Introduction
α-fetoprotein (AFP) is a significant serum protein
synthesized during fetal life, and the expression of the AFP gene
is dramatically reduced after birth. Numerous cases of
AFP-producing carcinomas have been reported, with stomach as the
most common site (1). It has been
demonstrated that AFP-producing gastric cancers are clinically
aggressive and have a poor prognosis (2). However, AFP-producing endometrial
tumors, with the exception of yolk sac tumors, are extremely rare,
and only 12 cases of the former have been described in the English
literature (3–14). In such cases, the serum level of AFP
is generally elevated and AFP production can be demonstrated by
immunohistochemical analysis of the cytoplasm, especially in
hepatoid adenocarcinoma (HC) cells. We report on a case of
AFP-producing, Grade 2 endometrioid adenocarcinoma without an
obvious hepatoid component.
Case report
The patient was a 59-year-old (gravida 2, para 2)
Japanese woman. Except for diabetes mellitus since the age of 57,
her family and past medical history were routine. Age of menopause
was 50 years. The patient presented at a local clinic complaining
of abdominal swelling that had persisted for a few months. Serum
AFP levels were elevated and a pelvic tumor was detected. The
patient was referred to our hospital. No vaginal bleeding nor
palpable lymphadenopathy were detected. A pelvic examination showed
a tumor approximately the same size as a 16-week gestational
uterus. No abnormalities were observed in the indices of the
complete blood count and serological tests. Serum levels of AFP and
CA-125 were 1292.8 ng/ml (normal <10 ng/ml) and 82.0 U/ml
(normal <35 U/ml), respectively, while those of other tumor
markers including CA 19-9, CA 72-4 and CEA were within the normal
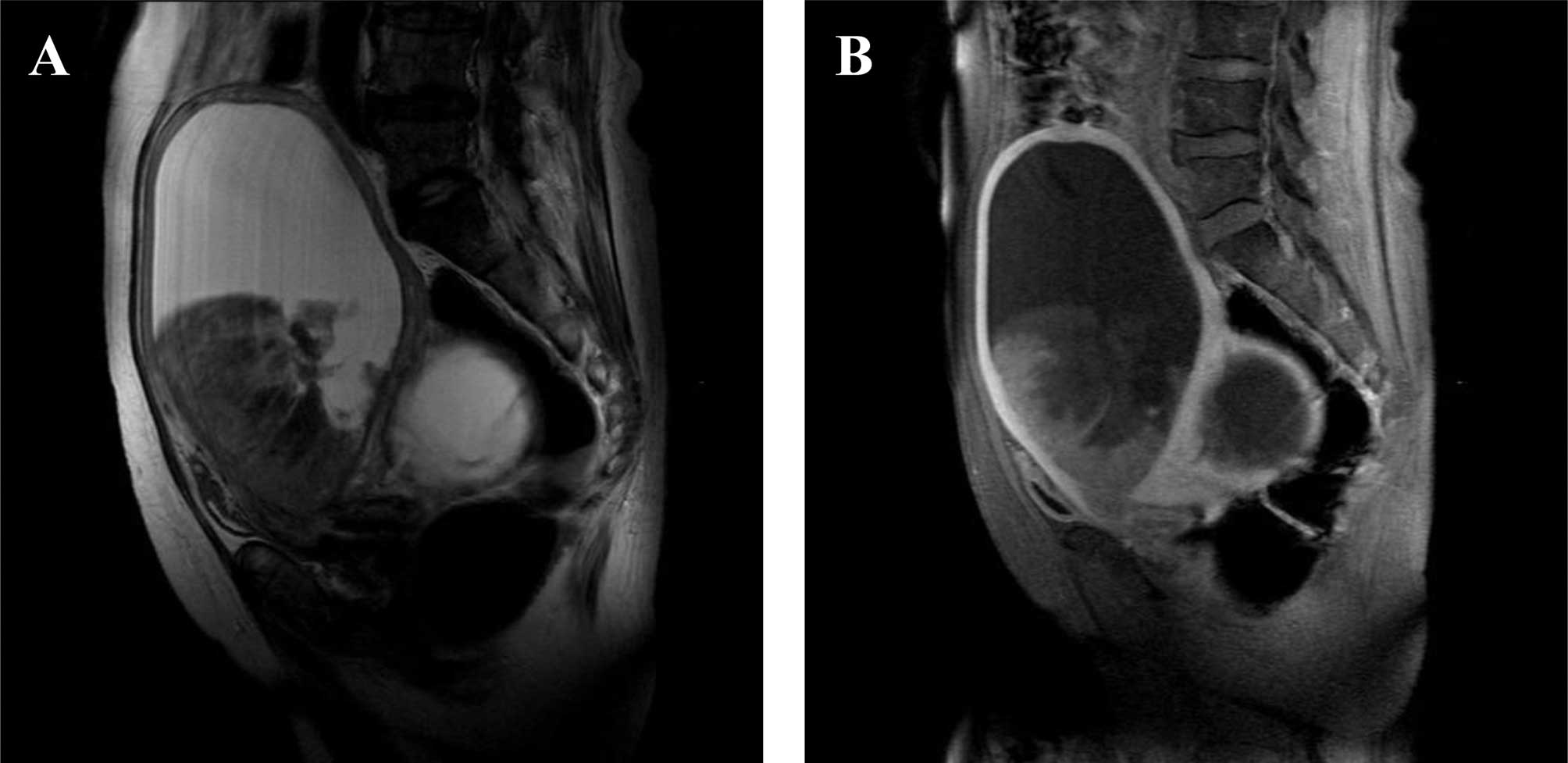
range. Magnetic resonance imaging of the pelvis showed a markedly
enlarged uterus, with a tumor growing exophytically within the
endometrial cavity along with fluid collection and an adnexal
tumor. The uterine tumor was heterogeneously hypointense with
scattered areas of slightly high signal intensity on T2-weighted
imaging (Fig. 1A) and appeared
hypointense to the myometrium on contrast-enhanced T1-weighted
imaging (Fig. 1B). Computerized
tomography showed no signs of lymph node, peritoneal, lung or liver
metastases.
Abdominal exploration revealed a markedly enlarged
uterus, and a left ovarian cyst and hydrosalpinx adhesive to the
posterior wall of the uterine corpus. A small amount of ascites was
present and the cytological examination was negative for malignant
cells. No dissemination was observed in the peritoneal cavity.
Total abdominal hysterectomy, bilateral salpingo-oophorectomy and
partial omentectomy were carried out. Lymphadenectomy was not
performed, since the adhesions in the pelvic cavity were extremely
strong. The uterus along with the left adnexal tumor weighed 1,850
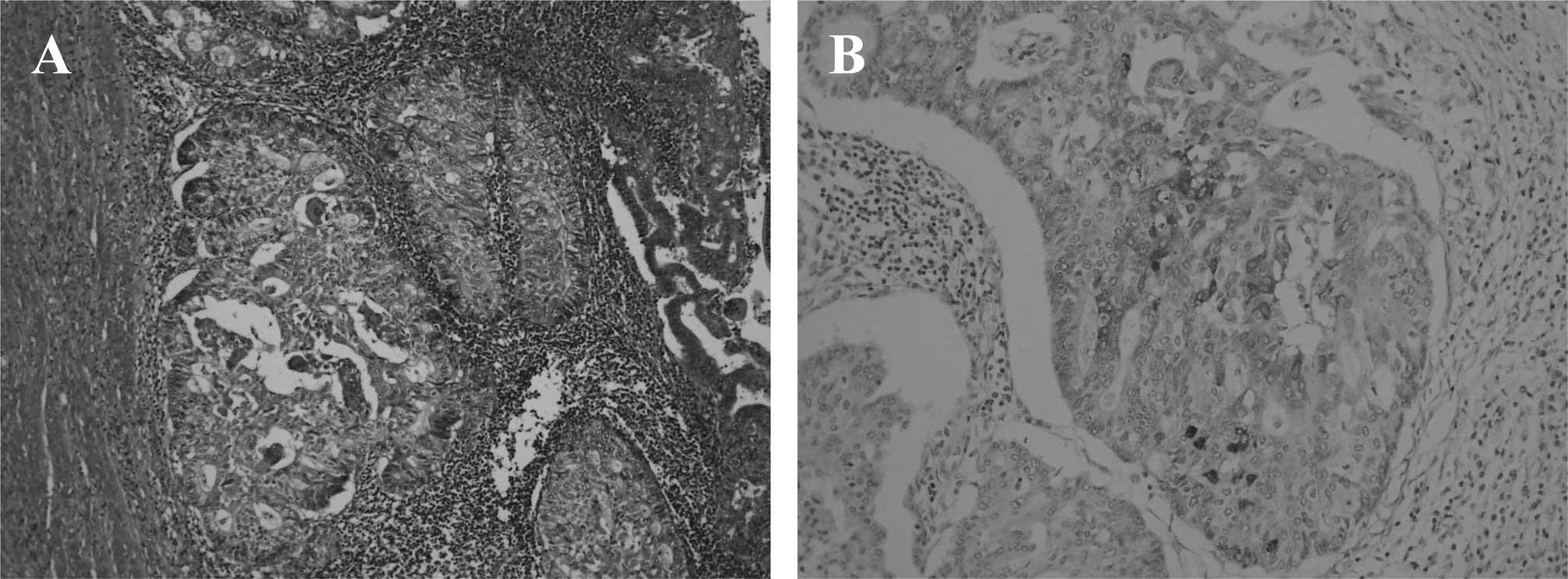
g. A histopathological examination showed a Grade 2 endometrioid
adenocarcinoma invading more than half of the thickness of the
myometrium, extending into the cervical mucosa, with lymphatic
invasion and no evidence of malignancy in the left adnexal tumor
(Fig. 2A). Hepatoid adenocarcinoma
cells were not detected in any of the tissue sections obtained. The
endometrioid adenocarcinoma cells were partially immunoreactive for
AFP (Fig. 2B). The disease was
consistent with Stage IIA endometrial cancer, according to the
International Federation of Gynecology and Obstetrics (FIGO)
classification system. The patient was intravenously administered
six courses of a multidrug regimen consisting of carboplatin,
therarubicin and paclitaxel. Levels of serum AFP declined to the
normal range on the 42nd postoperative day. Drug therapy was
well-tolerated and the patient has remained disease-free 60 months
after the operation.
Discussion
HC is a variant of adenocarcinoma that is composed
of tumor cells with extensive hepatic differentiation. The first
case of HC was reported in 1985 as an AFP-producing gastric
carcinoma with features of hepatic differentiation (15). HC usually generates an expanding
mass with scattered or extensive necrosis. Numerous cases of
carcinomas with hepatoid differentiation were reported in a variety
of primary organs, including the gastrointestinal tract, ovary,
pancreas, lung, kidney, urinary bladder and uterus (1). HC should essentially be diagnosed on
the basis of the histological features of the tumor. It is
characterized by the medullary or papillotubular arrangement of
tumor cells with eosiophilic and granular cytoplasm, thus
resembling hepatocellular carcinoma.
In 13 reported cases of AFP-producing endometrial
carcinomas, including the present case, the patients were elderly
(median 63 years; range 44–68 years) (3–14).
These tumors usually show an exophytic growth pattern and are
histologically high-grade. Furthermore, patients are diagnosed at
advanced stages and have poor prognosis. AFP expression has been
demonstrated in HC cells. However, previously published reports,
did not address AFP expression in HCs (4,5). In
the cases of carcinosarcoma, AFP was reported to be detected in the
carcinomatous but not in the sarcomatous components (3).
The present case was unusual in comparison with most
reported cases in that the tumor was not high-grade, and hepatoid
features were not detected in any of its sections. Yamamoto et
al reported that AFP expression was detected in endometrioid
adenocarcinoma cells and was particularly strong in the hepatoid
cells (7). The relationship between
endometrioid adenocarcinoma and hepatoid cells is completely
unknown. In our case, the aberrant differentiation of
adenocarcinoma cells into cells with phenotypes of hepatocytes may
be weak. Toyoda et al reported that it is difficult to
determine whether an endometrial tumor produces AFP solely on the
basis of the morphological features of the tumor (9). Nagai et al reported cases of
HCs and AFP-positive adenocarcinomas without heparoid features
(APC) of the stomach (16). The
incidence of venous invasion in HC was higher than that of APC.
There were no significant differences between the two tumors
regarding clinical features, macroscopic features and the incidence
of lymphatic permeation. As for advanced carcinomas, the prognosis
of patients with HC was poorer than that of patients with APC.
Therefore, previous investigators stated that HC should be
distinguished from APC. Our case suggests the occurrence of
endometrial AFP-positive adenocarcinomas without hepatoid
features.
Serum AFP does is not commonly examined in cases of
endometrial cancer. Therefore, the true frequency of endometrial
AFP-producing adenocarcinomas without obvious hepatoid features
remains unclear. The endometrium is a possible site of
AFP-producing carcinomas and further studies are essential to
clarify this issue.
References
|
1
|
Kishimoto T, Nagai Y, Kato K, Ozaki D and
Ishikura H: Hepatoid adenocarcinoma: a new clinicopathological
entity and the hypotheses on carcinogenesis. Med Electron Microsc.
33:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adachi Y, Tsuchidashi J, Shiraishi N,
Yasuda K, Etoh T and Kitao S: AFP-producing gastric carcinoma:
multivariate analysis of prognostic factors in 270 patients.
Oncology. 65:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawagoe K: A case of mixed mesodermal
tumor of the uterus with alpha-fetoprotein production. Jpn J Clin
Oncol. 15:577–583. 1985.PubMed/NCBI
|
|
4
|
Matsukuma K and Tsukamoto N:
Alpha-fetoprotein producing endometrial adenocarcinoma: report of a
case. Gynecol Oncol. 29:370–377. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubo K, Lee GH, Yamauchi K and Kitagawa T:
Alpha-fetoprotein-producing papillary adenocarcinomas originating
from a uterine body. A case report. Acta Pathol Jpn. 41:399–403.
1991.PubMed/NCBI
|
|
6
|
Hoshida Y, Nagakawa T, Mano S, Taguchi K
and Aozasa K: Hepatoid adenocarcinoma of the endometrium associated
with alpha-fetoprotein production. Int J Gynecol Pathol.
15:266–269. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto R, Ishikawa H, Azuma M, Hareyama
H, Makinoda S, Koyama Y, Nishi S and Fujimoto S: Alpha-fetoprotein
production by a hepatoid adenocarcinomas of the uterus. J Clin
Pathol. 49:422–425. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phillips KA, Scurry JP and Toner G:
Alpha-fetoprotein production by a malignant mixed mullerian tumor
of the uterus. J Clin pathol. 49:349–351. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toyoda H, Hirai T and Ishii E:
Alpha-fetoprotein producing uterine corpus carcinoma: a hepatoid
adenocarcinomas of the endometrium. Pathol Int. 50:847–852. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams SF, Yamada D, Montag A and Rotmensch
R: An alpha-fetoprotein-producing hepatoid adenocarcinoma of the
endometrium. Gynecol Oncol. 83:418–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takano M, Shibasaki T, Sato K, Aida S and
Kikuchi Y: Malignant mixed mullerian tumor of the uterine corpus
with alpha-fetoprotein-producing hepatoid adenocarcinoma component.
Gynecol Oncol. 91:444–448. 2003. View Article : Google Scholar
|
|
12
|
Takahashi Y and Inoue T: Hepatoid
carcinoma of the uterus that collided with carcinosarcoma. Pathol
Int. 53:323–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeuchi K, Kitazawa S, Hamanishi S,
Inagaki M and Murata K: A case of alpha-fetoprotein-producing
adenocarcinoma of the endometrium with a hepatoid component as a
potential source for alpha-fetoprotein in a postmenopausal woman.
Int J Gynecol Cancer. 16:1442–1445. 2006.PubMed/NCBI
|
|
14
|
Tran TA, Ortiz HB, Holloway RW, Bigsby GE
and Finkler NJ: Alpha-fetoprotein-producing serous carcinoma of the
uterus metastasizing to the ovaries, mimicking primary ovarian yolk
sac tumor: a case report and review of the literature. Int J
Gynecol Pathol. 26:68–70. 2007.PubMed/NCBI
|
|
15
|
Ishikura H, Fukasawa Y, Ogasawara K,
Natori T, Tsukada Y and Aizawa M: An AFP-producing gastric
carcinoma with features of hepatic differentiation: case report.
Cancer. 56:840–848. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinomas of the stomach. A clinicopathological and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar
|