Introduction
Primary cutaneous apocrine carcinoma (PCAC) is a
rare skin neoplasm. Although approximately 40 cases of PCAC have
been reported (1), no previous
reports regarding apocrine carcinoma within a congenital
melanocytic nevus exist.
The origin of PCAC remains unclear. Keratin (K) is
an essential marker of epithelial neoplasms used to evaluate the
origin of the tumor and stage of differentiation (2,3).
Filament-aggregating protein (filaggrin) is a marker of terminal
epidermal differentiation (4). To
elucidate the differentiation of PCAC, we studied the expression of
epithelial keratin and filaggrin in PCAC using 10 anti-keratin
antibodies and an anti-filaggrin antibody. Immunohistochemical
studies of PCAC using types of anti-keratin antibodies that
identify multiple keratins exist (5). However, this is the first systematic
study to investigate PCAC with detailed immunohistochemistry
results using monospecific anti-keratin antibodies and an
anti-filaggrin antibody.
Materials and methods
A 70-year-old male presented to our hospital with a
10-year history of a slow-growing, red-to-black nodule that
developed into a large black macule on his back. He had a pigmented
macule on his back at birth that had been treated 50 years earlier
with cryotherapy using liquid nitrogen. A clinical examination
showed a leaf-shaped black macule measuring 22×10.5 cm on the
patient’s back. On the left side of the macule, a 45×30 mm in
diameter red-to-black nodule with ulceration was observed (Fig. 1). The left axillary lymph node was
palpable. No other occult carcinomas, including breast carcinoma,
were detected. The macule, including the tumor, was totally excised
and a left axillary lymphadenectomy was performed. Each specimen
was fixed in formalin, embedded in paraffin and stained with
hematoxylin and eosin. Serially cut sections were used in the
immunohistochemical study. Informed consent was obtained from the
patient before the treatment.
An immunohistochemical study of gross cystic disease
fluid protein 15 (GCDFP-15), s100a and HMB-45 was performed. The
epithelial keratin and filaggrin expression was also studied using
immunohistochemical procedures. The anti-keratin and anti-filaggrin
antibodies used in this study were: 34βB4 (K1) (6), LP5K (K7) (7), LP3K (K8) (7), HP1 (K10) (8), LL002 (K14) (7), LHK15 (K15) (9), LL025 (K16) (7), E3 (K17) (7), 5D3 (K18) (7), b170 (K19) (10) and 15C10 (filaggrin) (4) (all from Novocastra Laboratories Ltd.,
Newcastle upon Tyne, UK). This immunohistochemical study employed
the labeled streptoavidin-biotin method (LSAB; Dako, Carpinteria,
CA, USA) as previously reported (11). Normal skin from the scalp was used
for control specimens.
Results
Hematoxylin and eosin staining
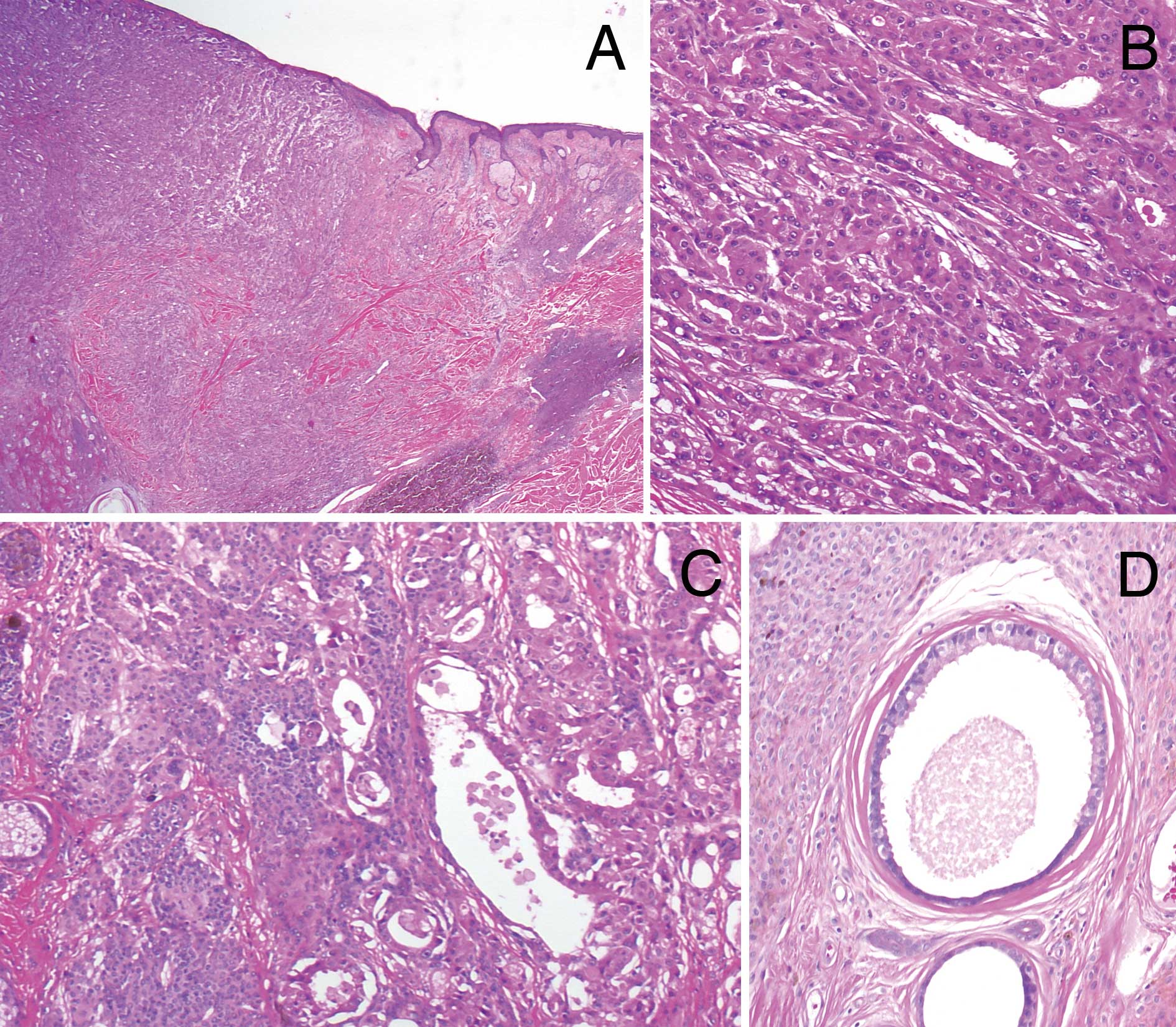
Including ulceration, the tumor occupied the dermis
and the upper subcutaneous fatty tissue (Fig. 2A). There was a complex glandular
arrangement including tubular, solid and cord-like areas. In the
upper dermis, large lumina with decapitation secretion were
observed, whereas invasive growth of smaller tumor nests in the
surrounding tissue was noted in the subcutaneous fat. The
neoplastic cells had abundant eosinophilic granular cytoplasm and
atypical round, hyperchromatic nuclei with prominent nucleoli
(Fig. 2B). Mitotic figures were
occasionally seen. The histopathological diagnosis was apocrine
carcinoma, due to the characteristic ‘apocrine snout’. According to
the subclassification of PCAC by Ackerman et al (12), the tumor was classified into
cutaneous apocrine ductal carcinoma. Metastasis was observed to the
left axillary lymph nodes.
Around the tumor and in the stroma between the tumor
nests, dense diffuse proliferation of small monomorphous
melanocytes without atypia was observed (Fig. 2C). The melanocytes demonstrated an
adnexocentric or angiocentric arrangement and were disposed in
single files between collagen bundles. These histopathological
features were consistent with congenital melanocytic nevus. Within
the nevus, ectopic apocrine glands, which were larger glands
without nuclear atypia showing decapitation secretion, were located
adjacent to the tumor (Fig. 2D).
Benign apocrine neoplasm was not found. Neither sebaceous
hyperplasia nor follicular anomaly, frequently noted in nevus
sebaceous of Jadasshon, was observed. We therefore considered this
case to be an apocrine carcinoma developed within a congenital
melanocytic nevus.
Immunohistochemical findings
The tumor cells were positive for GCDFP-15 and
negative for s100a and HMB-45. Table
I shows the pattern of keratin and filaggrin expression in the
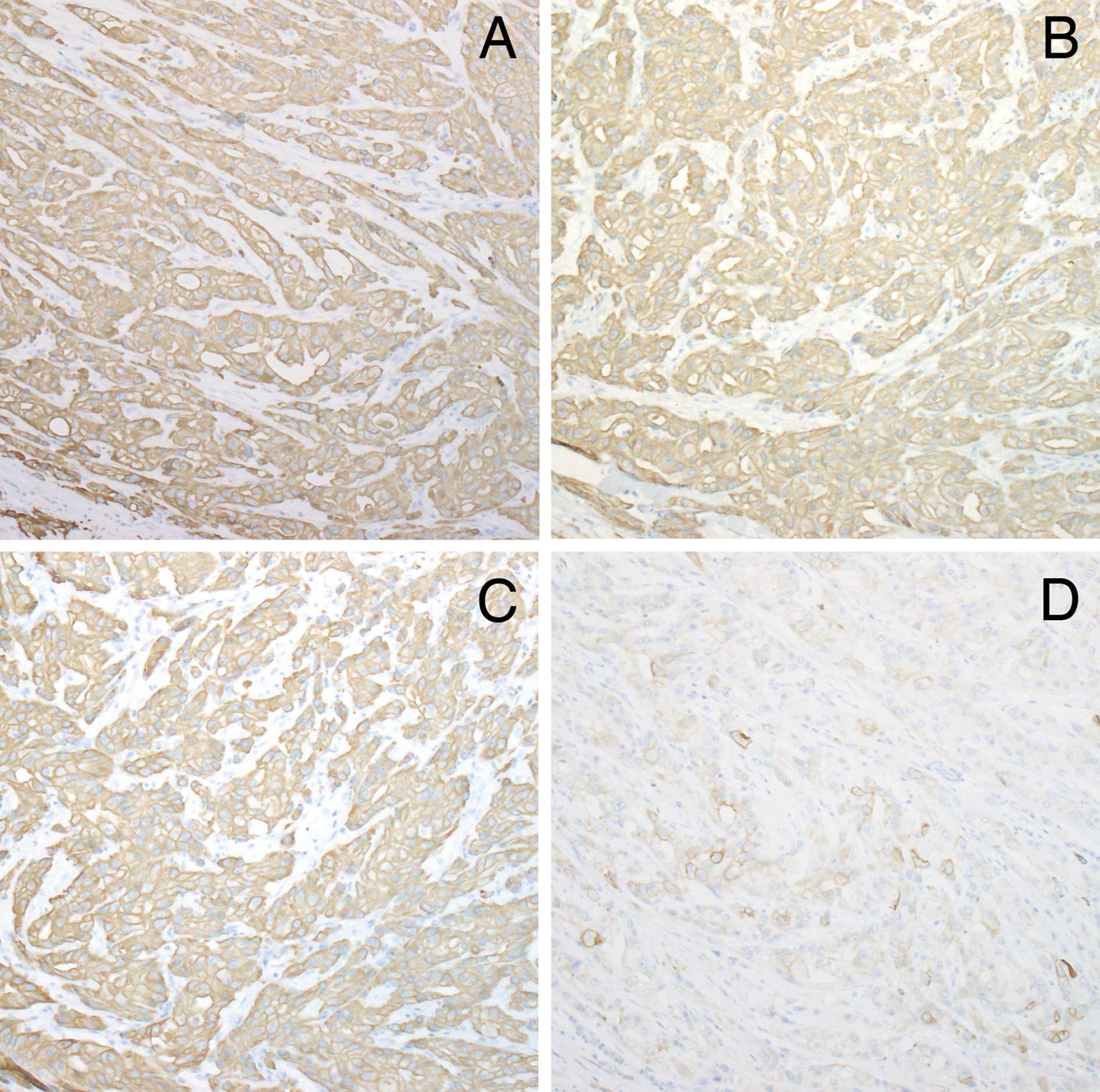
normal apocrine glands and tumor. The tumor demonstrated a diffuse
strong positivity for K7, 8, 18 and 19 (Figs. 3A-C). Tumor cells were negative for
K1, 14, 15, 16 and 17. K10 was focally distributed in ductal
structures within the tumor (Fig.
3D).
 | Table IKeratin expression in the normal
apocrine gland and carcinoma. |
Table I
Keratin expression in the normal
apocrine gland and carcinoma.
| Acrosyringium | Intradermal duct | Secretory
portion | Case |
|---|
|
|
|
| |
|---|
| Luminal cells | Periluminal
cells | Basal cells | Luminal cells | Basal cells | Secretory cells | Myoepithelial
cells | |
|---|
| K1 | + | + | − | + | − | − | − | − |
| K7 | − | − | − | − | − | + | − | ++ |
| K8 | − | − | − | − | − | + | − | ++ |
| K10 | + | + | − | + | − | − | − | − ~ (+)a |
| K14 | − | − | + | − | + | − | + | − |
| K15 | − | − | − | − | − | − | − | − |
| K16 | − | − | − | − | − | − | − | − |
| K17 | − | − | − | − | − | − | + | − |
| K18 | − | − | − | − | − | + | − | + |
| K19 | + | − | − | + | − | + | − | + |
| Filaggrin | + | − | − | − | − | − | − | − |
Discussion
Primary cutaneous apocrine carcinomas (PCAC) are
rare neoplasms and have yet to be fully investigated. Approximately
40 cases of PCAC have been reported (1). The majority of PCACs are relatively
indolent, but some are rapidly progressive (13). The prognosis of PCAC is generally
favorable and depends on the degree of tumor differentiation. PCAC
appears where normal apocrine glands exist including axilla,
eyelid, anogenital lesions, but rarely the chest and lip (14,15).
PCAC occasionally arises from ectopic apocrine glands in nevus
sebaceous (16,17) or from benign apocrine neoplasms such
as tubular apocrine adenoma (18,19),
cylindroma (20) and spiradenoma
(21). PCAC was located overlying a
large congenital melanocytic nevus in our case. To the best of our
knowledge, no reports of PCAC on the congenital melanocytic nevus
or other melanocytic nevi have been reported. Since ectopic
apocrine glands were observed within the nevus, we suggested that,
in our study, PCAC originated from these ectopic apocrine
glands.
The pattern of keratin and filaggrin expression in
normal apocrine glands is summarized in Table I (11,22,23).
In acrosyringium and dermal duct, luminal cells showed positivity
for K1, 10 and 19, whereas basal cells were positive for K14. In
the secretory portion of the apocrine gland, secretory cells
expressed K7, 8, 18 and 19, whereas myoepithelial cells expressed
K14 and 17. Keratin expression is unique in each section of the
normal apocrine sweat gland (luminal cells and basal cells of the
acrosyringium and intradermal duct, secretory cells and
myoepithelial cells of the secretory portion) Thus, the origin of
PCAC was determined according to the pattern of keratin
distribution.
The keratin profile of PCAC in our case is shown in
Table I. K7, K8, K18 and K19 were
detected in the tumor as well as in secretory cells in the normal
apocrine gland. We therefore speculated that PCAC differentiated
into the secretory cells in the apocrine glands. Focal expression
of K10 in the ductal structure within the tumor nests showed that
some PCAC exhibited differentiation into the apocrine dermal duct.
On the other hand, the tumor nests did not express K14 and 17 since
these keratins are normally distributed in myoepithelial cells in
the secretory portion of apocrine glands. This finding suggests
that PCAC is composed not of a proliferation of myoepithelial cells
but of secretory cells. PCAC did not express K14, 16 or 17. K14, a
basal keratin, is undifferentiated, whereas K16 and 17 are known to
hyperproliferative keratins. Lack of these keratins is consistent
with a clinical indolent course of PCAC (20).
Our findings are consistent with previous research
in the literature which showed that PCAC is negative for α-SMA
(smooth muscle actin), a useful marker of myoepithelial cells
(15,24). An ultrastructural study also found
that PCAC, which displayed microvilli on the surface of the tumor
cells under an electron microscope, showed differentiation into
secretory cells (14,25). Positivity for K7 and negativity for
K1 and K14 was the same as in Miyamoto’s previous report (5), but reactivity for K10 was different in
our case, most likely due to the degree of tumor
differentiation.
In conclusion, in our case, primary cutaneous
apocrine carcinoma arose from secretory cells of ectopic apocrine
glands within a congenital melanocytic nevus.
References
|
1
|
Pucevich B, Catinchi-Jaime S, Ho J and
Lukic DM: Invasive primary ductal apocrine adenocarcinoma of
axilla: a case report with immunohistochemical profiling and a
review of literature. Dermatol Online J. 14:52008.PubMed/NCBI
|
|
2
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schweizer J, Bowden PE, Coulombe PA, et
al: New consensus nomenclature for mammalian keratins. J Cell Biol.
174:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dale B and Ling SY: Immunologic
cross-reaction of stratum corneum basic protein; a keratohyaline
granule protein. J Invest Dermatol. 72:257–261. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyamoto T, Inoue S, Adachi K and Takada
R: Differential expression of mucin core proteins and keratins in
apocrine carcinoma, extramammary Paget’s disease and apocrine
nevus. J Cutan Pathol. 36:529–534. 2009.PubMed/NCBI
|
|
6
|
Gown AM and Vogel AM: Monoclonal
antibodies to intermediate filament proteins of human cells: unique
and cross-reactivity antibodies. J Cell Biol. 95:414–424. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lane EB, Bartek J, Purkis PE and Leigh IM:
Keratin antigens in differentiating skin. Ann NY Acad Sci.
455:241–258. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leigh I, Purkis PE, Whitehead P and Lane
EB: Monospecific monoclonal antibodies to keratin 1 carboxyterminal
(synthetic peptide) and to keratin 10 as markers of epithelial
differentiation. Br J Dermatol. 129:110–119. 1993. View Article : Google Scholar
|
|
9
|
Jih DM, Lyle S, Elenitsas R, Elder DE and
Cotsarelis G: Cytokeratin 15 expression in trichoepitheliomas and a
subset of basal cell epitheliomas suggests they originate from hair
follicle stem cells. J Cutan Pathol. 26:113–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindberg K and Rheinwald JG: Suprabasal 40
kd keratin (K19) expression as an immunohistologic marker of
pregnancy in oral epithelium. Am J Pathol. 134:89–98.
1989.PubMed/NCBI
|
|
11
|
Kurokawa I, Mizutani H, Kusumoto K,
Nishijima S, Tsujita-Kyutoku M, Shikata N and Tsubura A:
Cytokeratin, filaggrin and p63 expression in reepithelialization
during human cutaneous wound healing. Wound Repair Regen. 14:38–45.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Requena L, Kiryu H and Ackerman AB: Ductal
carcinoma. Neoplasms With Apocrine Differentiation. 1st edition.
Lippincott-Raven; Philadelphia: pp. 607–649. 1998
|
|
13
|
Shintaku M, Tsuta K, Yoshida H, Tsubura A,
Nakashima Y and Noda K: Apocrine adenocarcinoma of the eyelid with
aggressive biological behavior: report of a case. Pathol Int.
52:169–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katagiri Y and Ansai S: Two cases of
cutaneous apocrine ductal carcinoma of the axilla. Dermatology.
199:332–337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayes MM, Matisic JP and Weir L: Apocrine
carcinoma of the lip: a case report including immunohistochemical
and ultrastructural study, discussion of differential diagnosis and
review of the literature. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 82:193–199. 1996. View Article : Google Scholar
|
|
16
|
Dalle S, Skowron F, Balme B and Perrot H:
Apocrine carcinoma developed in nevus sebaceous of Jadassohn. Eur J
Dermatol. 13:487–489. 2003.PubMed/NCBI
|
|
17
|
Hugel H and Requena L: Ductal carcinoma
arising from a syringocystadenoma papilliferum in a nevus sebaceous
of Jadassohn. Am J Dermatopathol. 25:490–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyamoto T, Hagari Y, Inoue S, Watanabe T
and Yoshino T: Axillary apocrine carcinoma with benign apocrine
tumours: a case report involving a pathological and
immunohistochemical study and review of the literature. J Clin
Pathol. 58:757–761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amo Y and Kawano N: A case of ductal
apocrine carcinoma in the left axilla with tubular apocrine adenoma
in the right axilla. J Dermatol. 30:72–75. 2003.PubMed/NCBI
|
|
20
|
Paties C, Taccagni GL, Papotti M, Valente
G, Zangrandi A and Aloi F: Apocrine carcinoma of the skin. A
clinicopathologic, immunocytochemical, and ultrastructural study.
Cancer. 71:375–381. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsujino Y and Dekio S: Apocrine carcinoma:
a report of a case which formed a single tumor with eccrine
spiradenoma. Skin Cancer (in Japanese). 17:98–100. 2002. View Article : Google Scholar
|
|
22
|
Kurokawa I, Urakawa Y, Senba Y, et al:
Keratin profiles may differ between intraepidermal and intradermal
invasive eccrine porocarcinoma. Oncol Rep. 16:473–477.
2006.PubMed/NCBI
|
|
23
|
Ishida-Yamamoto A, Iizuka H and Eady RA:
Filaggrin immunoreactive composite keratohyalin granules specific
to acrosyringia and related tumours. Acta Derm Venereol. 74:37–42.
1999.
|
|
24
|
Castelli E, Wollina U, Anzarone A, Morello
V and Tomasino RM: Extramammary Paget disease of the axilla
associated with comedo-like apocrine carcinoma in situ. Am J
Dermatopathol. 24:351–357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto O, Haratake J, Hisaoka M, Asahi M
and Bhawan J: A unique case of apocrine carcinoma on the male pubic
skin: histopathologic and ultrastructural observations. J Cutan
Pathol. 20:378–383. 1993. View Article : Google Scholar : PubMed/NCBI
|