Introduction
Primary renal carcinoid tumor arising from renal
parenchyma or renal pelvis is a rare neoplasm, and approximately 80
cases with such a tumor have been reported thus far (1–12).
However, few studies report on the genetic characteristics of
primary renal carcinoid tumor. Previously, a case of primary renal
carcinoid tumor sharing common molecular abnormality with clear
cell renal cell carcinoma (RCC) was reported (5). Additionally, the numerical and
structural abnormality of chromosome 13 was described in primary
renal carcinoid tumor associated with horseshoe kidney (6). This study investigated the status of
chromosomes 3 and 13 in four primary renal carcinoid tumors using
fluorescence in situ hybridization (FISH), loss of
heterozygosity (LOH) of 3p and VHL gene analysis and
discussed the pathogenesis.
Materials and methods
Archive materials
Among pathology files exceeding 14,000 renal tumors
originating from Kochi Red Cross Hospital, Japan; Mexico Medical
Center, Mexico; Charles University Hospital Plzen, Czech Republic
and Kansai Medical University Hirakata Hospital, Japan, four cases
with renal carcinoid tumor arising from the renal pelvis (case 1,
Tables I and II) or renal parenchyma (cases 2–4,
Tables I and II) were selected for the present study.
Patient age ranged from 32 to 55 years, and the gender ratio of
male to female was 1:3. One patient was previously described
(12).
 | Table IImmunohistochemical results. |
Table I
Immunohistochemical results.
| Case 1 | Case 2 | Case 3 | Case 4 |
|---|
| Chromogranin A | f, + | f, + | d, + | d, + |
| Synaptophysin | f, + | f, + | d, + | d, + |
| CD56 | − | f, + | d, + | − |
 | Table IIResults of fluorescence in situ
hybridization, 3p LOH and VHL gene mutation analyses. |
Table II
Results of fluorescence in situ
hybridization, 3p LOH and VHL gene mutation analyses.
| Chromosome 3 | Chromosome 13 | D3S1300 | D3S666 | D3S1768 | VHL gene
mutation |
|---|
| Case 1 | Monosomy | Monosomy | | | | |
| Total cells | 507 | 599 | | | | |
| One signal | 261 (51.5%) | 403 (67.3%) | Failed | Failed | Failed | Failed |
| Two signals | 226 (44.6%) | 196 (32.7%) | | | | |
| Three signals | 20 (3.9%) | 0 (0%) | | | | |
| Case 2 | Monosomy | Disomy | | | | |
| Total cells | 518 | 512 | | | | |
| One signal | 404 (78.0%) | 85 (16.6%) | Negative | Negative | Negative | Not performed |
| Two signals | 112 (21.6%) | 417 (81.4%) | | | | |
| Three signals | 2 (0.2%) | 10 (2.0%) | | | | |
| Case 3 | Monosomy | Disomy | | | | |
| Total cells | 617 | 541 | | | | |
| One signal | 577 (93.5%) | 29 (5.4%) | LOH | Negative | LOH | Wild-type |
| Two signals | 37 (6.0%) | 492 (90.9%) | | | | |
| Three signals | 3 (9.5%) | 20 (3.7%) | | | | |
| Case 4 | Disomy | Disomy | | | | |
| Total cells | 366 | 334 | | | | |
| One signal | 43 (11.7%) | 27 (8.1%) | Failed | Failed | Failed | Wild-type |
| Two signals | 317 (86.6%) | 303 (90.7%) | | | | |
| Three signals | 6 (1.6%) | 4 (1.2%) | | | | |
Histological examination and
immunohistochemistry
Renal tumor tissues obtained from nephrectomy were
fixed in 10% formalin and embedded in paraffin. Sections (3-μm
thick) were stained with H&E. Additionally, immunohistochemical
staining was performed using a Histofine Simple Stain-PO (Multi)
kit (Nichirei, Tokyo, Japan). Antibodies against chromogranin A
(polyclonal; DakoCytomation, Glostrup, Denmark), synaptophysin
(polyclonal; DakoCytomation) and CD56 (N-CAM) (123C3, 1:40; Zymed
Laboratories, San Francisco, CA, USA) were employed in the present
study. Specimens of normal pancreas were used as positive controls
of the above described antibodies.
Fluorescence in situ hybridization
(FISH)
FISH was performed using probes detecting chromosome
3 (D3Z1) and chromosome 13 (D13S319/13q34). FISH was carried out in
the Cytogenetic Testing Group, Molecular Genetic Testing
Department, Clinical Testing Center, Mitsubishi Chemical Medience
Corporation, Kyoto, Japan. In each case, >300 neoplastic cells
were counted, and the percentages of one, two and three signals per
cell were calculated. The cut-off value for monosomy was judged as
>20%.
Analyses of 3p LOH and VHL gene
mutation
These analyses were performed in the Department of
Pathology, Charles University Hospital Plzen, Czech Republic.
Several sections (10-μm thick) were cut from each formalin-fixed,
paraffin-embedded block. The tumorous component was microdissected.
DNA was extracted by the NucleoSpin Tissue Kit (Macherey Nagel,
Duren, Germany) according to the manufacturer’s protocol.
Polymerase chain reaction (PCR) for LOH analysis of chromosome 3p
was performed. Short tandem repeat markers and primers were as
follows: D3S1300 F, AGCTCACATTCTAGTCAGCCT and R, GCCAATTCCCC AGATG;
D3S666 F, CAAGGCATTAAAGTGGCCACGC and R, GTTTGAACCAGTTTCCTACTGAG;
D3S1768 F, GGT TGCTGCCAAAGATTAGA and R, CACTGCATTTGCTGT TGGA.
Normal tissues of the same patients were used as a reference.
Reaction conditions were as follows: 12.5 μl of HotStart Taq PCR
Master Mix (Qiagen, Hilden, Germany), 10 pmol of each primer, 100
ng of template DNA, and distilled water up to 25 μl. The
amplification program for the fragments consisted of denaturation
at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C
for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1
min. The program was completed by incubation at 72°C for 7 min. The
annealing temperature for fragment D3S666 was 58°C. Additionally,
analysis of three coding exons and exon-intron junctions of the
VHL gene was performed by PCR and direct sequencing
according to the previously described method (13).
Results
Microscopic findings
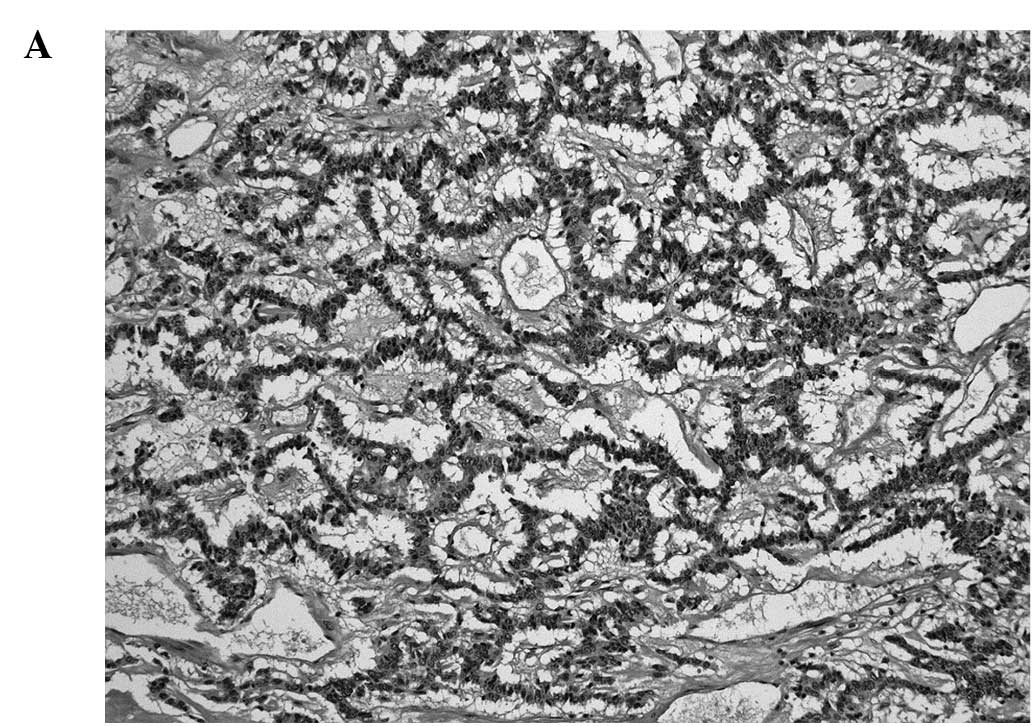
The tumors consisted of neoplastic cells with round
to oval nuclei. Various growth patterns such as tightly packed
cords and trabeculae, ribbon-like, trabecular (Fig. 1A), sheet-like or solid growth were
observed. The cell border was generally indistinct. Nuclear
chromatin exhibited a coarse and granular pattern (Fig. 1B). Necrosis or abnormal mitotic
figures were absent.
Immunohistochemical findings
Immunohistochemical results are summarized in
Table I. Four neoplastic cells were
positive for chromogranin A and synaptophysin. The positivity for
chromogranin A and synaptophysin in two tumors (cases 3 and 4) was
diffuse, and that of the remaining two tumors (cases 1 and 2) was
focal. Neoplastic cells were positive for CD56 in two tumors. The
positivity of one tumor (case 3) was diffuse, while that of the
other (case 2) was focal.
FISH findings
The results of the FISH analysis are summarized in
Table II. Tumorous cells of cases
1, 2 and 3 exhibited monosomy of chromosome 3 (Fig. 2), but one tumor (case 4) showed
disomy of chromosome 3. In case 1, neoplastic cells demonstrated a
monosomy of chromosome 13, but tumorous cells in cases 2, 3 and 4
showed a disomy of chromosome 13.
Findings of 3p LOH and VHL gene mutation
analyses
The results are summarized in Table II. Regarding 3p LOH, one tumor
showed LOH at two (D3S1300 and D3S1768) of three loci tested. One
tumor was not informative, and two tumors failed to provide better
results due to low DNA quality. Concerning VHL gene
analysis, two tumors showed wild-type and one tumor failed due to
low DNA quality. In one case, VHL gene analysis was not
performed (Fig. 3).
Discussion
Primary renal carcinoid tumor is a rare neoplasm,
and approximately 80 cases with such a tumor have been previously
reported. This tumor frequently occurs in patients <50 years of
age, affects male and female patients with equal frequency and does
not appear to present with carcinoid syndrome (11). It is well known that primary renal
carcinoid tumor is associated with other renal diseases including
horseshoe kidney (17.8%), teratoma (14.3%) or polycystic kidney
disease (1.8%) (6–10). However, no tumors in the present
study were associated with any other renal diseases. Poor patient
prognostic factors include an age >40 years, tumor size >4
cm, a purely solid tumor on the cut surface, a mitotic rate
>1/10 high power fields, metastases at initial diagnosis and
tumors extending into the extrarenal tissue (9). Patients with this tumor often present
with regional lymph node metastases which may progress to distant
organ metastases, but usually pursue a prolonged clinical course
despite widely metastastic disease (11). Accordingly, it is very important for
surgical pathologists to accurately recognize and diagnose renal
carcinoid tumors due to their peculiar clinical behavior.
Genetic studies of renal carcinoid tumor are
currently limited (5,6). In the present study, three of four
tumors (75%) demonstrated monosomy of chromosome 3 by FISH
analysis. Therefore, we suggest that the numerical loss of
chromosome 3 plays a crucial role in the pathogenesis of primary
renal carcinoid tumor. Previous cytogenetic studies of carcinoid
tumor of the respiratory area failed to show any abnormalities of
chromosome 3 (14,15). Therefore, it is possible that renal
carcinoid tumor is genetically different from respiratory carcinoid
tumor. On the other hand, primary renal carcinoid tumor showing the
abnormality of chromosome 3, characteristic of clear cell RCC, was
previously reported (5). In
pulmonary carcinoid tumor, Hurr et al (16) failed to detect any 3p deletion, and
some investigators detected allelic loss at a limited loci of small
number (17,18). From the results of the present
study, we can infer that LOH of chromosome 3p is involved in the
pathogenesis of certain cases of renal carcinoid tumors. A large
scale study is necessary to clarify the frequency and significance
of 3p LOH in renal carcinoid tumor. However, it is unlikely that
the VHL gene is associated with the pathogenesis of renal
carcinoid tumor. This suggests that renal carcinoid tumors do not
differentiate from clear cell RCC or that both tumors do not
originate from common stem cells. Additionally, only one of the
four tumors (25%) showed monosomy of chromosome 13 by FISH
analysis. The abnormality of chromosome 13 by G-band karyotype has
been reported in gastric carcinoid tumor (19). Although Van den Berg et al
(6) detected the numerical and
structural abnormality of chromosome 13 by a G-band karyotype, a
numerical abnormality of chromosome 13 in renal carcinoid tumor
appears to occasionally occur on the basis of our results. The rate
(25%) of abnormality of chromosome 13 in renal carcinoid tumor
using FISH analysis appears to be comparable with the rate (17%) of
abnormality of chromosome 13 in carcinoid tumor of various anatomic
sites using genome-wide single nucleotide polymorphism analysis
(20).
In conclusion, the abnormality of chromosome 3 may
be involved in the pathogenesis of some cases of renal carcinoid
tumor.
Acknowledgements
The authors are grateful to the Cytogenetic Testing
Group, Molecular Genetic Testing Department, Clinical Testing
Center, Mitsubishi Chemical Medience Corporation, Kyoto, Japan for
their technical assistance.
References
|
1
|
Toker C: Carcinoidal renal tumor. J Urol.
111:10–11. 1974.
|
|
2
|
Stahl RE and Sidhu GS: Primary carcinoid
tumor of the kidney. Light and electron microscopic study. Cancer.
44:1345–1349. 1974. View Article : Google Scholar
|
|
3
|
Ji X and Li W: Primary carcinoid of renal
pelvis. J Environ Pathol Toxicol Oncol. 13:269–271. 1994.
|
|
4
|
Rudrick B, Nguyen GK and Lakey WH:
Carcinoid tumor of the renal pelvis: report of a case with positive
urine cytology. Diagn Cytopathol. 12:360–363. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Naggar AK, Troncoso P and Ordonez NG:
Primary renal carcinoid tumor with molecular abnormality
characteristic of conventional renal cell neoplasms. Diagn Mol
Pathol. 4:48–53. 1995. View Article : Google Scholar
|
|
6
|
Van den Berg E, Gouw A, Oosterhuis JW,
Störkel S, Dijkhuizen T, Mensink HJ and de Jong B: Carcinoid in a
horseshoe kidney. Morphology, immunohistochemistry and
cytogenetics. Cancer Genet Cytogenet. 84:95–98. 1995.PubMed/NCBI
|
|
7
|
Krishnan B, Truong LD, Saleh G, Sirbasku M
and Slawin KM: Horseshoe kidney is associated with an increased
relative risk of primary carcinoid tumor. J Urol. 157:2059–2066.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo J, Park S, Lee HJ, Kang SJ and Kim BK:
Primary carcinoid tumor arising in a mature teratoma of the kidney.
A case report and review of the literature. Arch Pathol Lab Med.
126:979–981. 2002.PubMed/NCBI
|
|
9
|
Romero FR, Rais-Bahrami S, Permpongkosol
S, Fine SW, Kohanim S and Jarrett TW: Primary carcinoid tumor of
the kidney. J Urol. 176:2359–2366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murali R, Kneale K, Lalak N and Delprado
W: Carcinoid tumors of the urinary tract and prostate. Arch Pathol
Lab Med. 130:1693–1706. 2006.PubMed/NCBI
|
|
11
|
Hansel DE, Epstein JI, Berbescu E, Fine
SW, Young RH and Cheville JC: Renal carcinoid tumor: a
clinicopathologic study of 21 cases. Am J Surg Pathol.
31:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda N, Tamura M, Hes O, Michal M,
Hayashi Y and Lee GH: Carcinoid tumor of renal pelvis:
consideration on the histogenesis. Pathol Int. 58:51–54. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michal M, Hes O, Nemcova J, Sima R, Kuroda
N, Bulimbasic S, Franco M, Sakaida N, Danis D, Kazakov DV, Ohe C
and Hora M: Renal angiomyoadenomatous tumor: morphologic,
immunohistochemical and molecular genetic study of a distinct
entity. Virchows Arch. 454:89–99. 2009. View Article : Google Scholar
|
|
14
|
Teyssier JR, Sadrin R, Nou JM, Bureau G,
Adnet JJ, Bajolle F and Pigeon F: Trisomy 7 in a lung carcinoid
tumor. Precocious index of malignant transformation? Cancer Genet
Cytogenet. 15:277–282. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson M, Hein S, Mandahl N, Hambraeus
G, Johansson L and Mitelman F: Cytogenetic analysis of six
bronchial carcinoids. Cancer Genet Cytogenet. 66:33–38. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurr K, Kemp B, Silver SA and El-Naggar
AK: Microsattelite alteration at chromosome 3p loci in
neuroendocrine and non-neuroendocrine lung tumors. Am J Pathol.
149:613–620. 1996.PubMed/NCBI
|
|
17
|
Kovatich A, Friedland DM, Druck T,
Hadaczek P, Huebner K, Comis RL, Hauck W and McCue PA: Molecular
alterations to human chromosome 3p loci in neuroendocrine lung
tumors. Cancer. 83:1109–1117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Onuki N, Wistuba II, Travis W, Virmani AK,
Yashima K, Brambilla E, Hasleton P and Gazdar AF: Genetic changes
in the spectrum of neuroendocrine lung tumors. Cancer. 85:600–607.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panani AD, Malliaros S, Ferti A and Raptis
S: Cytogenetic study of a malignant carcinoid tumor. Cancer Genet
Cytogenet. 59:2201992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim DH, Nagano Y, Choi IS, White JA, Yao
JC and Rashid A: Allelic alterations in well-differentiated
neuroendocrine tumors (carcinoid tumors) identified by genome-wide
single nucleotide polymorphism analysis and comparison with
pancreatic endocrine tumors. Genes Chromosomes Cancer. 47:84–92.
2008. View Article : Google Scholar
|