by chronic inflammation,
leading to malignant transformation (9).
Clinically, PSCCL lacks specific symptoms and
laboratory markers, making it difficult to distinguish from other
hepatic malignancies. Liver function tests may show abnormalities
similar to those seen in HCC, such as elevated alanine
aminotransferase (10), AST
(11) and bilirubin (12) levels. Tumor markers such as
α-fetoprotein (13,14), SCC antigen and CA19-9 (15,16)
may be elevated, but are not specific to PSCCL. SCC antigen has
diagnostic value in SCC, but is mainly used for the diagnosis and
monitoring of lung (17), head and
neck (18), and cervical (19) cancer. To date, there remains a lack
of specific serum markers for diagnosing PSCCL. In the present
case, laboratory tests demonstrated significant elevations of CEA
and CA19-9, consistent with the characteristics of gastrointestinal
tract tumors, but other indices were normal.
CT imaging of PSCCL typically shows mild hypodense
shadows, occasionally with cystic components. Persistent
enhancement is observed in both the portal and delayed phases.
Enhanced imaging demonstrates enhanced lesion margins in the
arterial phase with lobulated features. Mild enhancement is
observed in the center of the lesion, with persistent enhancement
in the delayed phase (20). A CT
scan alone is insufficient to diagnose PSCCL. For example, in some
patients, no discernible hepatic mass is exhibited on CT, and only
hepatic cysts or intrahepatic stones are observed, but the
postoperative pathology indicates SCC. In the present case, the
initial CT of the patient suggested a hepatic space-occupying
lesion. To the best of our knowledge, limited literature exists on
the use of PET/CT in diagnosing PSCCL. The PET/CT images of the
present patient showed an irregular mass in the right lobe of the
liver, multiple intrahepatic hypodense nodules, multiple lymph node
metastases and bone metastases, helping to exclude metastasis from
other systems. Based on these findings, a final diagnosis of PSCCL
was made.
Histopathological examination remains the gold
standard for diagnosing PSCCL. Key pathological features include
keratinized cell clusters forming keratin pearls, polygonal cancer
cells with abundant cytoplasm and prominent nucleoli, evident
intercellular bridges, and positive immunohistochemical staining
for markers such as CK5/6 and p63. Metastasis from other SCCs must
be rigorously excluded (21).
In the present case, histopathological examination
of the biopsy tissue showed cancer cells arranged in sheets forming
nests, with polygonal cells, abundant cytoplasm, large nuclei,
nuclear pleomorphism, prominent nucleoli and numerous mitotic
figures. Keratinization was observed in the center of the nests,
arranged concentrically to form cancer beads. The tumor parenchyma
and stroma were clearly demarcated. Immunohistochemistry showed
positive results for CK5/6, CKpan and p63. No lesions were seen on
chest CT, leading to a final diagnosis of PSCCL.
Treatment options for PSCCL include surgery,
chemotherapy, radiotherapy and immunotherapy. Okuda et al
(22) reported that early stage
PSCCL can be surgically resected with no recurrence for >1.5
years postoperatively. Zhang et al (23) reported that among 19 surgically
resected cases, 8 survived for >12 months, while 11 died within
a year. Within this study, 1 patient experienced tumor recurrence
and died from metastatic disease 18 months after radical surgery
(23). Surgical resection is the
mainstay for early stage disease and can result in prolonged
survival times. However, most patients are diagnosed at advanced
stages when surgery is not feasible. Lee et al reported that
patients who refused surgery and were treated with chemotherapy
using carboplatin combined with 5-fluorouracil had an overall
survival time of >8 months (24). Regarding radiotherapy, it was
reported that patients with PSCCL who were physically unable to
undergo chemotherapy were treated with local radiotherapy and died
1 month after hospital discharge (25). Immunotherapy has shown efficacy in
esophageal (26), lung (27), head and neck (28), and skin (29) SCC.
At the time of presentation, the current patient had
multiple metastases and an advanced malignant tumor, making
surgical resection impossible. Since basic research has found that
SCCs are immunogenic, immune checkpoint inhibitors have become the
standard first-line treatment option for these tumor types. The
patient also had SCC, but PSCCL is a rare tumor, and there is a
lack of large-scale clinical research data for reference.
Therefore, the treatment selection was mainly based on the
histopathological type and referencing of other tumors, using
immune checkpoint inhibitors combined with paclitaxel and platinum
drugs commonly used in SCC (30–34).
Other studies suggest that immunotherapy combined with chemotherapy
can improve progression-free survival and overall survival (OS);
for example, gemcitabine combined with cisplatin and doxorubicin
extended the OS time from 11.5 to 12.8 months, showing a
statistically significant difference compared with conventional
treatment (chemotherapy, radiotherapy and surgery) (35). After considering the current
patient's age, physical condition and potential adverse effects,
and after communicating with the patient's family, the safer
(milder and more manageable side effects) PD-L1 inhibitor
envafolimab (36) combined with
albumin-bound paclitaxel plus cisplatin regimen was selected for
treatment.
The final regimen was envafolimab (200 mg
subcutaneously once a week) combined with albumin-paclitaxel (200
mg on day 1 and 100 mg on day 5) plus cisplatin (30 mg on days
1–3). Albumin-bound paclitaxel promotes the polymerization of
tubulin, inhibits mitosis of tumor cells and leads to cell
apoptosis. Cisplatin binds to DNA, interfering with replication and
transcription, exerting cytotoxic effects. The combination enhances
the antitumor effect and improves the objective response rate and
disease control rate. Envafolimab is a monoclonal antibody
targeting PD-L1; it binds to human PD-L1 protein, blocking its
interaction with the receptor programmed cell death protein 1
(PD-1). This mechanism can relieve the suppression of T cells by
tumors through the PD-1/PD-L1 pathway, mobilize the antitumor
activity of the immune system and thereby kill tumor cells
(37,38). During two treatment cycles, efficacy
was assessed as a PR. Follow-up to December 2024 (a total of 18
months) showed a sustained PR.
It has been demonstrated that patients with
digestive system tumors exhibiting one or more of the following
characteristics respond better to immunotherapy: Positive PD-L1
expression, high microsatellite instability (MSI-H) and defective
mismatch repair (dMMR) (39). These
patients are expected to achieve longer survival times with
immunotherapy. The present patient had a CPS of 5, which may be
advantageous in immunotherapy. If financial conditions permit,
comprehensive genome sequencing of tumor samples using genetic
testing and next-generation sequencing (NGS) can identify specific
mutations associated with the tumor, aiding in the early diagnosis
of rare tumors and identifying patients with high tumor mutational
burden (TMB-H) (40) who will
benefit from immunotherapy. Therefore, some scholars have proposed
that rare tumors should undergo NGS testing (41). For example, data from a domestic
phase II pivotal clinical trial demonstrated that envafolimab had
favorable therapeutic efficacy in patients with MSI-H/dMMR advanced
solid tumors (42). Clinical trials
investigating the efficacy of immunotherapy in patients with
advanced solid tumors and TMB-H have demonstrated that patients
with TMB-H (TMB ≥20 mutations/Mb) may derive greater benefit from
this approach, compared with those with TMB-L (43,44).
In conclusion, PSCCL is an extremely rare malignant
tumor of the liver with an unclear pathogenesis. The majority of
patients have a poor prognosis, often with survival times <1
year, typically ranging from 4 to 6 months (45). The clinical symptoms and laboratory
tests for PSCCL lack specificity, and imaging examinations such as
CT help in the preliminary diagnosis. However, a definitive
diagnosis relies on histopathology and immunohistochemistry, making
PSCCL challenging to diagnose. Additionally, clinicians often lack
sufficient awareness of the disease, leading to late diagnoses and
a lack of treatment guidelines, further complicating prognosis.
Currently, the main treatment modality for PSCCL is surgery,
supplemented by radiotherapy and immunotherapy. However, given the
considerable variability in the treatment approaches among
patients, there is an urgent need to further explore and optimize
therapeutic strategies for PSCCL, including chemo-immunotherapy and
immunotherapy alone. If financial conditions allow, genetic testing
and NGS can provide more precise guidance for the treatment of
PSCCL, offering additional information for the selection of
clinical drugs.
Acknowledgements
Not applicable.
Funding
This study was supported by the Wu Jieping Medical Foundation
Clinical Research Special Grant Fund (Project Name: Application of
T-Cell Subsets Combined with Dynamic Monitoring of Blood ctDNA in
Predicting the Efficacy of Immunotherapy in Advanced NSCLC
Patients;grant no. 320.6750.2023-17-23) and the Xi'an Science and
Technology Program (Construction of a Prediction Model for
Immunotherapy in Advanced NSCLC Based on ctDNA and Peripheral Blood
T-cell Subsets; grant no. 2024JH-YLYB-0176).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JJ was responsible for manuscript writing, data
organization and data analysis. SL, JB, JM, and ZZ provided
critical writing guidance and made revisions to the manuscript.
Contributions included input on the study design, data analysis and
interpretation, and ensuring the accuracy and integrity of the
content. GD and JH were responsible for data collection and
analysis, and the provision of medical images (PET/CT and CT
scans). HG was responsible for immunohistochemical image analysis,
data extraction and interpretation, result verification, anomaly
investigation and manuscript revision. ZZ was responsible for the
final review, overall supervision and funding acquisition. All
authors have read and approved the final manuscript. SL and ZZ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in accordance with ethical
standards.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao J, Ma L, Li J, Yin B, Liang J and
Wang J: Primary squamous cell carcinoma of the liver is rare but
hostile: Case series and comprehensive review of the literature.
Cancer Manag Res. 13:829–837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giorgio A, De Luca M, Gatti P, Matteucci P
and Giorgio V: CEUS LI-RADS categories to distinguish
hepatocellular carcinoma and non-hepatocellular carcinoma
malignancies. Radiology. 296:E121–E122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang LM, Yu DP, Zheng Y and Zhou YH:
Primary squamous cell carcinoma of the liver: A case report. World
J Clin Cases. 10:6744–6749. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsieh CB, Chen CJ, Yu JC, Chang TM, Gao HW
and Liu YC: Primary squamous cell carcinoma of the liver arising
from a complex liver cyst: Report of a case. Surg Today.
35:328–331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieweg O, Slooff MJ and Grond J: A case of
primary squamous cell carcinoma of the liver arising in a solitary
cyst. HPB Surg. 5:203–208. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson JM, Groeschl R, George B, Turaga
KK, Patel PJ, Saeian K and Gamblin TC: Ciliated hepatic cyst
leading to squamous cell carcinoma of the liver-a case report and
review of the literature. Int J Surg Case Rep. 4:972–975. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu KL, Li DY and Jiang CB: Primary
squamous cell carcinoma of the liver associated with
hepatolithiasis: A case report. World J Gastroenterol.
18:5830–5832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arase Y, Endo Y, Hara M, Kumada H, Ikeda K
and Yoshiba A: Hepatic squamous cell carcinoma with hypercalcemia
in liver cirrhosis. Acta Pathol Jpn. 38:643–650. 1988.PubMed/NCBI
|
|
9
|
Shi G, Ye X, Yang F, Wang Z and Ma X:
Hepatic squamous cell carcinoma initially presenting as
cholecystitis misdiagnosed as cholangiocarcinoma: A case report.
Oncol Lett. 29:32024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du Y, Du B, Fang X, Shu M, Zhang Y, Chung
H, Sun Y, Teng J, Visalath P, Qiu H and Cai W: ALT flare predicts
hepatocellular carcinoma among antiviral treated patients with
chronic hepatitis B: A cross-country cohort study. Front Oncol.
10:6152032021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carr BI, Bag HG, Ince V, Akbulut S, Ersan
V, Usta S, Isik B, Ogut Z, Tuncer A and Yilmaz S: A combination of
blood lymphocytes and AST levels distinguishes patients with small
hepatocellular carcinomas from non-cancer patients. J Gastrointest
Cancer. 52:1211–1216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toyoda H and Johnson PJ: The ALBI score:
From liver function in patients with HCC to a general measure of
liver function. JHEP Rep. 4:1005572022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazza S, Frigerio C, Alfieri D, Mauro A,
Torello Viera F, Scalvini D, Barteselli C, Sgarlata C, Veronese L,
Bardone M, et al: Prognostic role of basal serum alpha-fetoprotein
in patients with hepatocellular carcinoma suitable for curative
treatment. Medicina (Kaunas). 60:6922024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo P, Wu S, Yu Y, Ming X, Li S, Zuo X and
Tu J: Current status and perspective biomarkers in AFP negative
HCC: Towards screening for and diagnosing hepatocellular carcinoma
at an earlier stage. Pathol Oncol Res. 26:599–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng P, Li H, Chen Y, Pei H and Zhang L:
Serum CA199 levels are significantly increased in patients
suffering from liver, lung, and other diseases. Prog Mol Biol
Transl Sci. 162:253–264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong Y, Jing Y, Sun H and Zhou S: The
diagnostic value of contrast-enhanced ultrasound and enhanced CT
combined with tumor markers AFP and CA199 in liver cancer. J
Healthc Eng. 2022:50745712022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu LH, Chen L, Wang QY and Wang YT:
Correlation between HRCT signs and levels of CA125, SCCA, and NSE
for different pathological types of lung cancer. Eur Rev Med
Pharmacol Sci. 27:4162–4168. 2023.PubMed/NCBI
|
|
18
|
Schepens EJA, Al-Mamgani A, Karssemakers
LHE, van den Broek D, van den Brekel MWM and Lopez-Yurda M:
Squamous cell carcinoma antigen in the follow-up of patients with
head and neck cancer. Otolaryngol Head Neck Surg. 170:422–430.
2024. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tony V, Sathyamurthy A, Ramireddy JK,
Iswarya SJ, Gowri SM, Thomas A, Peedicayil A and Ram TS: Role of
squamous cell carcinoma antigen in prognostication, monitoring of
treatment response, and surveillance of locally advanced cervical
carcinoma. J Cancer Res Ther. 19:1236–1240. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song Y, Shi J, Zhang X, Qiao M, Sun Z and
Tian S: Diagnostic value of imaging modalities in primary squamous
cell carcinoma of the liver. J Clin Ultrasound. 51:887–897. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao R, Zhu K, Wang R, Gao J, Cui K, Yu F,
Zhang B and Li S: Primary squamous cell carcinoma of the liver: A
case report and review of the literature. Oncol Lett. 4:1163–1166.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okuda Y, Abe T, Ikeda M, Kurihara K,
Shimizu A, Oshita A, Yonehara S and Hanada K: Curative surgery for
primary squamous cell carcinoma of the liver: A rare case study.
Clin J Gastroenterol. 16:263–269. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XF, Du ZQ, Liu XM and Lv Y: Primary
squamous cell carcinoma of liver: Case series and review of
literatures. Medicine (Baltimore). 94:e8682015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HL, Fu CK, Chien LY and Chen LM:
Primary squamous cell carcinoma of the liver with good response to
carboplatin and 5-flurouracil: A case report. Medicina (Kaunas).
58:18642022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoo TK, Kim BI, Han EN, Kim DH, Yoo JH,
Lee SJ, Cho YK and Kim HJ: Primary squamous cell carcinoma of the
liver: A case report. Clin Mol Hepatol. 22:177–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei DD, Fang JM, Wang HZ, Chen J, Kong S,
Jiang YY and Jiang Y: Perioperative immunotherapy for esophageal
squamous cell carcinoma. Front Immunol. 15:13307852024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desai A and Peters S: Immunotherapy-based
combinations in metastatic NSCLC. Cancer Treat Rev. 116:1025452023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daste A, Larroquette M, Gibson N, Lasserre
M and Domblides C: Immunotherapy for head and neck squamous cell
carcinoma: Current status and perspectives. Immunotherapy.
16:187–197. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmults CD, Blitzblau R, Aasi SZ, Alam M,
Andersen JS, Baumann BC, Bordeaux J, Chen PL, Chin R, Contreras CM,
et al: NCCN guidelines® insights: Squamous cell skin
cancer, version 1.2022. J Natl Compr Canc Netw. 19:1382–1394. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang Q, Xu P, Cao F, Wu D and Liu X: PD-1
Inhibitors combined with paclitaxel (Albumin-bound) and cisplatin
for larynx preservation in locally advanced laryngeal and
hypopharyngeal squamous cell carcinoma: A retrospective study.
Cancer Immunol Immunother. 72:4161–4168. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adkins D, Ley J, Atiq O, Powell S, Spanos
WC, Gitau M, Rigden C, Palka K, Liu J and Oppelt P: Nanoparticle
albumin-bound paclitaxel with cetuximab and carboplatin as
first-line therapy for recurrent or metastatic head and neck
cancer: A single-arm, multicenter, phase 2 trial. Oral Oncol.
115:1051732021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu H, Wang W, Yin J, Song C, Li L and Sun
Z: Efficacy and safety of the PD-1 inhibitor combined with
albumin-bound paclitaxel and nedaplatin in preoperative neoadjuvant
therapy of unresectable stage III lung squamous cell carcinoma.
Drug Des Devel Ther. 16:4269–4277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng J, Luo G, Yu Y, Ning K and Liu X:
Retrospective assessment of neoadjuvant camrelizumab combined with
induction chemotherapy: Efficacy in laryngeal preservation for
advanced hypopharyngeal and laryngeal squamous cell carcinoma.
Cancer Immunol Immunother. 73:542024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Black CM, Zheng D, Hair GM, Ai L, Wang L,
Goto D, Lerman N, Bidadi B and Hanna GJ: Real-world use of
first-line pembrolizumab + platinum + taxane combination regimens
in recurrent/metastatic head and neck squamous cell carcinoma.
Front Oncol. 14:13480452024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Araki T, Muranushi R, Takagi K, Tanaka H,
Shibuya K, Ando T, Yoshioka I, Hirabayashi K, Yasuda I and Fujii T:
A case of successful conversion surgery for unresectable
gallbladder cancer treated with durvalumab in combination with
gemcitabine plus cisplatin. Clin J Gastroenterol. 18:161–168. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Markham A: Envafolimab: First approval.
Drugs. 82:235–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qian Y, Tang L, Yao J, Zhu Y, Zhang Y, Lu
H, Li W, An C and Gui L: Pembrolizumab with chemotherapy for
patients with recurrent or metastatic nasal cavity and paranasal
sinus squamous cell carcinoma: A prospective phase ll study. Clin
Cancer Res. Feb 24–2025.(Epub ahead of print). View Article : Google Scholar
|
|
38
|
Yang Y, Luo X, Dai L, He T, Luo S, Zhou Y,
Wang H, Yan Z, Wang Q and Jin X: A case report of envafolimab in
the treatment of microsatellite stable (MSS) metastatic colon
cancer. Onco Targets Ther. 17:1137–1144. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhamidipati D and Subbiah V:
Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends
Cancer. 9:828–839. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoon HH, Jin Z, Kour O, Kankeu Fonkoua LA,
Shitara K, Gibson MK, Prokop LJ, Moehler M, Kang YK, Shi Q and
Ajani JA: Association of PD-L1 expression and other variables with
benefit from immune checkpoint inhibition in advanced
gastroesophageal cancer: Systematic review and meta-analysis of 17
phase 3 randomized clinical trials. JAMA Oncol. 8:1456–1465. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bishop JA, Nakaguro M, Weinreb I,
Palsgrove D, Rooper LM, Vandergriff TW, Carlile B, Sorelle JA,
Gagan J and Nagao T: Comprehensive next generation sequencing
reveals that purported primary squamous cell carcinomas of the
parotid gland are genetically heterogeneous. Head Neck Pathol.
18:1062024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen M, Jiang M, Wang X, Shen L and Li J:
Envafolimab-first PD-1/PD-L1 antibody to be administered by
subcutaneous injection for microsatellite instability-high or
deficient mismatch repair advanced solid tumors. Expert Opin Biol
Ther. 22:1227–1232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmed J, Das B, Shin S and Chen A:
Challenges and future directions in the management of tumor
mutational burden-high (TMB-H) advanced solid malignancies. Cancers
(Basel). 15:58412023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Altomare NJ, Li Y, Neill C, Hussain M and
VanderWeele DJ: Response to pembrolizumab in advanced prostate
cancer with predictive biomarkers. Oncologist. 30:oyaf0252025.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao L, Zhou Y, Ding J, Qin Z, Zhou H and
Jing X: Primary hepatic squamous cell carcinoma: Case report and
systematic review of the literature. Front Oncol. 13:12299362023.
View Article : Google Scholar : PubMed/NCBI
|