Introduction
Neoadjuvant chemotherapy (NAC) is currently
recognized as an important component of breast cancer treatment for
patients who i) are candidates for breast-conserving surgery, ii)
have advanced-stage disease but are not candidates and iii) refuse
surgery. Among the various tools for monitoring the response to NAC
for breast cancer, contrast-enhanced magnetic resonance imaging
(MRI) is considered to be superior to any other imaging modality
due to its high spatial resolution (1–6).
Developments have enabled diffusion-weighted imaging
(DWI) to be used more widely in the study of breast cancer
(7–11). Previous reports described the use of
DWI in the differentiation of malignant from benign breast cancer
(7,9–12).
Apparent diffusion coefficient (ADC) obtained in DWI is reported to
be useful for predicting early tumor response to NAC (12–14).
In contrast to conventional MRI, DWI does not require the use of
contrast-enhancing material and has good temporal resolution.
Breast cancer can be classified into several
patterns based on morphology (5,15–17).
In addition, the pattern of response to NAC depends largely on the
initial morphology (15,16). Focal or solitary nodular type tumors
usually shrink centrically, while non-focal or unlocalized tumors
shrink in a multicentric manner. Therefore, the response to NAC
does not always correspond to shrinkage over the extent of the
tumor. Optimal response in the solid nodular pattern is shrinkage
of the tumor, which may have the potential to scatter small
components within the range of the initial tumor bed in the
multiple nodular and/or unlocalized dendritic patterns (16). Breast cancer treatment therefore
requires different approaches for the two morphological patterns
(15,16). According to previous reports, focal
or solitary nodular type tumors may correspond to a focal mass,
while non-focal or multiple nodular and/or unlocalized dendritic
type tumors may correspond to multiple masses and/or non-mass-like
tumor, based on the Breast Imaging Reporting and Data System
(BI-RADS) (18).
We hypothesized that the effectiveness of imaging
parameters to gauge response to NAC in breast cancer may not be
uniform across all subclasses of tumors. Therefore, the aim was to
assess the efficacy of DWI of the breast to evaluate the response
to NAC in breast cancer patients based on tumor morphology on
MRI.
Materials and methods
Patients
Between April 2007 and September 2008, breast MRI
was performed at our hospital on 200 patients with breast cancer.
This retrospective study comprised 35 breast cancer patients (36
lesions) who underwent breast MRI before and after NAC. The time
interval between MRI and the last course of chemotherapy ranged
from 3 to 15 days (mean 7). Patient age ranged from 26 to 69 years
(mean 54). According to the tumor-node-metastases (TNM)
classification, the clinical stages of the tumors were IIA (n=17),
IIB (n=8), IIIA (n=7), IIIB (n=2) and IIIC (n=2). Patients were
treated with anthracycline-based (EC) chemotherapy, and received
four courses of epirubicine at 100 mg/m2 and
cyclophosphamide at 600 mg/m2. Written informed consent
was obtained from all subjects.
Magnetic resonance imaging
MRI was performed using a 1.5 T system (Signa HDx;
General Electric Healthcare, USA). The patients were examined in
the prone position using a breast coil. Dynamic MRI using a
three-dimensional fast spoiled gradient-echo sequence (VIBRANT,
volume imaging for breast imaging; TR 5.0 ms; TE 2.7 ms; flip angle
10°; FOV 28×28 cm; matrix 512×256; slice thickness 3 mm; space 0
mm; NEX 1) was obtained before and 8 times (every 30 sec) after a
bolus injection of 0.1 mmol gadolinium-diethylenetriamine
pentaacetic acid (Gd-DTPA)/kg by automatic injector at a rate of 3
ml/sec, followed by a 50 ml saline flush. Bilateral transverse
diffusion-weighted images with b-values of 0 and 1,500
s/mm2 were acquired before the administration of
contrast material (TR 5000 ms; TE 68.0 ms; flip angle 90°; FOV
36×36 cm; matrix 160×160; slice thickness 3 mm; space 0 mm; NEX
4).
MRI data analysis
Delayed enhanced images were classified as focal
mass (FM) or multiple masses and/or non-mass (MM/NM), based on
BI-RADS (18). ADC values were
calculated according to the formula: ADC = [In
S(h)/S(l)]/(b(h)-b(l)), where In is the natural log, and S(h) and
S(l) are the signal intensities in each region of interest (ROI),
placed on sections that correspond to two different b factors
(b=1500, 0 s/mm2]. In obtaining ADC values of the
lesions, ROIs were placed carefully within the largest section of
the tumor on DWI. The change in ADC following NAC was calculated
as: (ADC after NAC - ADC before NAC)/(ADC before NAC) ×100. The
largest possible ROI was used according to the size and morphology
of each tumor. The same radiologist calculated the ADCs of the
tumors before and after NAC. Tumor sizes were measured on delayed
enhanced MRI using the image with maximum tumor diameter and signal
intensity of the tumor relative to the signal intensity of
surrounding breast tissue. Tumor size was calculated as the
bi-axial diameter product using maximum and orthogonal diameter on
the maximum dimension of each tumor. Tumor response to NAC was
calculated as: (tumor size before NAC - tumor size after
NAC)/(tumor size before NAC) ×100.
Dynamic enhancement was evaluated by visual
assessment of the resulting curve, and delayed-enhancement patterns
were classified as washout, plateau or persistent, based on BI-RADS
(18). A ROI was placed manually on
the portion of the lesion with the most rapid enhancement or the
portion with the most suspicious washout curve.
Tumor morphologies and enhancement patterns were
evaluated independently by one radiologist, and DWI by another. The
two radiologists had >10 years of experience in the field of
breast MRI.
Surgical approach and histological
evaluation
In breast-conserving surgery, tumors were generally
resected with a 2-cm surgical margin. Breast specimens were
evaluated by routine pathologic examination. The specimens were
processed by serial gross sectioning at ~1-cm intervals. The
closest margin was evaluated histologically. In cases where it was
difficult to distinguish the extent of residual tumor on MRI after
NAC, a skin marker (alfacalcidol capsule of 0.5 μg for
osteoporosis) was placed on the skin immediately before scanning in
the supine position. Skin markers were helpful in evaluating the
correlation between MRI measurement and histological size.
Statistical analysis
Pearson’s correlation test was used to measure the
linear association between ADC and clinical tumor measurements. A
non-parametric test was used to assess significance in measuring
the differences between groups. The Mann-Whitney U test was
employed to measure the differences between the mean ADC of
responders and non-responders before and after NAC. P<0.01 was
assumed to indicate a statistically significant difference.
Results
Patient characteristics and MRI findings are
summarized in Table I.
Breast-conserving surgery was performed in 29 patients (30
lesions), total glandectomy in 3 and total mastectomy in 3. The
histological types of carcinoma included invasive ductal (n=34),
mucinous (n=1) and invasive lobular carcinoma (n=1). Assessment of
the tumor response by MRI was in agreement with the pathological
findings in 83% of cases (FM 23/26; MM/NM, 7/10). Imaging
assessment overestimated the degree of response in 1 case (FM) with
extensive intraductal components and in 3 cases (MM/NM) with
scattered invasive foci surrounding the lesions. The degree of
response was underestimated in 2 cases (FM) of pathological
complete response (pCR). In cases of MN/NM with a high ADC
response, despite a low MRI response, extensive scattering of the
residual tumor was identified histologically.
 | Table IClinical manifestation and imaging
findings of the cases examined. |
Table I
Clinical manifestation and imaging
findings of the cases examined.
| Characteristic | FM | MM/NM |
|---|
| Age (years) |
| Median | 55 | 56 |
| Range | 26–69 | 44–68 |
| TNM |
| IIA | 17 | 0 |
| IIB | 6 | 2 |
| IIIA | 1 | 6 |
| IIIB | 1 | 1 |
| IIIC | 1 | 1 |
| ADC (×10−3
mm2/s) |
| Before NAC |
| Average | 0.833 | 0.815 |
| SD | 0.153 | 0.146 |
| After NAC |
| Average | 0.963 | 1.137 |
| SD | 0.216 | 0.245 |
| Change in ADC
(%) |
| Average | 17 | 50.1 |
| SD | 23.6 | 58.9 |
| Size in MRI
(mm2) |
| Before NAC |
| Average | 531.1 | 2,088 |
| SD | 524.4 | 1,583 |
| After NAC |
| Average | 288.4 | 1,376.2 |
| SD | 277.2 | 1,048 |
| Response rate
(%) |
| Average | 44.5 | 34.2 |
| SD | 27.7 | 17.6 |
The tumors were well visualized on delayed
enhancement MRI before and after NAC. Before NAC, the average
maximum tumor size on MRI was 531.1 mm2 for FM
(SD=524.4; n=26) and 2,088 mm2 for MM/NM (SD=1,583;
n=10). Of the FM tumors, all except 2 had the appearance of a focal
mass after NAC, while 9 of the 10 MM/NM tumors had multiple masses
or a non-mass-like appearance after NAC. After NAC, the average
maximum tumor size was 288.4 mm2 for FM (SD=277.2) and
1,376.2 mm2 for MM/NM (SD=1048). The average response
rate according to tumor size on MRI was 44.5% for FM (SD=27.7) and
34.2% for MM/NM (SD=17.6).
The tumors were clearly visualized on DWI before
NAC; all except one were visualized after NAC. The average ADC
value before NAC was 0.833×10−3 mm2/s for FM
(SD=0.153) and 0.815×10−3 mm2/s for MM/NM
(SD=0.146). The average ADC value after NAC was
0.963×10−3 mm2/s for FM (SD=0.216) and
1.137×10−3 mm2/s for MM/NM (SD=0.245). The
average change in ADC was 17% for FM (SD=23.6) and 50.1% for MM/NM
(SD=58.9). An MM/NM tumor that was not observed on DWI after NAC
was clearly visualized on conventional MRI as having a 20% response
rate, which was confirmed by the pathological findings.
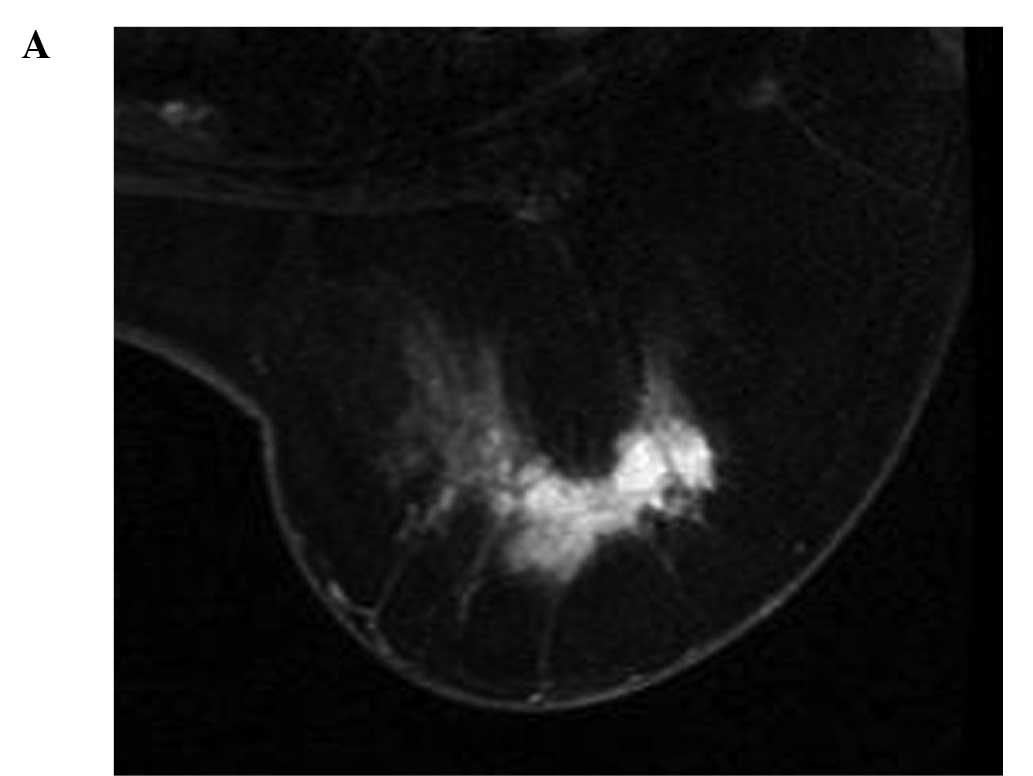
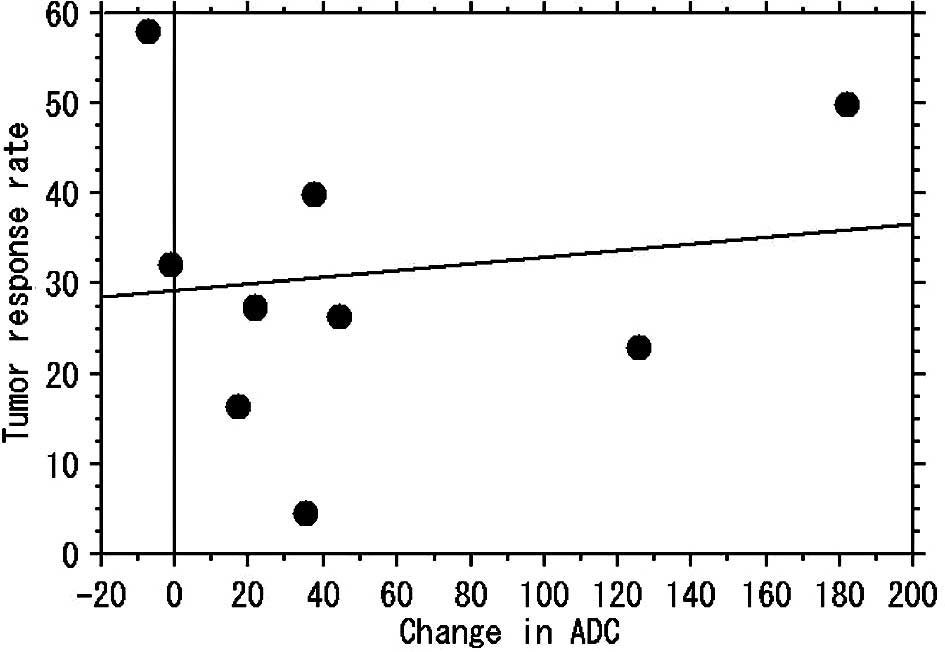
For the MM/NM tumors, no correlation was found
between change in ADC and the tumor reduction rate (r=0.141,
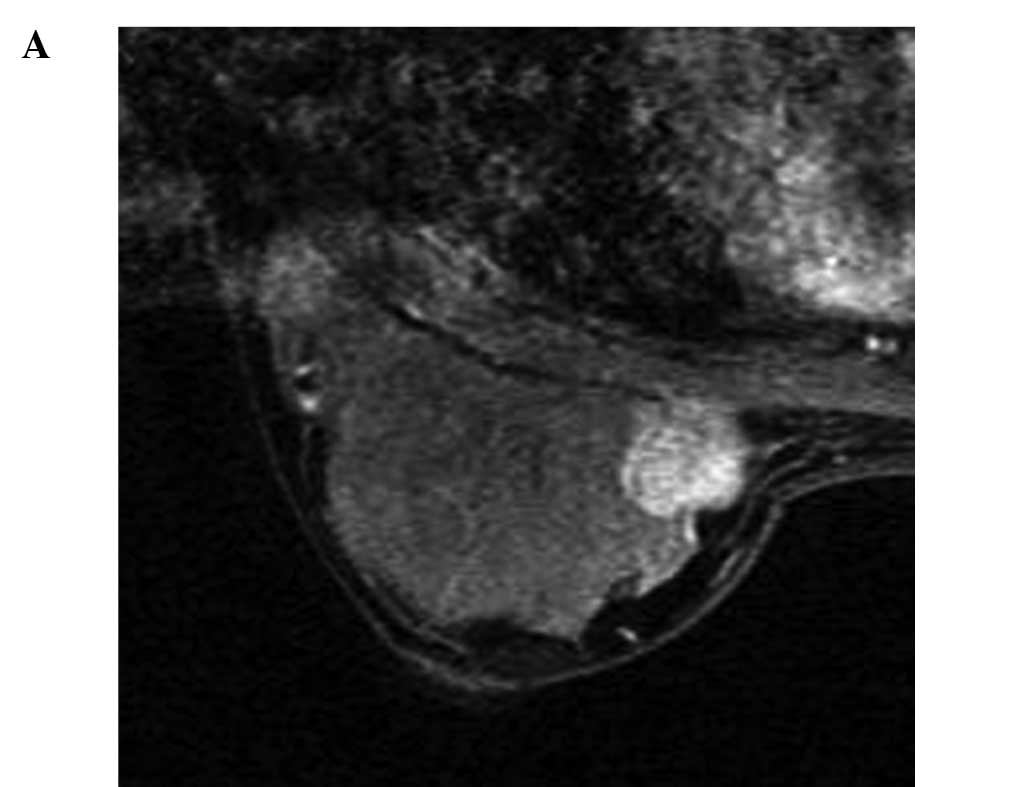
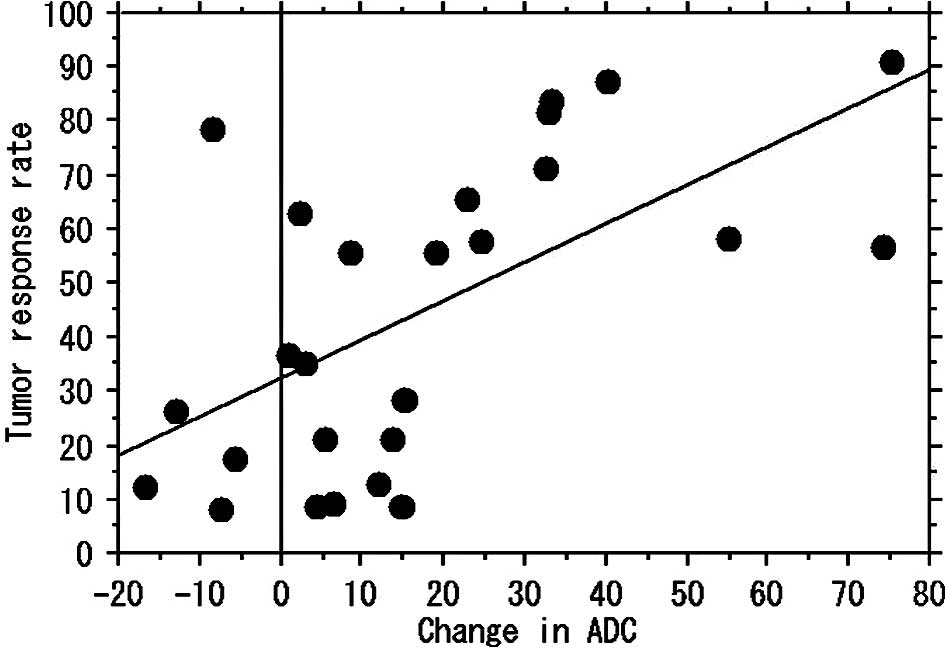
p=0.717) (Figs. 1 and 3). For 26 localized tumors, a positive
correlation was found between change in ADC and the tumor reduction
rate (r=0.608, p<0.001) (Figs. 2
and 4). Assessment of the delayed
enhancement patterns from dynamic MRI before NAC for the 36 tumors
revealed washout in 5 and plateau in 31 tumors. Persistent delayed
enhancement pattern was displayed in 12 cases of FM and 8 cases of
MM/NM; the remaining 16 (FM, 14; MM/NM, 2) tumors demonstrated
washout or plateau after NAC. For FM, change in the delayed
enhancement pattern was significantly correlated with the response
rate (p=0.008) and change in ADC (p=0.004). However, no such
correlations were found for MM/NM (p=0.431, p=0.449).
Discussion
MRI-based assessment commonly under- or
overestimates the extent of residual breast cancer following NAC
(6,19). However, among the various imaging
modalities, dynamic-enhanced MRI is recognized as the most
sensitive and has the highest spatial resolution. The use of
morphological concepts can reduce the discrepancy between MRI
assessment and pathological findings with regard to the extent of
residual tumors (15,16). The six under- or overestimated cases
of MRI in the present study are considered to be an exception,
particularly in the FM cases with extensive intraductal components
and in the three MM/NM cases with scattered invasive foci
surrounding the tumors. Multiple nodular and/or unlocalized
dendritic tumors usually demonstrate dendritic or multicentric
shrinking and remain extensively in the original tumor beds
(15,16). In the two cases of pCR, the
conventional MRI and DWI findings may represent so-called
pseudotumors corresponding to xanthomatous change (20).
The clinical usefulness of DWI has already been
established in patients with cerebral infarction in the acute stage
(21–23). Tumors are depicted as regions of
high signal intensity, since their ADC values are lower than those
for normal tissue. The applications of DWI have expanded to various
organs (24–27). In DWI, ADC reflects the biological
character of the tissue. Thus, ADC values in the breast are useful
in differentiating malignant from benign tumors, or in predicting
response to chemotherapy (7,9–14).
Despite its excellent contrast resolution, DWI is
vastly inferior to conventional studies, since its spatial
resolution is reduced by susceptibility and chemical shift
artifacts (28). Furthermore, cell
density is reportedly related to ADC (7,29).
Previous studies reported that DWI was not able to depict a small
compartment of focus of ductal carcinoma in situ neighboring
a main tumor, comedo-type ductal carcinoma in situ
containing notable bleeding or necrosis (9,11). In
the present study, one case was not visualized on DWI after NAC.
However, enhanced MRI showed an obvious residual tumor that was
able to be proven pathologically. In two other cases with a
discrepancy between the change in ADC and response to NAC on MRI,
extensive scattering of the residual tumor was observed in the
original tumor bed, although the central regions of tumors and
tumor cell density were not analyzed histologically in these
cases.
In MM/NM tumors, the response to NAC is not always
related to the shrinking of tumor extent. However, in FM tumors,
large changes in ADC indicate shrinking of the residual tumor. FM
tumors most commonly decreased in size following NAC, and were
centric to the initial tumor. Changes in the ADC of FM tumors may
thus be correlated to the reduction rates of the tumor extent and
density. In the case of FM, which could not be visualized on DWI
after NAC, we believed the tumors to be pCR or near-pCR.
Changes in the delayed enhancement pattern
correlated with the response rate and changes in ADC for FM but not
for MM/NM. In FM, changes in ADC may reflect quantitative changes
in cellularity and vascularity. In contrast, the response to NAC
may be heterogeneous and complex if the initial morphological
features in MM/NM are considered.
The present results suggest that change in ADC is
useful in assessing the response to NAC in FM tumors. In FM tumors,
DWI may be used instead of sequential conventional MRI to assess
response to NAC. This method may be both an effective and low-cost
means of assessing the tumor reduction rate. Since chemotherapy
regimens usually change according to the stage of cancer or
expression of human epidermal growth factor, the imaging tool used
to monitor the response to NAC may be selected on a morphological
basis.
To the best of our knowledge, no studies exist that
report on the relationship between changes in ADC and the effect of
NAC based on tumor morphology. The present results indicated that
change in ADC is correlated with the tumor reduction rate in FM-
but not MM/NM-type tumors.
Although the results prove the usefulness of DWI for
detecting the response to NAC for localized-type breast cancer,
several limitations were noted. First, the study population was
relatively small, with a much smaller number of MM/NM compared to
FM tumors. Our results therefore need to be confirmed in a larger
clinical study. Second, we used the bi-axial diameter product as
tumor size, although the three-dimensional product from tumor
morphology would also be acceptable. Third, only two b-factors of 0
and 1,500 s/mm2 were used. The use of multiple b-values,
including those higher and lower than 1,500, would have enabled a
more detailed discussion to be added to our results. However, ADC
by DWI using a high b-value would be less affected by perfusion
(30). Thus the smaller effect of
perfusion at a high b-value (1,500 s/mm2) used needs to
be considered. In addition, we measured mean ADC values, which may
have underestimated the global ADC response in heterogeneous
tumors. Further studies using any alternative measurements, such as
minimum, are needed to support our findings.
In conclusion, changes in ADC are well correlated
with the tumor reduction rate in NAC of breast cancer in FM tumors.
In MM/NM tumors, it is possible that changes in ADC do not indicate
a reduction in tumor extent but reflect tumor density of the
residual tumor. We therefore propose DWI as a potential tool for
NAC assessment based on morphological concepts.
Acknowledgements
We thank the radiological technologists of the MRI
division, Kazuo Morio, Hiroaki Yasunami, Shin Yaogawa and Ichiro
Morita for the technical support, and Mr. Ryo Akema and Ms. Shiho
Tokuhiro for assistance with the tables and figures.
References
|
1
|
Gilles R, Guinebretiere J-M, Toussaint C,
Spielman M, Rietjens M, Petit J-Y, Contesso G, Masselot J and Vael
D: Locally advanced breast cancer: contrast-enhanced subtraction MR
imaging of response to preoperative chemotherapy. Radiology.
191:633–638. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuboi N, Ogawa Y, Inomata T, Yoshida D,
Yoshida S, Moriki T and Kumon M: Changes in the findings of dynamic
MRI by preoperative CAF chemotherapy for patients with breast
cancer of stage II and III: Pathologic correlation. Oncol Rep.
6:727–732. 1998.PubMed/NCBI
|
|
3
|
Weatherall PT, Evans GF, Metzger GJ,
Saborrian MH and Leith AM: MRI vs. histologic measurement of breast
cancer following chemotherapy: comparison with X-ray mammography
and palpation. J Magn Reson Imaging. 13:868–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balu-Maestro C, Chapellier C, Bleuse A,
Chanalet I, Chauvel C and Largillier R: Imaging in evaluation of
response to neoadjuvant breast cancer treatment benefits of MRI.
Breast Cancer Res Treat. 72:145–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen EL, Blackwell KL, Bakser JA, Soo MS,
Bentley RC, Yu D, Samulski TV and Dewhirst MW: Accuracy of MRI in
the detection of residual breast cancer after neoadjuvant
chemotherapy. AJR Am J Roentgenol. 181:1275–1282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baudu LD, Murakami J, Murayama S,
Hashiguchi N, Sakai S Masuda K, Toyoshima S, Kuroki S and Ohno S:
Breast lesions correlation of contrast medium enhancement patterns
on MR images with histopathologic findings and tumor angiogenesis.
Radiology. 200:639–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma
L, Mahankali S and Gao JH: Differentiation of clinically benign and
malignant breast lesions using diffusion-weighted imaging. J Magn
Reson Imaging. 16:172–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Englander SA, Ulung AM, Brem R, Glickson
JD and Zijl PCM: Diffusion imaging of human breast. NMR Biomed.
10:348–352. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuroki Y, Nasu K, Kuroki S, Murakami K,
Hayashi T, Sekiguchi R and Nawano S: Diffusion-weighted imaging of
breast cancer with the sensitivity encoding technique: analysis of
the apparent diffusion coefficient value. Magn Reon Med Sci.
3:79–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woodhams R, Matsunaga K, Kan S, Hata H,
Ozaki M, Iwabuchi K, Kuranami M, Watanabe M and Hayakawa K: ADC
mapping of benign and malignant breast tumors. Magn Reon Med Sci.
4:35–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woodhams R, Matsunaga K, Iwabuchi K, Kan
S, Hata H, Kuranami M, Watanabe M and Hayakawa K: Diffusion
weighted imaging of malignant breast tumors: the usefulness of
apparent diffusion coefficient (ADC) value and ADC map for the
detection of malignant breast tumors and evaluation of cancer
extension. J Comput Assist Tomogr. 29:644–649. 2005.PubMed/NCBI
|
|
12
|
Rubesova E, Grell AS, De Maertelaer V,
Metens T, Chao S and Land Lemort M: Quantitive diffusion imaging in
breast cancer: a clinical prospective study. J Magn Reson Imaging.
24:319–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galons JP, Altbach MI, Paine-Murrieta GD,
Taylor CW and Gillies RJ: Early increases in breast tumor xenograft
water mobility in response to pacritaxel therapy detected by
non-invasive diffusion magnetic resonance imaging. Neoplasia.
1:113–117. 1999. View Article : Google Scholar
|
|
14
|
Pickles MD, Gibbs P, Lowry M and Turnbull
LW: Diffusion changes precede size reduction in neoadjuvant
treatment of breast cancer. Magn Reson Imaging. 24:843–847. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura S, Kenjo H, Nishio T, Kaxama T
and Doi O: Efficacy of 3D-MR mammography for breast conserving
surgery after neoadjuvant chemotherapy. Breast Cancer. 9:15–19.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata Y, Ogawa Y, Yoshida S, Kubota K,
Itoh S, Fukumoto M, Nishioka A, Moriki T, Maeda H and Tanaka Y:
Utility of initial MRI for predicting extent of residual disease
after neoadjuvant chemotherapy: Analysis of 70 breast cancer
patients. Oncol Rep. 12:1257–1262. 2004.
|
|
17
|
Tozaki M, Kobayashi T, Uno S, Aiba K,
Takeyama H, Shioya H, Tabei I, Toriumi Y, Suzuki M and Fukuda K:
Breast-conserving surgery after chemotherapy: value of MDCT for
determining tumor distribution and shrinkage pattern. AJR Am J
Roentogenol. 186:431–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
American College of Radiology. Breast
Imaging Reporting and Data System (BI-RADS). 4th edition. American
College of Radiology; Reston, VA: pp. 79–89. 2003
|
|
19
|
Rieber A, Zeitler H, Rosenthal H, Görich
J, Kreienberg R, Brambs HJ and Tomczak R: MRI of breast cancer:
influence of chemotherapy on sensitivity. Br J Radiol. 70:452–458.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stehling MK, Turner R and Mansfield P:
Echo-planar imaging: magnetic resonance imaging in a fraction of a
second. Science. 254:43–50. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan KB, Thamboo TP and Rju GC:
Xanthomatous pseudotumor: a usual postchemothrapy phenomenon in
breast cancer. Arch Pathol Lab Med. 127:739–741. 2003.PubMed/NCBI
|
|
22
|
Basser PJ, Pajevic S, Pierpaoli C, Dura J
and Aldroubi A: In vivo fiber tractography using DT-MRI data. Magn
Reson Med. 44:625–632. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rovira A, Rovira-Gols A, Pedraza S, Grive
E, Molina C and Alvarez-Sobin J: Diffusion-weighted MR imaging in
the acute phase of transient ischemic attacks. AJNR Am J
Neuroradiol. 23:77–83. 2002.PubMed/NCBI
|
|
24
|
Yamashita Y, Tang Y and Takahashi M:
Ultrafast MR imaging of the abdomen: echo plannar imaging and
diffusion-weighted imaging. J Magn Reson Imaging. 8:367–374. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ichikawa T, Haradome H, Hachiya J,
Nitatori T and Araki T: Diffusion-weighted MR imaging with
single-shot echo-plannar imaging in the upper abdomen: preliminary
clinical experience in 61 patients. Abdom Imaging. 24:456–461.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ries M, Joones RA, Basseau F, Moonen CT
and Grenier N: Diffusion tensor MRI of the human kidney. J Magn
Reson Imaging. 14:42–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahara T, Imai Y, Yamashita T, Yasuda S,
Nasu S and van Cauteren M: Diffusion weighted whole body imaging
with background body signal suppression (DWIBS): technical
improvement using free breathing, STIR and high resolusion 3D
display. Radiat Med. 22:275–282. 2004.PubMed/NCBI
|
|
28
|
Nonomura Y, Yasumoto M, Yoshimura R,
Haraguchi K, Ito S, Akashi T and Ohashi I: Relationship between
bone marrow cellularity and apparent diffusion coefficient. J Magn
Reon Imaging. 13:757–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmithorst VJ, Dardzinski BJ and Holland
SK: Simultaneous correlation of ghost and geometric distortion
artifacts in EPI using a multiecho reference scan. IEEE Tran Med
Imaging. 20:535–539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Bihan D, Breton E, Lallemand D, Aubin
ML, Vignaud J and Laval-Jeantet M: Separation of diffusion and
perfusion in intraoxel incoherent motion MR imaging. Radiology.
168:692–698. 1998.PubMed/NCBI
|