Introduction
In recent years, more prostatic carcinoma have been
detected at an earlier stage because of the widespread use of
prostate-specific antigen and transrectal ultrasonography-guided
systematic biopsy. Therefore, a low cancer volume has been
increasingly observed in radical prostatectomy specimens. Despite
this observation, the true natural history of prostatic carcinoma
remains largely uncertain (1).
Subsequently, this study investigated the change of histological
characteristics associated with increasing total cancer volume to
demonstrate morphological original and infiltrating patterns of
prostatic carcinoma which increase total cancer volume.
Materials and methods
Tissue specimens from 248 histopathological cases,
obtained between September 2001 and October 2007 at the Kyushu
Cancer Center, were reviewed in embedded whole-mount antegrade
radical prostatectomy specimens with adenocarcinoma. Fifty-two
cases were excluded from the investigation because of past hormonal
therapy or incomplete sections. The subjects were Japanese, aged
between 47 and 78 years (mean 66.8, median 68) and the value of the
prostate-specific antigen ranged from 0.8 to 49.6 ng/ml (mean 10.2,
median 7.5). The radical prostatectomy specimens were fixed in 10%
neutral-buffered formalin for 48–96 h. Whole organ prostate
specimens were serially sectioned perpendicular to the rectal
surface at 5 mm intervals. The most caudal and cephalic sections
were cut in sagittal planes at 5 mm intervals to assess the bladder
neck and apical margins. The specimens were embedded in paraffin,
and the sections were cut into 5 μm slices and then stained with
hematoxylin and eosin. The area of each tumor focus was determined
by computer planimetry and multiplied by the section thickness. The
calculated volume was multiplied by a factor of 1.33 to correct for
tissue shrinkage in processing (2).
In multifocal tumors the volumes of the foci were calculated to
determine the radical prostatectomy tumor volume. Three groups
(<0.5 cm3, ≤0.5 cm3 <1 cm and ≥1
cm3) were identified based on the total cancer volume,
and a histological study was conducted on each group. Based on the
2005 International Society of Urological Pathology (ISUP) Consensus
Conference on Gleason Grading of Prostatic Carcinoma (3), the pathological evaluations were
performed by one pathologist. Statistical methods used included the
Kruskal-Wallis test.
Results
In an evaluation of the Gleason primary pattern, 45
cases with a cancer volume of <0.5 cm3, or 64.4% of
the lesions, exhibited Gleason pattern 3, while Gleason pattern 4
was observed in 26.7%; a total of 91.1%. When lesions with Gleason
pattern 5 were included, the Gleason primary pattern totaled 95.5%
(Table I). Similarly, secondary
Gleason patterns 3 and 4 were observed in 53.3 and 42.2% (a total
of 95.5%) of cases, respectively, for lesions with a cancer volume
of <0.5 cm3. The Gleason secondary pattern included
97.7% when the lesions with Gleason pattern 5 were included
(Table II). In addition, no
cribriform carcinoma was identified in Gleason pattern 3 in lesions
with a cancer volume of <0.5 cm3 (Table III). In the group with a cancer
volume <0.5 cm3, both the Gleason primary and
secondary patterns were consistent with a carcinoma with a small
size acini. When the density of those acini was compared with that
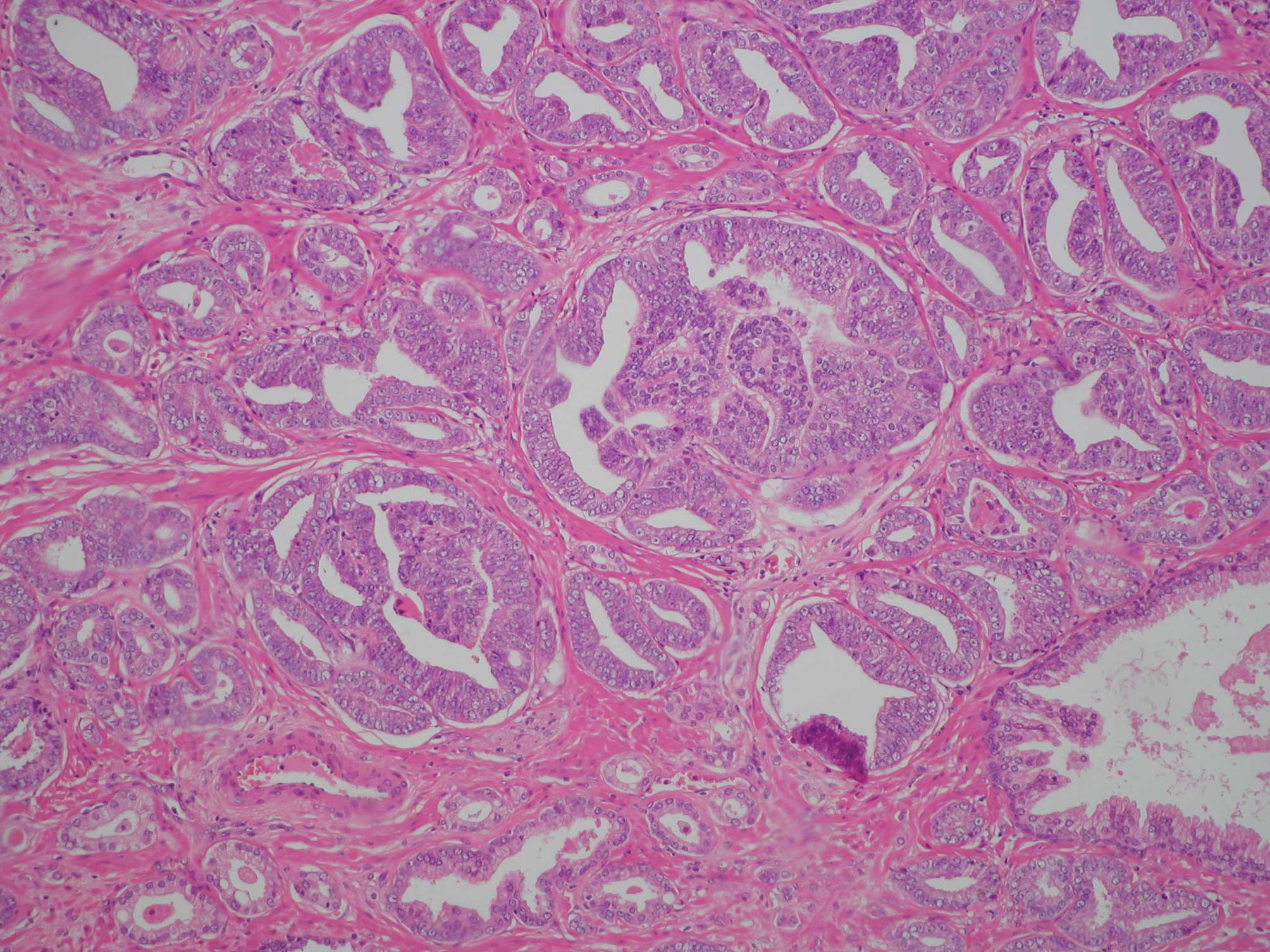
of ambient normal acini, it proved to be much higher (Fig. 1). With the increase in total cancer
volume, Gleason primary pattern 4 and the cribriform carcinoma
classified as Gleason pattern 3 increased significantly (p<0.01;
Tables I and III), while the Gleason primary pattern 3
alone decreased (p<0.01; Table
II). Over 95% of the total cancer volume ≥1 cm3
contain cribriform carcinoma classified as Gleason pattern 3.
 | Table ISummary of the Gleason primary
pattern. |
Table I
Summary of the Gleason primary
pattern.
| Gleason primary
pattern | |
|---|
|
| |
|---|
| Cancer volume | 2 | 3 | 4 | 5 | Total |
|---|
| <0.5
cm3 | 2 (4.4%) | 29 (64.4%) | 12 (26.7%) | 2 (4.40%) | 45 (23.0%) |
| ≤0.5 cm3,
<1 cm3 | 0 (0.0%) | 16 (53.3%) | 13 (43.3%) | 1 (3.30%) | 30 (15.3%) |
| ≥1
cm3 | 1 (0.8%) | 38 (31.4%) | 65 (53.7%) | 17 (14.00%) | 121 (61.7%) |
| p-value | | p<0.01 | p<0.01 | p=0.08 | |
 | Table IISummary of the Gleason secondary
pattern. |
Table II
Summary of the Gleason secondary
pattern.
| Gleason secondary
pattern | |
|---|
|
| |
|---|
| Cancer volume | 2 | 3 | 4 | 5 | Total |
|---|
| <0.5
cm3 | 1 (2.2%) | 24 (53.3%) | 19 (42.2%) | 1 (2.2%) | 45 (23.0%) |
| ≤0.5 cm3,
<1 cm3 | 0 (0.0%) | 11 (36.7%) | 15 (50.0%) | 4 (13.3%) | 30 (15.3%) |
| ≥1
cm3 | 1 (0.8%) | 36 (29.8%) | 55 (45.5%) | 29 (24.0%) | 121 (61.7%) |
| p-value | | p=0.02 | p=0.08 | p<0.01 | |
 | Table IIIRelationship between cribriform
carcinoma classified as Gleason pattern 3 and total cancer
volume. |
Table III
Relationship between cribriform
carcinoma classified as Gleason pattern 3 and total cancer
volume.
| Cancer volume | GP3CC (+) | GP3CC (−) | Total |
|---|
| <0.5
cm3 | 0 (0.0%) | 45 (100.0%) | 45 |
| ≤0.5 cm3,
<1 cm3 | 9 (30.0%) | 21 (70.0%) | 30 |
| ≥1
cm3 | 116 (95.9%) | 5 (4.1%) | 121 |
Discussion
We aimed to investigate the pattern in which
prostatic carcinoma originates and how it invades tissue. Untreated
radical prostatectomy specimens archived in this hospital were
therefore histologically examined.
Initially the specimens were evaluated based on the
2005 ISUP Consensus Conference on Gleason Grading of Prostatic
Carcinoma (3). McNeal et al
reported that evaluation of the whole-tumor histological
characteristics may contribute to a better appreciation of the role
of histological differentiation compared with other factors in
determining the behavior of prostate cancer (4). Hence, the total cancer volume was then
estimated. The specimens were then classified into three groups
based on the total cancer volume.
To demonstrate the original patterns of prostatic
carcinoma, lesions with a cancer volume of <0.5 cm3
were evaluated using the definitions of insignificant cancer
described by Stamey et al (5). Gleason primary patterns 3 and 4
accounted for 91.1% of the smaller tumors, while Gleason secondary
patterns 3 and 4 were found in 95.5% of the lesions. These data
indicate that the classification of prostatic carcinoma with a
cancer volume of <0.5 cm3 is predominantly Gleason
pattern 3 and 4. In this group, Gleason pattern 3 did not exhibit a
cribriform pattern and consisted of assembled acini, which were
independent and smaller in size than those characteristic of
Gleason patterns 1 or 2. Gleason pattern 4 exhibited independent
and ill-defined acini and did not contain large cribriform acini.
In other words, the acini of prostate carcinoma with a cancer
volume of <0.5 cm3 consisted mostly of independent
acini of a small size. Furthermore, these acini proliferated
without disrupting the structure of smooth muscle. The acini were
quite dense in comparison to the surrounding non-neoplastic acini
(Fig. 1). Based on these
observations, it would appear that small acini of prostatic
carcinoma are dense because they invade not only the surrounding
acini and small ducts, but also the stroma at a quite early stage.
Humphrey reported that the natural history of prostatic carcinoma
begins with the invasion of prostatic carcinoma cells in the
stroma. Most prostatic carcinomas fail to elicit a stromal
response. Subsequently, the malignant acini appear to be embedded
within normal-appearing fibromuscular stroma (6). These descriptions are consistent with
observations in the present study. Prostatic carcinomas originate
morphologically in independent small-sized acini. Therefore,
prostatic carcinomas are dense in comparison with non-neoplastic
acini because of the invasion not only into the surrounding acini
and small ducts, but also in the stroma at a relative early
stage.
The study also aimed to clarify how the mass of
small acini increased by reviewing the changes in the histological
features associated with increasing total cancer volume. The
primary focus was on the cribriform pattern of the Gleason pattern
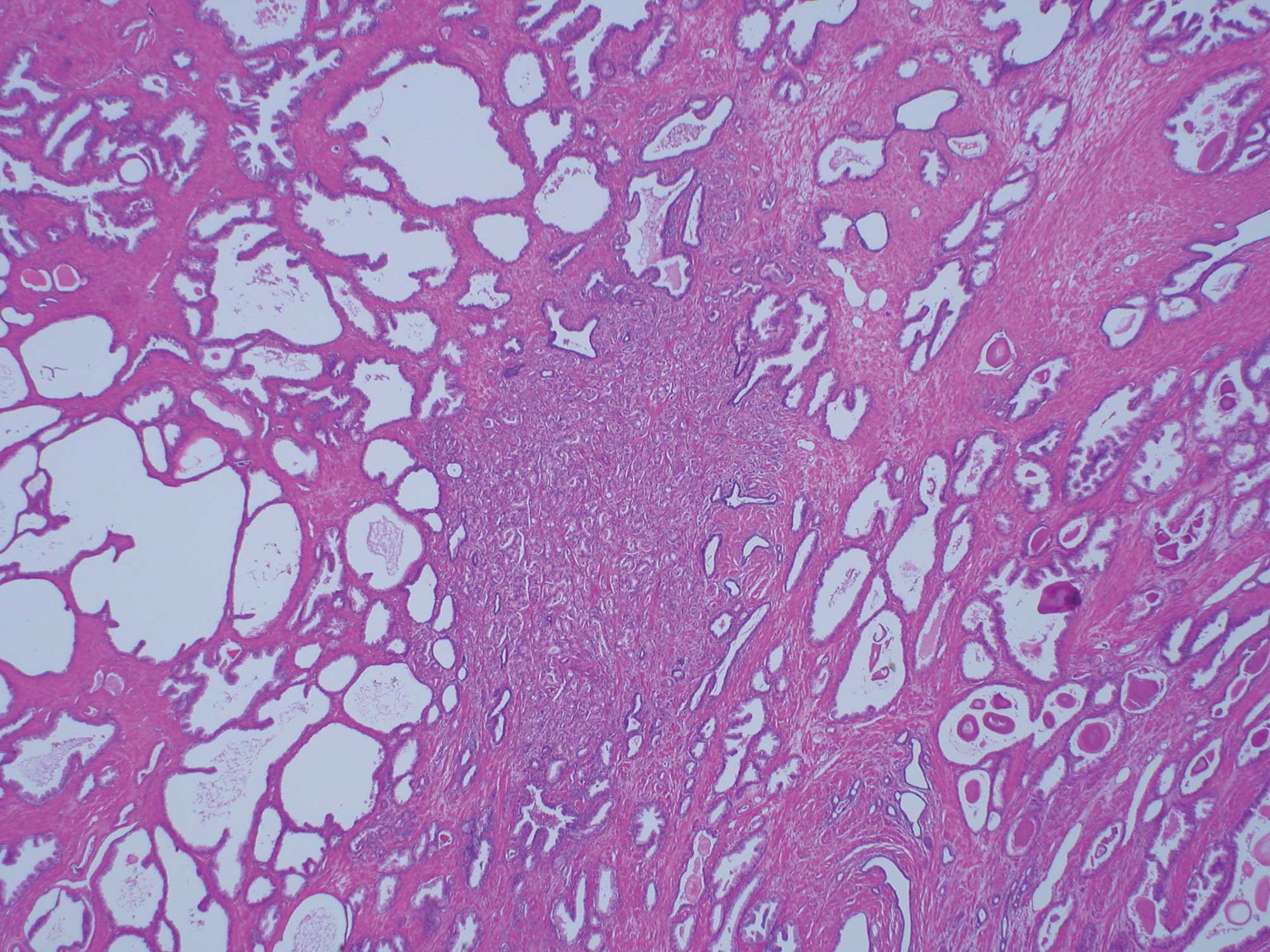
3 cribriform carcinoma in particular (Fig. 2). Cribriform carcinoma classified as
Gleason pattern 3 was not observed in any of the 45 cases with a
cancer volume of <0.5 cm3. McNeal et al
reported that the histological features of the cribriform grade 3
pattern based on Gleason’s original illustrations (7–9) may
often represent cancer growing within the lumens of preexisting
ducts and acini rather than a pattern of invasive tumor (10–12).
Within Gleason’s original illustrations, cribriform pattern 3 is
depicted as large cribriform acini. However, most of the cribriform
patterns in this study were diagnosed to be Gleason pattern 4
(Fig. 3). The 2005 ISUP reported
that it was the consensus that most of the cribriform patterns
should be diagnosed as pattern 4, with only rare cribriform lesions
satisfying the diagnostic criteria for Gleason pattern 3. Moreover,
the criteria used to diagnose Gleason pattern 3 were rounded,
well-circumscribed glands of the same size as normal glands
(3). In the present study, the
proportion including cribriform carcinoma classified as Gleason
pattern 3 increased significantly with the increase of cancer
volume (p<0.01). Thus, >95% of the total cancer volume ≥1
cm3 contain cribriform carcinoma classified as Gleason
pattern 3. Based on this observation, the growth of the small acini
of prostatic carcinoma within the lumens of preexisting ducts and
acini is considered to increase the tumor volume.
It was thought that Gleason pattern 3 cribriform
carcinoma expressed an invasion to the terminal acini or small
ducts, since they were nearly equal to normal acini in size. In
addition, it appeared that Gleason pattern 3 cribriform carcinoma
expressed infiltrating preexisting acini and ducts that did not
invade peripherally as the lesions were rounded and
well-circumscribed. McNeal et al noted that with regard to
the cribriform pattern, the relationship between total tumor volume
and presence of cribriform elements was statistically significant
(p<0.001) (11). The current
results are consistent with those of McNeal et al.
Therefore, it appears that the cribriform pattern of prostatic
carcinoma categorized as Gleason pattern 3 is characteristic of
tumors infiltrating the terminal ducts and acini, accelerating the
increase of tumor volume. We estimate that the Gleason pattern 4
cribriform carcinoma may express invasion to larger acini and ducts
in comparison with the Gleason pattern 3 cribriform carcinoma. As a
result, we hypothesize that the Gleason pattern 4 cribriform
carcinoma demonstrates the structure of the cribriform pattern
which has either a large size or shows marginal irregularity.
In summary, the original pattern of prostatic
carcinoma was mainly composed of small acini. In addition, the
prostatic carcinoma infiltrated surrounding stroma at quite an
early stage. This infiltration caused an increase in the tumor
volume in both the stroma and preexisting acini and ducts. However,
infiltration of the preexisting ducts and acini is considered to
lead to a greater increase in the tumor volume than direct
infiltration of the stroma.
Acknowledgements
The authors thank Mr. Brian Quinn for linguistic
comments and help with the manuscript.
References
|
1
|
Ekman P, Adolfsson J and Gronberg H: The
natural history of prostate cancer. Martin Dunitz; London: pp.
1–16. 1996
|
|
2
|
Elgamal AA, Van Poppel HP, Van De Voorde
WN, Van Dorpe JA, Oyen RH and Baert LV: Impalpable invisible stage
T1c prostate cancer: characteristics and clinical relevance in 100
radical prostatectomy specimens - a different view. J Urol.
157:244–250. 1997. View Article : Google Scholar
|
|
3
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; the ISUP Grading Committee. The 2005 International
Society of Urological Pathology (ISUP) Consensus Conference on
Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol.
29:1228–1242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNeal JE, Villers AA, Redwine EA, Freiha
FS and Stamey TA: Histologic differentiation, cancer volume and
pelvic lymph node metastasis in adenocarcinoma of the prostate.
Cancer. 66:1225–1233. 1990.PubMed/NCBI
|
|
5
|
Stamey TA, Freiha FS, McNeal JE, et al:
Localized prostate cancer: relationship of tumor volume to clinical
significance for treatment of prostate cancer. Cancer. 71:933–938.
1993.PubMed/NCBI
|
|
6
|
Humphrey PA: Natural history and
mortality. Prostate Pathology. Amer Society of Clinical Pathology;
Chicago: pp. 241–249. 2003
|
|
7
|
Bailar JC III, Mellinger GT and Gleason
DF: Survival rates of patients with prostatic cancer, tumor stage
and differentiation: preliminary report. Cancer Chemother Rep.
50:129–136. 1966.PubMed/NCBI
|
|
8
|
Gleason DF: Classification of prostatic
carcinomas. Cancer Chemother Rep. 50:125–128. 1966.PubMed/NCBI
|
|
9
|
Mellinger GT, Gleason D and Bailar JC III:
The histology and prognosis of prostatic cancer. J Urol.
97:331–337. 1967.PubMed/NCBI
|
|
10
|
Kovi J, Jackson MA and Heshmat MY: Ductal
spread in prostatic carcinoma. Cancer. 56:1566–1573. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McNeal JE, Reese JH, Redwine EA, Freiha FS
and Stamey TA: Cribriform adenocarcinoma of the prostate. Cancer.
58:1714–1719. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McNeal JE and Cheryl EM: Spread of
adenocarcinoma within prostatic ducts and acini. Am J Surg Pathol.
20:802–814. 1996. View Article : Google Scholar : PubMed/NCBI
|