Introduction
Treatment strategies for breast cancers have been
successfully designed in an integrated manner with biological
molecules including the estrogen receptor (ER), the progesterone
receptor (PgR) and the human epidermal growth factor receptor-2
(HER2) (1). Endocrine therapies,
such as tamoxifen or letrozole, and the HER2 antibody (trastuzumab)
are used clinically for hormonal receptor (HR)- and HER2-positive
patients, respectively, and have been found to prolong the survival
of breast cancer patients (2). On
the other hand, approximately 15–20% of breast cancer patients who
have triple-negative breast cancer (TNBC; ER-, PgR- and
HER2-negative) are generally subjected to chemotherapeutic agents.
Sørlie et al showed that breast cancer can be clustered into
four subtypes: luminal (A,B), HER2-positive, basal-like and normal
breast-like cancer. The findings were based on a hierarchical
clustering study of gene expression (3–5). The
basal-like subtype is one of the categories for which therapeutic
strategies are being reconsidered, and many drugs have been
proposed as candidates. DNA-damaging drugs including platinum or
anthracyclines, DNA-repairing inhibitors including polyA-ribose 1
(PARP1), and targeted drugs for epidermal growth factor receptor
(EGFR) are expected to have benefits for this group. The basal-like
subtype was originally a genotypic concept. However, studies have
increasingly defined basal-like breast cancer (BLBC) to be a type
of breast cancer with the immunophenotype of TNBC and positive for
EGFR and/or cytokeratin (CK)5/6 expression (6). TNBC expresses a basal phenotype in 56%
of cases compared with non-TNBC (11.5%) (7). Thus, TNBC and BLBC are not identical
but closely related, and both are associated with poor clinical
outcome and lack the benefit of a targeted systemic therapy.
Capecitabine is a widely used chemotherapeutic agent
for breast cancer patients (8).
Capecitabine was designed as a prodrug which is selectively
converted to 5-fluorouracil (5-FU) by thymidine phosphorylase (TP)
in tumors (9). TP was first
described as an enzyme responsible for nucleoside metabolism, but
was later found to be identical to the enzyme extracted from human
platelets known as platelet-derived endothelial cell growth factor
(PD-ECGF) (10,11) and was found to be involved in
anti-apoptotic activity and angiogenesis (12). The efficacy of capecitabine may
correlate to TP expression in the tumor (13). Some case reports have indicated that
capecitabine and docetaxel combination therapy is useful for the
treatment of TNBC (14,15). However, it is not well documented
whether or not TP expression and the intrinsic subtype of breast
cancer are related. Hence, we focused on TP expression in tumor
specimens obtained from breast cancer patients with differentially
expressed ER/HER2 status in relation to TNBC and BLBC.
Patients and methods
Patients and tumor specimens
We serially collected 40 tumor specimens consisting
of 10 samples in each of the four groups defined by the
immunohistochemical expression of ER and HER2:
ER-positive/HER2-positive, ER-positive/HER2-negative,
ER-negative/HER2-positive and ER-negative/HER2-negative, and
analyzed the specimens using an oligonucleotide microarray. A total
of 40 tumor tissues including 39 invasive ductal carcinomas and 1
ductal carcinoma in situ were surgically obtained from
breast cancer patients following informed consent. This experiment
was approved by the ethics committees of both Tokai University and
Chugai Pharmaceutical Co. Ltd. The clinicopathological features are
shown in Table I. Specimens were
resected from the main tumor mass, avoiding areas with massive
necrosis and areas intermingling with non-neoplastic breast tissue.
Tumor tissue samples were divided into two specimens; one was
snap-frozen in liquid nitrogen and stored at -80°C for gene
expression analysis and the other was fixed in 10% formalin within
48 h and embedded in paraffin for immunohistochemical analysis. For
clinical diagnosis, ER was stained by an automated machine for
immunohistochemistry (IHC) (Benchmark; Ventana Japan, Yokohama,
Japan), and HER2 was stained using a HER2 kit (Dako HercepTest;
DakoCytomation, Carpinteria, CA, USA) according to the
manufacturer’s protocols. The ER-IHC results were interpreted as
positive when >10% of the cancer cells immunoreacted. HER2-IHC
was assigned scores according to the following ranking: 3+
(positive), 2+ (equivocal) and 0 or 1+ (negative). HER2 was
interpreted as positive when IHC 3+ or the HER2/CEP17 signal ratio
was ≥2.2 from fluorescence in situ hybridization (FISH) for
IHC 2+ tumors. None of the patients had received any pre-operative
adjuvant hormone treatment or chemotherapy.
 | Table IClinicopathological features and
immunohistochemical phenotype of the breast cancers examined. |
Table I
Clinicopathological features and
immunohistochemical phenotype of the breast cancers examined.
|
ER-positive/HER2-negative |
ER-positive/HER2-positive |
ER-negative/HER2-positive |
ER-negative/HER2-negative |
|---|
| Tumor size |
| T1 | 7 | 3 | 4 | 4 |
| T2 | 2 | 6 | 2 | 4 |
| T3 | 1 | 1 | 3 | 2 |
| Lymph node
metastasis |
| Present | 4 | 4 | 6 | 5 |
| Absent | 6 | 6 | 4 | 5 |
| Histological
grade |
| 1 | 5 | 0 | 1 | 0 |
| 2 | 5 | 8 | 5 | 4 |
| 3 | 0 | 2 | 4 | 6 |
| Immunohistochemical
phenotype |
| PgR-positive | 10 | 7 | 0 | 0 |
| EGFR-positive | 0 | 2 | 7 | 7 |
| CK5/6-positive | 1 | 1 | 4 | 8 |
| TP IHC score |
| Cancer cell | 1.3 | 1.1 | 1.5 | 1.4 |
| Stroma | 1.4 | 1.7 | 1.9 | 2.0 |
| Total | 2.7 | 2.9 | 3.4 | 3.4 |
Evaluation of gene expression
Details of the oligonucleotide microarray analysis
were as previously described (16).
Briefly, total RNA was extracted from the frozen tissues with
Sepazol-I (Wako, Osaka, Japan). The RNA was reverse-transcribed to
cDNA using T7-(dT)24 primer. Biotin-labeled cRNA was synthesized
from cDNA using a MegaScript In Vitro Transcript Kit (Ambion,
Austin, TX, USA), and then hybridized to human U95Av2
GeneChip® (Affymetrix, Santa Clara, CA, USA). The
hybridized oligonucleotide microarrays were scanned using a
confocal scanner (Affymetrix) and analyzed using Affymetrix
software (LIMS 5.0). The signal intensity of TP was expressed as a
logarithmic value.
Immunohistochemical analysis for
thymidine phosphorylase and the basal phenotype
Specimen sections (4 μm) were mounted on
silane-coated glass slides, deparaffinized in graded xylene and
dehydrated in ethanol. After the inhibition of intrinsic peroxidase
by immersion in methanol with 0.3% H2O2 for
30 min, antigen retrieval pretreatment was carried out by twice
heating in a microwave oven for 5 min (500 W) in 0.01 M citrate
buffer, pH 6.0. Anti-human mouse TP monoclonal antibody (1C6-203)
(16), anti-human EGFR antibody
(clone 2-18C9, EGFR pharmDx Kit; DakoCytomation), anti-human CK5/6
antibody (clone D5/16B4; DakoCytomation) and anti-human PgR were
used in this study. After incubation of the TP primary antibody,
mouse IgG (ICN Pharmaceuticals, Inc., Costa Mesa, CA, USA) was
soaked in 0.1 M phosphate buffer (pH 7.4) containing 3% blocking
serum and 0.3% Triton X-100 and incubated at 4°C overnight. The
slides were washed with PBS, incubated with biotin-labeled
secondary antibody and detected using a Vectastain Elite ABC kit
(Vector Laboratories, Inc., Burlingame, CA, USA). The levels of
immunostained TP in the tumor or stromal cells were independently
assigned a score (0, 1, 2, or 3, from lowest to highest) based on a
previous report (18). Total TP
expression was estimated from the sum of the scores observed in the
tumor and stromal cells. PgR was stained by an automated machine
for IHC, and EGFR was stained according to the manufacturer’s
protocols. Breast cancers that were negative for the expression of
ER, PgR and HER2 were determined to be TNBCs, and breast cancer
samples lacking an expression of ER, PgR and HER2 but expressing
EGFR and/or CK5/6 were considered to be BLBCs (6).
Statistics
Statistical analysis was performed using StatView
5.0 (Hulinks Inc.) on a Windows PC. P<0.05 was considered to be
of statistical significance.
Results
Thymidine phosphorylase mRNA
expression
As shown in Fig. 1A,
TP expression (array signal intensity of logarithm, 2.8184; 95% CI
2.7071–2.8833) was significantly higher in the tumors than that in
adjacent normal tissues (2.4260; 95% CI 2.1112–2.5975) (p=0.0061,
Mann-Whitney U test). To investigate the relationship between TP
expression and ER or HER2 status, the expression levels of TP were
compared between ER-positive and ER-negative (Fig. 1B), and between HER2-positive and
HER2-negative (Fig. 1C) breast
tumors. ER-negative breast tumors (2.9270; 95% CI 2.8352–3.0171)
showed a significantly higher TP expression than ER-positive breast
tumors (2.7529; 95% CI 2.5370–2.7914) (p=0.0035, Mann-Whitney U
test), whereas there was no significant difference in TP expression
between the HER2-positive and HER2-negative breast tumors
(p=0.2448, Mann-Whitney U test). To further investigate, we
compared TP expression between the ER-positive/HER2-positive,
ER-positive/HER2-negative, ER-negative/HER2-positive and the
ER-negative/HER2-negative tumor subtypes. In the multivariate
analysis (Fig. 1D), TP expression
in ER-negative/HER2-negative tumors (3.0641; 95% CI 2.8886–3.1392)
was higher than that in ER-positive/HER2-positive (2.7179; 95% CI
2.3667–2.8321) and ER-positive/HER2-negative tumors (2.7529; 95% CI
2.6435–2.8137). In addition to the ER and HER2 status, the
expression of PgR, EGFR and CK5/6 is summarized in Table I. The ER-negative/HER2-negative
tumors were also negative for PgR and were considered to be TNBC.
Moreover, 8/10 of the ER-negative/HER2-negative tumors were BLBC,
expressing EGFR and/or CK5/6. We then compared TP expression of
TNBC or BLBC to that of the other tumor groups. TP expression was
higher in TNBC (p=0.0030, Mann-Whitney U test) (Fig. 1E) and BLBC (p=0.0019, Mann-Whitney U
test) (Fig. 1F) than in non-TNBC
and non-BLBC tumors, respectively.
Immunohistochemical analysis of thymidine
phosphorylase
In addition to comparing the mRNA levels, we
investigated TP expression immunohistochemically in breast cancer
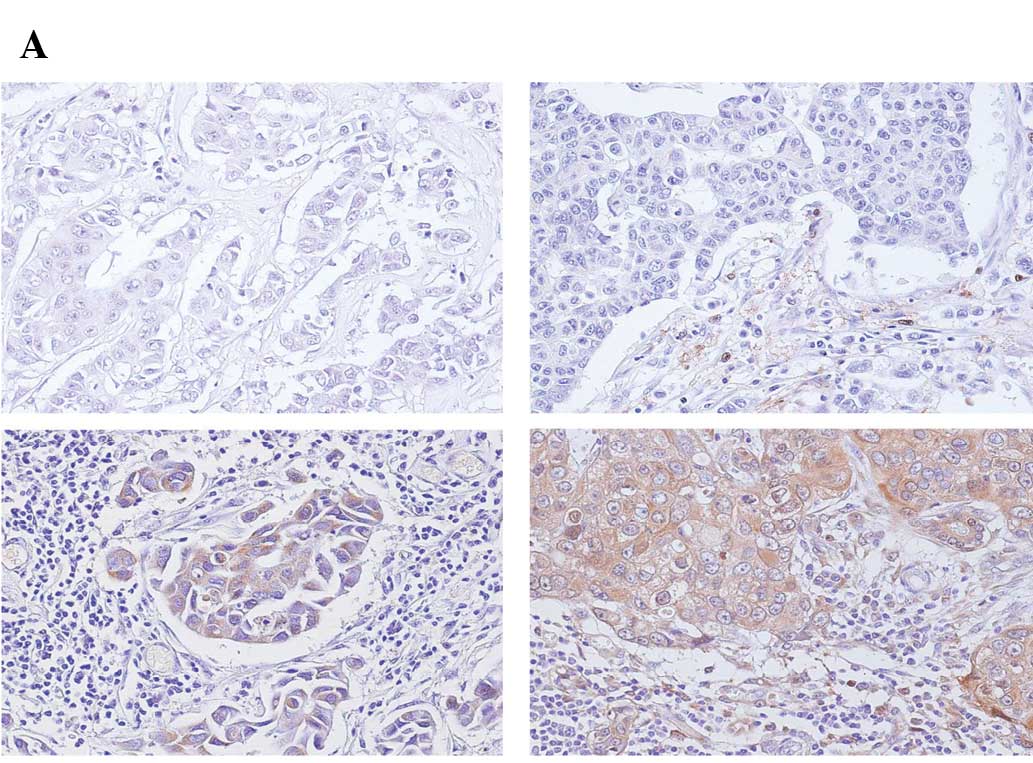
tissues. Typical patterns of TP expression in cancers are shown in
Fig. 2A including no expression
(top left), expression in stroma (top right), expression in cancer
cells (bottom left), and expression in both stroma and cancer cells
(bottom right). The proportion of TP expression in cancer cells and
stroma varied from tumor to tumor in the four groups of breast
tumors. Subsequently, the tumor samples were evaluated according to
previously reported scores (13).
Among the 40 specimens, total immunohistochemical TP staining
scores weakly but significantly correlated to mRNA expression
(r=0.34, p=0.034) (Table I,
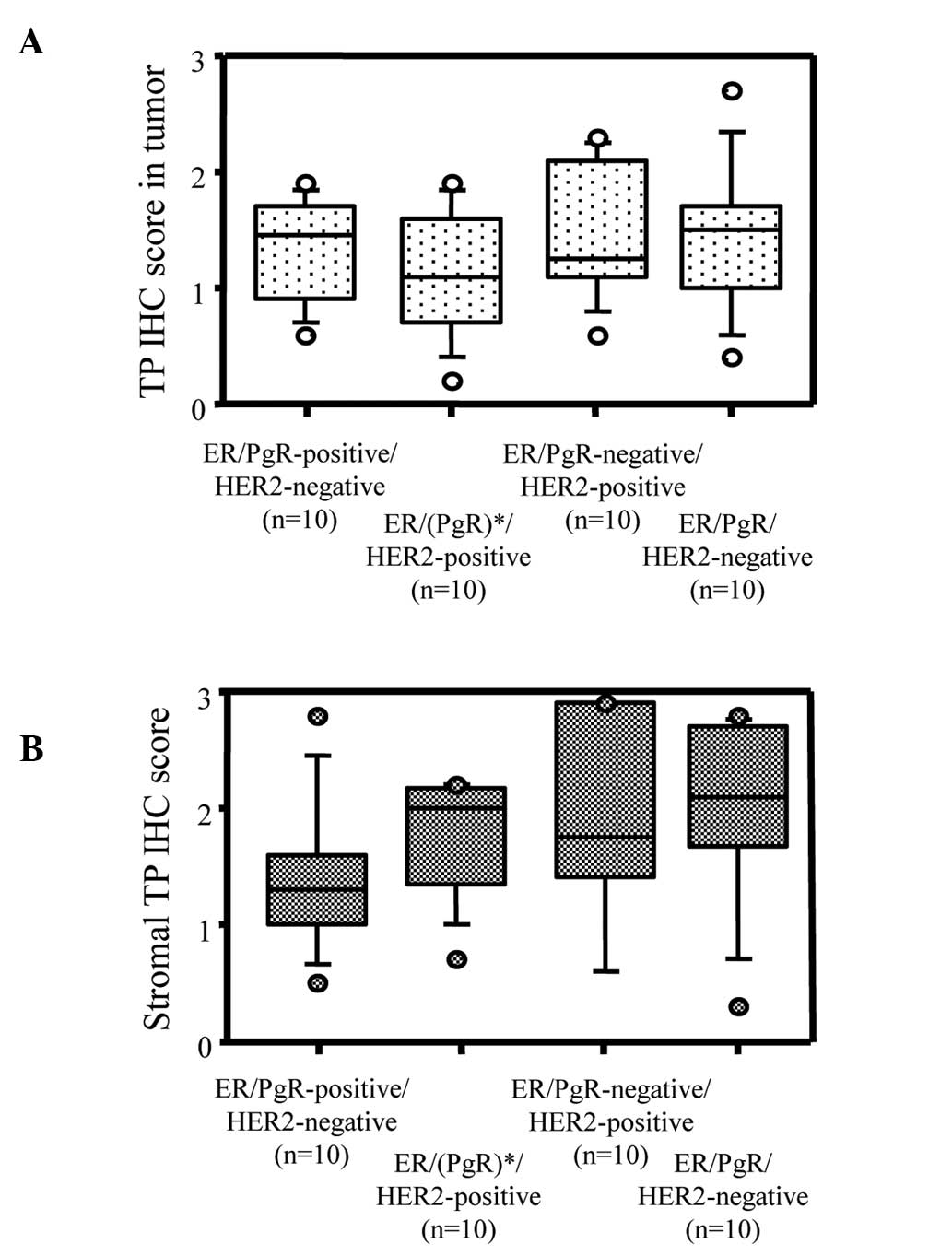
Fig. 2B). Although total TP
expression scores showed no differences among the
ER/PgR/HER2-positive, ER/(PgR)-positive/HER2-negative,
ER/PgR-negative/HER2-positive and ER/PgR/HER2-negative breast
tumors (Fig. 3A), TP expression in
the stromal cells tended to be higher in ER-negative breast cancer
than in ER-positive breast cancer (Fig.
3B).
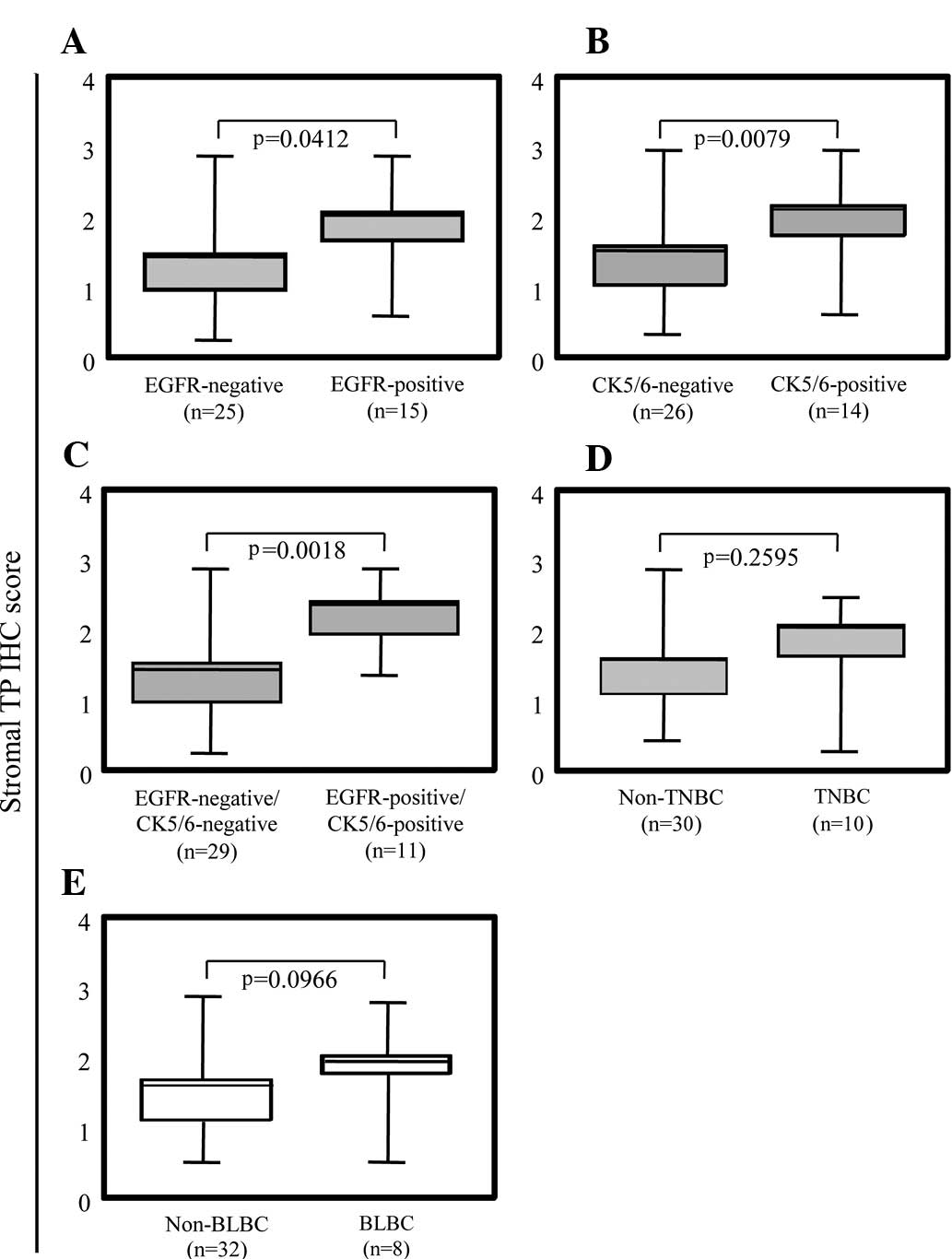
We further analyzed the correlation between TP
expression in the stromal cells and basal phenotypes of breast
cancer. TP expression in the stromal cells of the EGFR- and
CK5/6-positive breast tumors was significantly higher than that in
each negative group (Fig. 4A and
B). Notably, both EGFR- and CK5/6-positive breast tumors showed
extensively higher TP expression in the stromal cells (Fig. 4C). TP expression in the stromal
cells demonstrated a tendency related to BLBC (p=0.0979,
Mann-Whitney U test) (Fig. 4E),
even though TNBC showed no tendency with TP expression in stromal
cells (Fig. 4D). Taken together,
the high level of expression of TP in the stromal cells correlated
with ER-negative breast cancers expressing CK5/6 and EGFR.
Discussion
The present study demonstrated that the expression
of the TP gene was higher in TNBC and BLBC. Immunohistochemical
analysis results showed that a higher TP expression in the stroma
significantly correlated with ER-negative breast cancers expressing
EGFR and CK5/6. Intracellular signaling via ER or HER2 is
considered essential for tumor growth and/or anti-apoptosis, and
there are therapeutic strategies to inhibit these receptors such as
tamoxifen, letrozol and trastuzumab. However, in
ER-negative/HER2-negative breast tumors, the transmitted signals
that result in tumorigenesis and anti-apoptosis remain unclear.
TNBC and BLBC share clinical features such as a poor patient
outcome. Moreover, they lack the benefit of a targeted therapy
(3–5,19). It
is widely accepted that TNBC is heterogeneous and includes BLBC, as
a significant subgroup, as well as non-basal phenotypes. Among the
specific markers for BLBC such as EGFR, CK5/6, BRCA1, c-kit and p53
mutation (3–5), EGFR or c-kit may be candidate
treatment targets. Although EGFR overexpression has been observed
in approximately half (57%) of BLBCs (19), at present insufficient evidence
exists regarding a treatment targeting EGFR in BLBC patients
(20,21). TP exerts tumorigenic, anti-apoptotic
and angiogenic functions (12) and,
considering the results of the present study, TP is a reasonable
target for patients with BLBC.
Our results contributed to the elucidation of the
relationship between cancer cells and stroma. The expression level
of the TP gene in tumor subtypes correlated with TP IHC scores in
the stroma, which were higher. Stromal tissue is presumed to be a
significant source of TP gene expression throughout the entire
tumor. Since the stromal compartment has been reported to be
associated with breast tumor initiation and progression (22), many investigators recently focused
on this relationship. Stromal gene expression profiles can predict
clinical outcome for patients with breast tumors (23). Stromal cells play an important role
in breast tumors, and the microenvironment that includes the tumor
and stromal cells needs to be considered when evaluating drug
sensitivity to breast tumors. With regard to TP expression, it was
demonstrated that human TP cDNA transfection in colorectal
(24) and non-small lung cancer
cells (25) increased sensitivity
to 5′-DFUR and induced a bystander effect towards co-cultured
parental cells with increased 5′-DFUR sensitivity. Therefore, it is
theoretically expected that stromal TP would induce a bystander
effect towards adjacent tumor cells when treated with capecitabine.
Since capecitabine was designed as a pro-drug that is selectively
converted to 5-FU by TP in tumor tissue (9), it is expected to be more efficiently
converted to 5-FU in a tumor microenvironment with a higher
expression level of TP even though it is expressed in the
stroma.
It remains unclear why the expression of TP in
stromal and cancer cells is higher in ER-negative expressing EGFR-
and CK5/6-positive breast cancers. TP expression is induced by
hypoxia and several cytokines such as tumor necrosis factor α,
interleukin 1, or interferon γ (26). One possible mechanism is that
ER-negative and EGFR/CK5/6-positive cancer cells secrete cytokines
that may cause the stromal cells to induce a high TP in the stroma.
Notably, there were no significant differences between the TP IHC
scores for TNBC/non-TNBC and BLBC/non-BLBC, possibly because there
is no correlation between TP expression and HER2 that offsets the
difference between TNBC/non-TNBC and BLBC/non-BLBC. It is
noteworthy that EGFR- and CK5/6-positive cancer cells are more
intimately related to stromal TP expression than HER2-positive
breast cancers.
The hypoxic mechanism of TP induction is one of the
explanations for the efficacy of capecitabine in tumors after
treatment with other anticancer drugs (12). In preclinical studies, capecitabine
showed at least an additive effect in human breast cancer xenograft
models in combination with trastuzumab (23) and endocrine therapies (28). In human cancer tissues of various
organs, TP expression was demonstrated to be significantly higher
in tumors than in adjacent normal tissues, regardless of the ER or
HER2 status [Kataoka et al, Cancer Res 69 (Suppl 2): abs.
2012, 2009]. In the clinical setting, capecitabine is often
administered in combination with other anticancer drugs for breast
cancers, and combination therapies have achieved a more favorable
response rate, time-to-progression, and/or overall survival in
advanced or metastatic breast cancer patients than capecitabine
monotherapy (29). Collectively,
capecitabine is expected to be effective for BLBC and HER2-positive
breast cancers expressing EGFR and CK5/6 in combination with
chemotherapy and trastuzumab.
In conclusion, the expression of TP was higher in
BLBC, and capecitabine-based chemotherapy is a likely candidate for
the treatment of patients with BLBC. The results warrant clinical
trials of capecitabine in combination with chemotherapy or
trastuzumab based on hormonal/HER2 receptor status.
References
|
1
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Panel members: Thresholds for
therapies: highlights of the St Gallen International expert
consensus on the primary therapy of early breast cancer 22009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar
|
|
2
|
Early Breast Cancer Trialists
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI
|
|
3
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
4
|
Van’t Veer LJ, Dai H, van de Vijver MJ, et
al: Gene expression profiling predicts clinical outcome of breast
cancer. Nature. 415:530–536. 2002.PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rakha EA, El-Sayed ME, Green AR, et al:
Prognostic markers in triple-negative breast cancer. Cancer.
109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blum JL: Xeloda in the treatment of
metastatic breast cancer. Oncology. 57(Suppl 1): 16–20. 1999.
View Article : Google Scholar
|
|
9
|
Miwa M, Ura M, Nishida M, et al: Design of
a novel oral fluoropyrimidine carbamate, capecitabine, which
generates 5-fluorouracil selectively in tumours by enzymes
concentrated in human liver and cancer tissue. Eur J Cancer.
34:1274–1281. 1998. View Article : Google Scholar
|
|
10
|
Fiedkin M and Roberts D: The enzymatic
synthesis of nucleosides. I. Thymidine phosphorylase in mammalian
tissue. J Biol Chem. 207:245–256. 1954.PubMed/NCBI
|
|
11
|
Moghaddam A and Bicknell R: Expression of
platelet-derived endothelial cell growth factor in Escherichia
coli and confirmation of its thymidine phosphorylase activity.
Biochemistry. 31:12141–12146. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toi M, Atiqur Rahman M, Bando H, et al:
Thymidine phosphorylase (platelet-derived endothelial-cell growth
factor) in cancer biology and treatment. Lancet Oncol. 6:158–166.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa T, Sekiguchi F, Fukase Y, et al:
Positive correlation between the efficacy of capecitabine and
doxifluridine and the ration of thymidine phosphorylase to
dihydropyrimidine dehydrogenase activities in tumors in human
cancer xenografts. Cancer Res. 58:685–690. 1998.
|
|
14
|
Sawada Y, Fujii T, Takahashi H, et al: A
case of triple negative chest wall recurrent breast cancer treated
with capecitabine and docetaxel combination therapy (XT therapy).
Gan To Kagaku Ryoho. 36:815–817. 2009.PubMed/NCBI
|
|
15
|
Hachisuka Y, Kamei Y, Umeoka T, et al: A
case of triple negative recurrent breast cancer successfully
treated with capecitabine + docetaxel combination chemotherapy. Gan
To Kagaku Ryoho. 35:475–458. 2008.PubMed/NCBI
|
|
16
|
Umemura S, Shirane M, Takekoshi S, et al:
Overexpression of E2F-5 correlates with a pathological basal
phenotype and a worse clinical outcome. Br J Cancer. 100:764–771.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kono T, Nishida M, Inagaki N, et al:
Development and characterization of 1C6–203, a new monoclonal
antibody specific to human thymidine phosphorylase. J Histochem
Cytochem. 49:131–138. 2001.
|
|
18
|
Tsuda H, Akiyama F, Kurosumi M, et al:
Reproducible immuno-histochemical criteria based on multiple
raters’ judgments for expression of thymidine phosphorylase in
breast cancer tissue. Breast Cancer Res Treat. 86:215–223.
2004.PubMed/NCBI
|
|
19
|
Nielsen TO, Hsu FD, Jensen K, et al:
Immunohistochemical and clinical characterization of the basal-like
subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baselga J, Albanell J and Ruiz A: Phase II
and tumor pharmaco-dynamic study of gefitinib in patients with
advanced breast cancer. J Clin Oncol. 23:5323–5333. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiu KK, Tan DS and Reis-Filho JS:
Development of therapeutic approaches to ‘triple negative’
phenotype breast cancer. Expert Opin Ther Targets. 12:1123–1137.
2008.
|
|
22
|
Kim JB, Stein R and O’Hare MJ:
Tumour-stromal interactions in breast cancer: the role of stroma in
tumourigenesis. Tumour Biol. 26:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Finak G, Bertos N, Pepin F, et al: Stromal
gene expression predicts clinical outcome in breast cancer. Nat
Med. 14:518–527. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evrard A, Cuq P, Ciccolini J, et al:
Increased cytotoxicity and bystander effect of 5-fluorouracil and
5-deoxy-5-fluorouridine in human colorectal cancer cells
transfected with thymidine phosphorylase. Br J Cancer.
80:1726–1733. 1999. View Article : Google Scholar
|
|
25
|
Kato Y, Matsukawa S, Muraoka R, et al:
Enhancement of drug sensitivity and a bystander effect in PC-9
cells transfected with a platelet-derived endothelial cell growth
factor thymidine phosphorylase cDNA. Br J Cancer. 75:506–511. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eda H, Fujimoto K, Watanabe S, et al:
Cytokines induce thymidine phosphorylase expression in tumor cells
and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer
Chemother Pharmacol. 32:333–338. 1993.PubMed/NCBI
|
|
27
|
Tripathy D: Capecitabine in combination
with novel targeted agents in the management of metastatic breast
cancer: underlying rationale and results of clinical trials.
Oncologist. 12:375–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujimoto-Ouchi K, Sekiguchi F and Tanaka
Y: Antitumor activity of combinations of anti-HER-2 antibody
trastuzumab and oral fluoropyrimidines capecitabine/5′-dFUrd in
human breast cancer models. Cancer Chemother Pharmacol. 49:211–216.
2002.PubMed/NCBI
|
|
29
|
Mori K, Hasegawa M, Nishida M, et al:
Expression levels of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in various human tumor tissues. Int J Oncol.
17:33–38. 2000.PubMed/NCBI
|