Introduction
The inflammatory cytokines IL-6, IL-8, TNF-α and
IL-1β play roles in mediating the cellular injury and pathogenesis
of chronic inflammatory diseases (1–3). TNF-α
and IL-1β initiate the cascade of destructive events, in part,
through the activation of transcription factor NF-κB, which in turn
induces several proinflammatory genes. In addition,
mitogen-activated protein kinases (MAPKs) regulate key
proinflammatory pathways following stimulation with UV and TNF-α
(4,5). Three MAPK proteins, i.e.,
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK) and p38 MAPK are thought to play different roles in
chronic inflammatory diseases and homeostasis in the skin (6–8).
Glucosamine, an amino sugar, plays a role in
improving arthritis in patients due to the anti-inflammatory action
of glucosamine compounds that are associated with the suppression
of neutrophil functions and proinflammatory cytokines (9–11).
Moreover, structural modifications to glucosamine by introducing
new functional groups can be expected to improve its therapeutic
effects (12). As in the case of
glucosamine, curcumin, extracted from C. longa, is a
promising anti-inflammatory agent under various experimental
conditions (13,14). Curcumin attenuates the expression of
TNF-α or ultraviolet-induced inflammatory cytokines in cells
(15–17). However, it is still largely unknown
whether glucosamine inhibits the TNF-α-induced expression of
inflammatory cytokines in the HaCaT keratinocyte cell line. Thus,
the present study investigated the anti-inflammatory effect of
glucosamine in HaCaT keratinocyte cells with or without TNF-α
treatment. In addition, the inhibitory effects of glucosamine were
compared to those of curcumin in the HaCaT keratinocyte cell
line.
Materials and methods
Materials
Curcumin, glucosamine and TNF-α were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies against phospho-ERK
(p-ERK), ERK, phospho-p38 (p-p38), p38, phospho-JNK (p-JNK) and JNK
were purchased from Cell Signaling (Beverly, MA, USA).
Cell culture
The HaCaT keratinocyte cell line was maintained at
37°C in a humidified atmosphere of 95% air and 5% CO2 in
Dulbecco’s modified Eagle’s medium supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin. For the experiments, cells
(5×104/ml) were seeded in a culture dish and maintained
in the tissue culture incubator.
Chemical agent treatment
Cells were cultured and treated with glucosamine
(1–10 mM), curcumin (1–20 μM) or TNF-α (20 ng/ml) for 24 h.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cells using RNAzol™
B (Biotech Laboratories, Houston, TX, USA) according to the
manufacturer’s instructions and then quantitated with a
spectrophotometer. Total RNA (1 μg) was reverse transcribed using
M-MLV Reverse Transcriptase (Promega Co., Madison, WI, USA). The
PCR reaction was carried out under the conditions recommended by
the manufacturer (Takara Co., Otsu, Japan). The primer sequences
and product sizes were as follows: GAPDH forward, 5′-CGT CTT CAC
CAC CAT GGA GA-3′; reverse, 5′-CGG CCA TCA CGC CAC AGT TT-3′; IL-6
forward, 5′-GTG TGA AAG CAG CAA AGA GGC-3′; reverse, 5′-CTG GAG GTA
CTC TAG GTA TAC-3′; IL-8 forward, 5′-ATG ACT TCC AAG CTG GGC CGT
G-3′; reverse, 5′-TAT GAA TTC TCA GCC CTC TTC AAAA-3′; TNF-α
forward, 5′-CAA AGT AGA CCT GCC CAG AC-3′; reverse, 5′-GAC CTC TCT
CTA ATC AGC CC-3′; IL-1β forward, 5′-AAA AGC TTG GTG ATG TCT GG-3′;
reverse, 5′-TTT CAA CAC GCA GGA CAG G-3′.
Western blot analysis
Cells were lysed in lysis buffer [10 mM Tris (pH
7.4), 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, 10 μg/ml PMSF, 10
μg/ml aprotinin, 10 μg/ml leupeptin, 5 mM phenanthroline and 28 mM
benzamidine-HCl] for 30 min on ice. Lysates were clarified by
centrifugation and quantitated using the Bradford assay (Life
Science Co., CA, USA) with bovine serum albumin as a reference
standard. Proteins (35 μg) were resolved by sodium dodecyl
sulfate-polyacrylamide gels and transferred to an Immobilon-P
Transfer Membrane (Millipore Co., Billerica, MA, USA). After
incubation with the primary antibodies, proteins were visualized by
incubation with horseradish peroxidase-conjugated secondary
antibodies, followed by ECL according to the manufacturer’s
instructions (Amersham Life Science Co., Buckinghamshire, UK).
Results
Effect of glucosamine and curcumin on the
expression of proinflammatory cytokines in HaCaT cells
To study the effect of glucosamine and curcumin
treatment on the expression of IL-6, IL-8, TNF-α and IL-1β, HaCaT
cells were exposed to glucosamine at doses ranging from 1 to 10 mM,
and curcumin at doses ranging from 1 to 20 μM. Cells were then
harvested for 24 h after treatment for RT-PCR analysis. As shown in
Fig. 1A, IL-6, IL-8, TNF-α and
IL-1β expression was clearly decreased in a dose-dependent manner
in the glucosamine-treated HaCaT cells. In contrast to glucosamine
treatment, the expression of IL-8, TNF-α and IL-1β was increased in
the curcumin-treated HaCaT cells (Fig.
1B). Increased expression of IL-8 and IL-1β was clearly
visualized at >10 μM curcumin treatment, following a sustained
increased expression of IL-8 and IL-1β. The results indicate that
glucosamine induces the down-regulation of IL-6, IL-8, TNF-α and
IL-1β, while curcumin induces the up-regulation of IL-8 and IL-1β
in HaCaT cells.
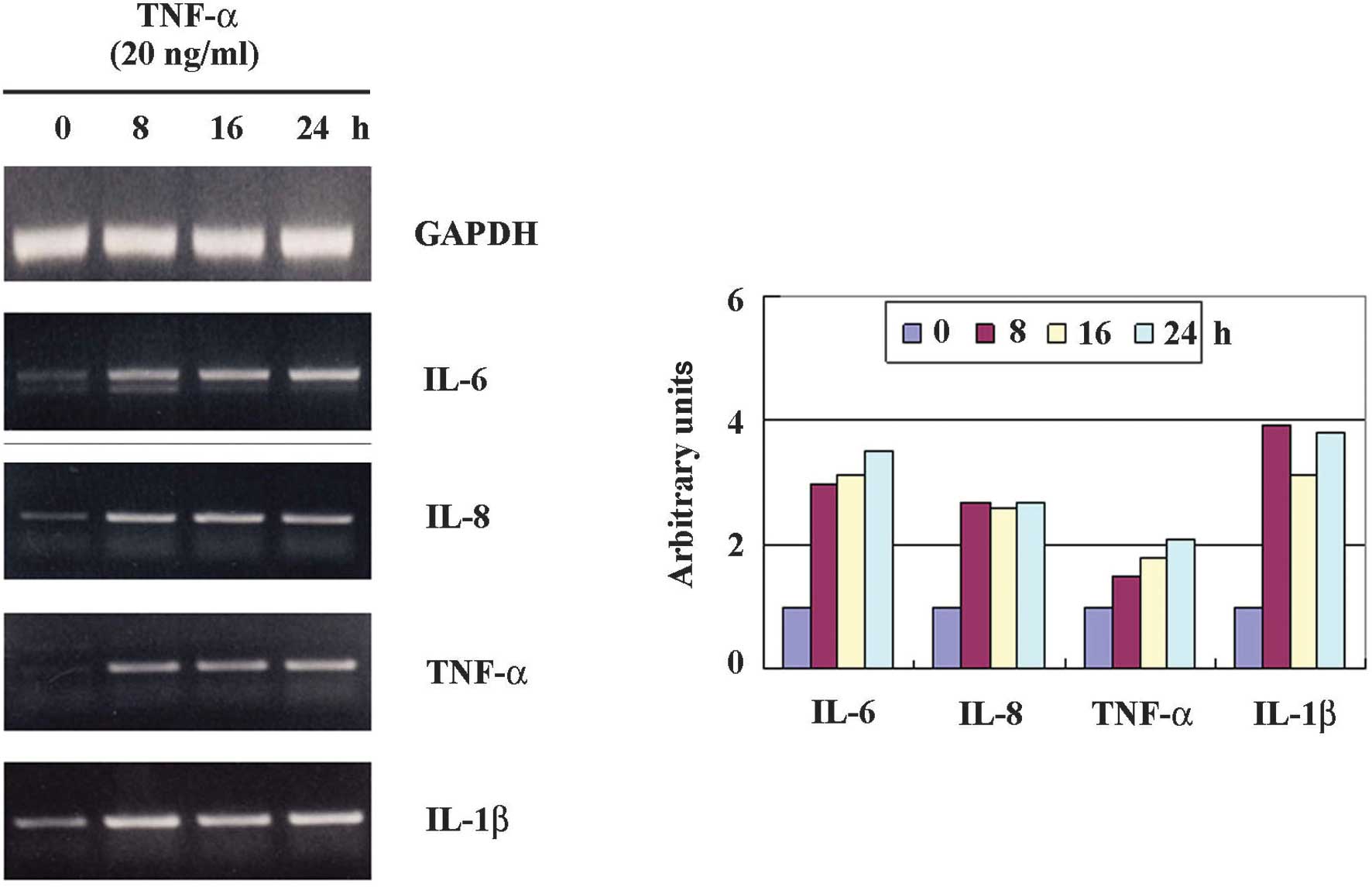
Effect of TNF-α on the expression of
proinflammatory cytokines in HaCaT cells
To study the effect of TNF-α treatment on the
expression of IL-6, IL-8, TNF-α and IL-1β, HaCaT cells were exposed
to TNF-α at doses of 20 ng/ml. The cells were then harvested for 24
h after treatment for RT-PCR analysis. As shown in Fig. 2, IL-6, IL-8, TNF-α and IL-1β
expression was increased and visualized at 8 h, following the
sustained increased expression. Thus, we investigated whether
glucosamine inhibits the TNF-α-induced up-regulation of IL-6, IL-8,
TNF-α and IL-1β in HaCaT cells.
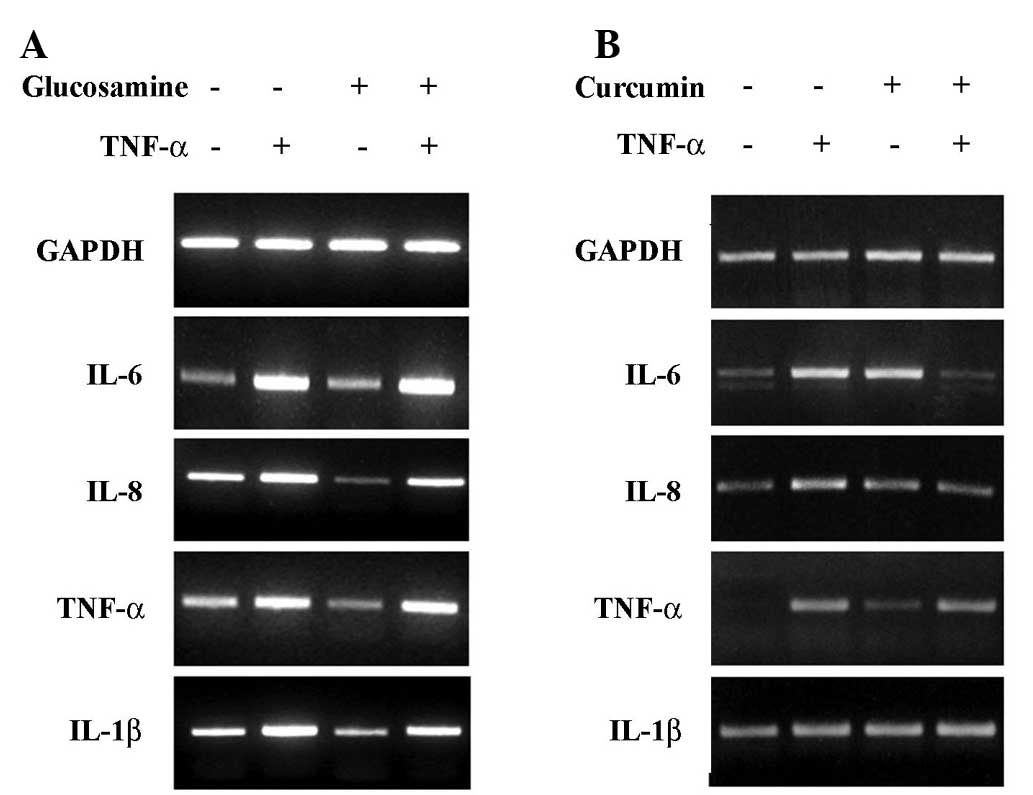
Effect of glucosamine on TNF-α-induced
expression of proinflammatory cytokines in HaCaT cells
To investigate whether glucosamine inhibits
TNF-α-induced IL-6, IL-8, TNF-α and IL-1β expression, HaCaT cells
were treated with TNF-α (20 ng/ml) with or without glucosamine (10
mM). These results were compared to those of curcumin treatment. As
shown in Fig. 3A, the up-regulation
of IL-6, IL-8, TNF-α and IL-1β by TNF-α treatment was not decreased
by glucosamine treatment. These results indicate that, even though
glucosamine reduces the expression of proinflammatory cytokines in
cells, the glucosamine-induced inhibitory effect on the expression
of proinflammatory cytokines, such as IL-6, IL-8, TNF-α and IL-1β,
is eliminated by the presence of a potent inflammatory stimulus
such as TNF-α. However, the up-regulation of IL-6, IL-8, TNF-α and
IL-1β by TNF-α treatment was decreased by curcumin treatment
(Fig. 3B).
Effect of glucosamine on TNF-α induced
the activation of MAPKs
Accumulating data suggest that TNF-α-treated cells
show an increased expression of IL-6, IL-8, TNF-α and IL-1β through
MAPK-dependent pathways such as p38 MAPK and JNK. However, it is
unknown whether glucosamine modulates the expression of IL-6, IL-8
and IL-1β by inhibiting the MAPK pathways in TNF-α-treated HaCaT
cells. We compared the MPK inhibitory effects between glucosamine
and curcumin. We examined the effect of glucosamine (10 mM) or
curcumin (10 μM) on the activation of p38 MAPK, JNK and ERK in
HaCaT cells. As shown in Fig. 4,
treatment of glucosamine (10 mM) did not inhibit the activation of
p38, JNK and ERK in HaCaT cells treated with TNF-α. However, in the
presence of curcumin (20 μM), TNF-α-induced JNK and ERK activation
was dramatically inhibited, evidenced by decreased phospho-p38 and
ERK. These results suggest that TNF-α-induced MAPK activation is
not inhibited by glucosamine treatment in HaCaT cells, but is
inhibited by curcumin. Stripping and reprobing the same membrane
with antibodies against p38, JNK and ERK revealed no change in the
total protein levels of each kinase (data not shown).
Discussion
Glucosamine is thought to exert anti-inflammatory
effects, including the suppression of inflammatory cytokines under
various conditions. In addition, glucosamine was found to inhibit
inducible nitric oxide synthase and cyclooxygenase-2 in
lipopolysaccharide (LPS)-stimulated RAW264.7 cells (18) and suppress the proliferation of
human prostate carcinoma DU145 cells through the inhibition of
STAT3 signaling (19). Numerous
studies have repoted on the anti-inflammatory effects of
glucosamine and chondroitin sulfate. The two sulfates are agents
that relieve pain, improve functional activity and slow disease
progression in osteoarthritis especially that of the hip and knee
(20). However, the
anti-inflammatory effect of glucosamine in inflammatory skin
disease still remains unknown. Our data showed that glucosamine
inhibited the growth of the HaCaT keratinocyte cell line and
induced the down-regulation of IL-6, IL-8, TNF-α and IL-1β mRNA,
suggesting that glucosamine also plays a role in alleviating
inflammatory skin diseases.
TNF-α is a primary inflammatory cytokine with a
broad range of proinflammatory and immunostimulatory activities.
TNF-α plays an important role in the development of inflammatory
skin diseases such as psoriasis and atopic dermatitis (21–23).
Down-stream inflammatory responses to TNF-α are mediated through
the up-regulation of IL-1, IL-2, IL-6, IL-10 and IFN-γ (5,24).
Thus, broad anti-inflammatory effects may be achieved through the
inhibition of the TNF-α pathways. However, we did not observe an
inhibitory effect of glucosamine on the TNF-α-induced up-regulation
of IL-6, IL-8, TNF-α and IL-1β in HaCaT cells. In addition, the
activation of MAPKs such as p38, JNK and ERK in TNF-α-treated HaCaT
cells was not inhibited by glucosamine. These results were similar
to a previous study in which glucosamine was noted to have no
effect on the activation of p38 MAPK, JNK-1/2 and ERK-1/2 in
IL-1β-stimulated A549 cells (9). In
contrast, glucosamine was shown to block LPS-induced activation of
p38 MAPK and JNK in RAW264.7 cells (18). Thus, the glucosamine-induced
down-regulation of the MAPK pathway requires further study under
various conditions. Curcumin is known to exert its
anti-inflammatory activity in various cell lines through the
inhibition of the MAPK pathways. In this study, curcumin attenuated
the expression of TNF-α-induced IL-6 and IL-8 in HaCaT cells, as
well as the inhibition of TNF-α-induced JNK and ERK activation.
In conclusion, the findings of the present study
demonstrate, for the first time, to the best of our knowledge, that
glucosamine inhibits the expression of IL-6, IL-8, TNF-α and IL-1β
in HaCaT cells, but not in the presence of TNF-α. These results
suggest that glucosamine can be used as a candidate
immunomodulatory agent in inflammatory skin disease, while its use
in the case of TNF-α-overexpressing skin disease should be further
investigated.
References
|
1
|
Damjanov N and Vojinovic J: Biologic
therapy of rheumatoid arthritis. Srp Arh Celok Lek. 137:205–210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding C, Cicuttini F, Li J and Jones G:
Targeting IL-6 in the treatment of inflammatory and autoimmune
diseases. Expert Opin Investig Drugs. 18:1457–1466. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kovarikova M, Hofmanova J, Soucek K and
Kozubik A: The effects of TNF-alpha and inhibitors of arachidonic
acid metabolism on human colon HT-29 cells depend on
differentiation status. Differentiation. 72:23–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carlsen H, Alexander G, Austenaa LM,
Ebihara K and Blomhoff R: Molecular imaging of the transcription
factor NF-kappaB, a primary regulator of stress response. Mutat
Res. 551:199–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muthusamy V and Piva TJ: The UV response
of the skin: a review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways. Arch Dermatol Res. Sep 16–2009.(Epub ahead
of print).
|
|
6
|
Leng H, Luo X, Ma L, Kang K and Zheng Z:
Reversal of ultraviolet B-induced immunosuppression by inhibition
of the extracellular signal-regulated mitogen-activated protein
kinase. Photodermatol Photoimmunol Photomed. 25:264–269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neves BM, Cruz MT, Francisco V,
Garcia-Rodriguez C, Silvestre R, Cordeiro-da-Silva A, Dinis AM,
Batista MT, Duarte CB and Lopes MC: Differential roles of
PI3-kinase, MAPKs and NF-kappaB on the manipulation of dendritic
cell T(h)1/T(h)2 cytokine/chemokine polarizing profile. Mol
Immunol. 46:2481–2492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanova IA, Nakrieko KA and Dagnino L:
Phosphorylation by p38 MAP kinase is required for E2F1 degradation
and keratinocyte differentiation. Oncogene. 28:52–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong H, Park YK, Choi MS, Ryu NH, Song DK,
Suh SI, Nam KY, Park GY and Jang BC: Differential down-regulation
of COX-2 and MMP-13 in human skin fibroblasts by
glucosamine-hydrochloride. J Dermatol Sci. 56:43–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang SM, Chen CY, Chen SS and Chen JC:
Chitinous materials inhibit nitric oxide production by activated
RAW 264.7 macrophages. Biochem Biophys Res Commun. 271:229–233.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing D, Feng W, Not LG, Miller AP, Zhang
Y, Chen YF, Majid-Hassan E, Chatham JC and Oparil S: Increased
protein O-GlcNAc modification inhibits inflammatory and neointimal
responses to acute endoluminal arterial injury. Am J Physiol Heart
Circ Physiol. 295:335–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kneass ZT and Marchase RB: Protein
O-GlcNAc modulates motility-associated signaling intermediates in
neutrophils. J Biol Chem. 280:14579–14585. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha R, Anderson DE, McDonald SS and
Greenwald P: Cancer risk and diet in India. J Postgrad Med.
49:222–228. 2003.PubMed/NCBI
|
|
14
|
Pari L, Tewas D and Eckel J: Role of
curcumin in health and disease. Arch Physiol Biochem. 114:127–149.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang ZY, Zou L, Shi SS, Lu YR, Dong J,
Yang CH, Lu YC and Dai GK: Effects of curcumin on TNF-alpha and
TGF-beta1 in serum and lung tissue of SiO(2)-induced fibrosis in
mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 25:399–401.
2009.PubMed/NCBI
|
|
16
|
Reuter S, Charlet J, Juncker T, Teiten MH,
Dicato M and Diederich M: Effect of curcumin on nuclear factor
kappaB signaling pathways in human chronic myelogenous K562
leukemia cells. Ann NY Acad Sci. 1171:436–447. 2009. View Article : Google Scholar
|
|
17
|
Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li
ST and Sun CH: Curcumin, a potential inhibitor of up-regulation of
TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes
through NF-kappaB and JNK pathway. Biomed Environ Sci. 22:32–39.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rafi MM, Yadav PN and Rossi AO:
Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse
macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and
transcription factor NF-kappaB. Mol Nutr Food Res. 51:587–593.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chesnokov V, Sun C and Itakura K:
Glucosamine suppresses proliferation of human prostate carcinoma
DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int.
9:25–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fox BA and Stephens MM:
Glucosamine/chondroitin/primorine combination therapy for
osteoarthritis. Drugs Today. 45:21–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonness S and Bieber T: Molecular basis of
atopic dermatitis. Curr Opin Allergy Clin Immunol. 7:382–386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capon F, Trembath RC and Barker JN: An
update on the genetics of psoriasis. Dermatol Clin. 22:339–347.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deane JA and Hickey MJ: Molecular
mechanisms of leukocyte trafficking in T-cell-mediated skin
inflammation: insights from intravital imaging. Expert Rev Mol Med.
11:e252009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escobar J, Pereda J, Arduini A, Sandoval
J, Sabater L, Aparisi L, Lopez-Rodas G and Sastre J: Cross-talk
between oxidative stress and pro-inflammatory cytokines in acute
pancreatitis: a key role for protein phosphatases. Curr Pharm Des.
15:3027–3042. 2009. View Article : Google Scholar : PubMed/NCBI
|