Introduction
Malignant tumors of the small intestine account for
only 2.1% of all malignant tumors of the gastrointestinal tract in
Japanese patients and are seldom encountered in clinical practice
(1–3). While adenocarcinoma and carcinoid
tumors comprise 80% of the primary malignant tumors of the small
intestine in the US, adenocarcinoma and malignant lymphoma
constitute 47 and 30%, respectively, in Japan (4).
In most patients with small intestinal
adenocarcinoma (SIA), the disease is already advanced by the time
of diagnosis. Delay of the diagnosis is due to non-specific
presentation, lack of awareness of the disease and inaccessibility
of the tumor to clinical examination. The clinical staging reflects
this delay, with stages I to IV being reported to account for 4,
20, 39 and 35%, respectively. The 5-year survival rate of patients
with stage I-III disease has been reported to be 36%, compared to
5% for stage IV disease, and the median survival time of stage IV
patients is only 11 months (5).
While surgical resection of the primary tumor and
the regional lymph nodes is the preferred treatment for patients in
stages I-III, chemotherapy is administered to patients with stage
IV disease. However, a standard chemotherapy regimen for SIA has
yet to be established, and the regimens employed are usually those
designed for advanced colorectal or gastric adenocarcinoma
(6–10). The clinical effectiveness of such
regimens for the two latter cancer types is well established;
nevertheless, their efficacy for the treatment of SIA has yet to be
determined.
This study described a patient who was diagnosed
with SIA with lung and lymph node metastases plus invasion of the
transverse colon. She was treated with surgery and irinotecan-based
chemotherapy, resulting in a partial response of the disease.
Case report
A 67-year-old woman consulted her doctor in 2002
complaining of fatigue and headache. As her blood count showed
hypochromic anemia (hemoglobin 4.4 g/dl) and her stool was positive
for occult blood, bleeding from the digestive tract was suspected.
However, gastroduodenal endoscopy and colonoscopy revealed no
abnormalities. Since her anemia persisted, these endoscopic
examinations were repeated in August 2003 along with chest computed
tomography (CT), abdominal CT and a gynecologic examination. A
uterine myoma was diagnosed, and she was given a blood transfusion
and an oral iron supplement. In August 2004, repeat endoscopy again
revealed no abnormalities. The patient had lost 12 kg of weight
over the preceding 4 years, and began to complain of abdominal
pain, after which she was referred to our department. The study was
prepared following ethics guidelines of clinical study issued by
the Ministry of Health, Labor and Welfare of Japan.
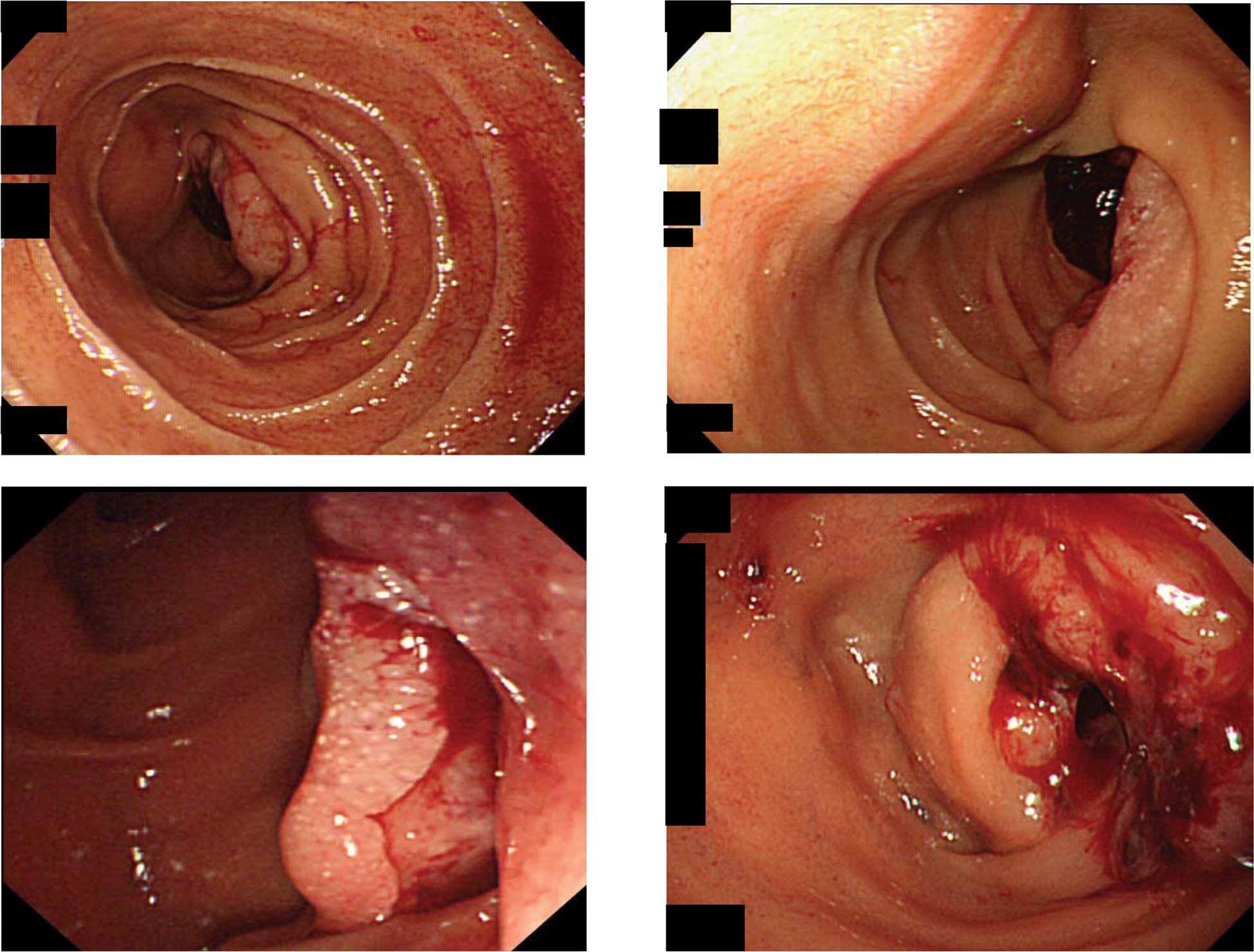
Upper gastrointestinal endoscopy revealed a large
ulcerated tumor covered with clots in the distal third of the
duodenum (Fig. 1A and B). A clear
border was observed between the tumor and the surrounding mucosa.
Side and oblique endoscopic views showed that the tumor occupied
almost the entire lumen and was causing obstruction (Fig. 1C and D). Histological examination of
a biopsy specimen showed that the tumor was a well- to moderately
differentiated adenocarcinoma. A barium meal showed that the distal
part of the tumor had an overhanging edge. Abdominal CT scans
revealed a mass near the ligament of Treitz and invasion of the
mesentery. The patient’s chest X-ray film and CT scans showed
multiple metastatic nodules in the lungs, but no lymphadenopathy. A
blood count and bone marrow biopsy showed iron-deficiency anemia,
and her plasma C-reactive protein level was 4.87 mg/dl. The plasma
levels of tumor markers (carcinoembryonic antigen and CA 19-9) were
within normal limits. The results of other biochemical analyses
showed no abnormalities.
Radical resection of this invasive tumor was
impossible; thus, partial removal of the involved upper jejunum and
duodenojejunostomy was performed to decrease the risk of
obstruction. No peritoneal dissemination or liver metastases were
noted. The postoperative course was uneventful. One month after the
operation, chemotherapy was commenced, consisting of infusion of
irinotecan (CPT-11) at 100 mg/m2 and l-leucovorin (l-LV)
at 20 mg/m2, plus bolus infusion of 5-fluorouracil
(5-FU) at 500 mg/m2 on days 1, 8 and 15. Administration
on day 15 was postponed as grade 2 leukopenia was detected on day
14. After one course of chemotherapy, no non-hematological
toxicities were noted, and a chest X-ray film showed that the lung
metastases had shrunk. Due to persistent leukopenia, after the
second course of chemotherapy, the dose of CPT-11 was reduced to 80
mg/m2 on days 1 and 8 every 3 weeks. Grade 2 leukopenia
recurred during the subsequent courses, but was quickly resolved.
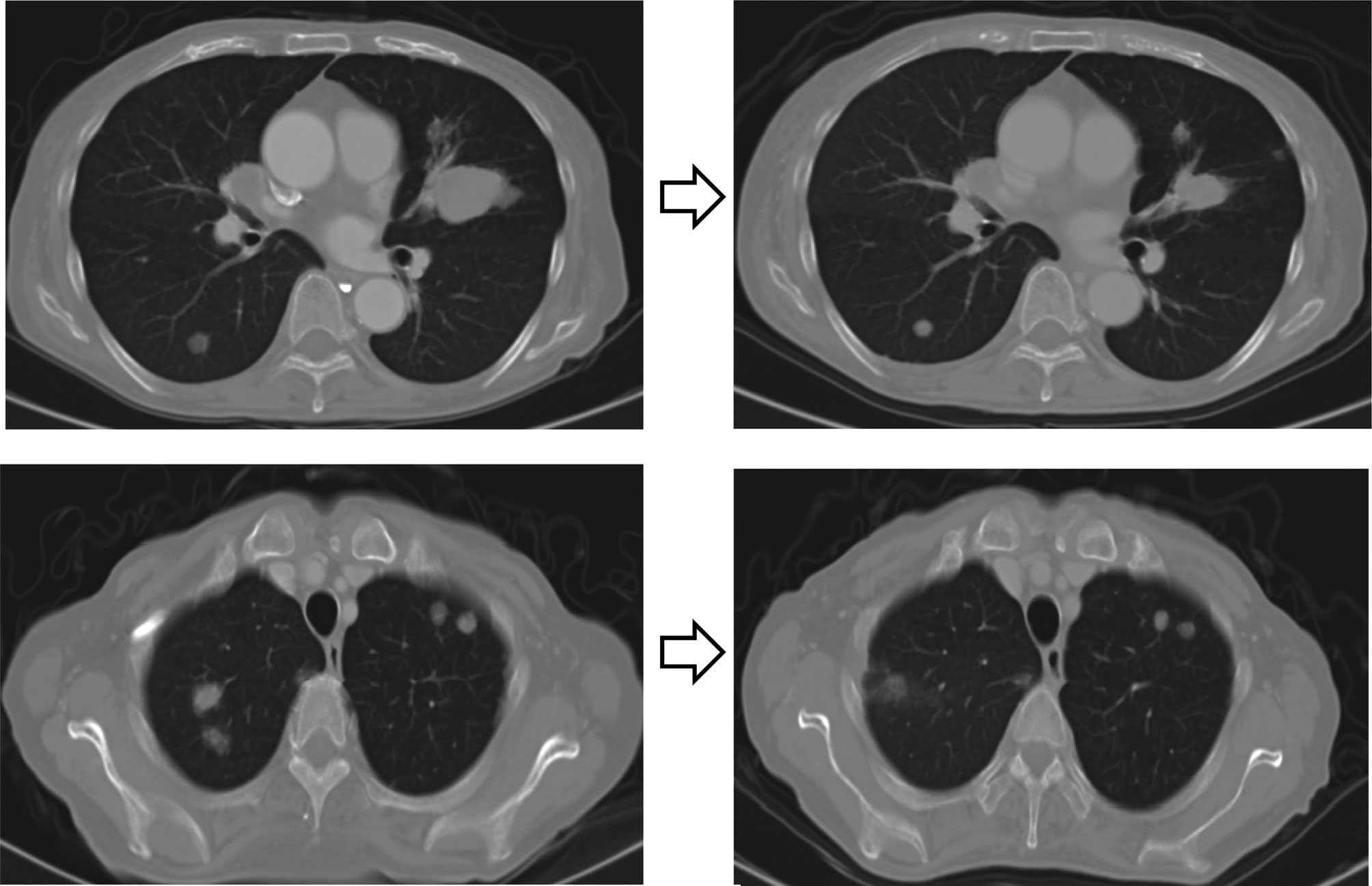
Two courses of chemotherapy produced 65% tumor regression (Fig. 2), which was maintained over six
courses. However, regrowth was observed after the six courses,
leading to obstruction of the jejunum. The patient succumbed to the
disease in December 2004, 12 months after the initial
diagnosis.
Discussion
Chemotherapy is administered to treat stage IV SIA
when metastases or the location of the tumor renders the tumor
inoperable. The median survival time of stage IV patients is only
11 months.
Recently, Overman et al reported a
retrospective analysis of 80 patients with primary SIA (11). They found that the response rate and
progression-free survival were superior in patients treated with
5-FU plus platinum compared to those administered with 5-FU alone
(46 vs. 16%, and 8.7 vs. 3.9 months, respectively). Another
retrospective study of 44 SIA patients treated with various
chemotherapy regimens showed a median response rate of 29% and
survival time of 18.6 months (12).
Few prospective studies exist for the treatment of
SIA. A phase II study of 5-FU, doxorubicin and mitomycin C (FAM)
showed that the response rate was 18% and the median survival time
was 8 months (13). Combination
chemotherapy with capecitabine and oxaliplatin in a phase II trial
achieved a response rate of 50% and median survival time of 20.3
months (11). These reports suggest
that combination chemotherapy that employs new anticancer agents
may improve the outcome of patients with SIA.
Most of the chemotherapy regimens for SIA
investigated thus far have been based on those designed for
colorectal or gastric adenocarcinoma, while irinotecan-based
chemotherapy has not been widely used. In the above-mentioned
retrospective study of Overman et al, only 2 patients
received irinotecan (14). Ono
et al reported that only 1 out of 8 SIA patients treated
with irinotecan plus cisplatin achieved partial response (15). Two small phase I studies have also
been reported. These studies investigated 5-FU, cisplatin and
irinotecan in 5 patients as well as 5-FU, oxaliplatin and
irinotecan in 4 patients, respectively. Thus, the efficacy of
irinotecan-based regimens for SIA remains to be established. It is
likely that both irinotecan- and platinum-based chemotherapy are
equally effective against SIA.
When we initiated chemotherapy for the present
patient in 2004, the standard first-line regimen for metastatic
colorectal cancer (CRC) was a bolus 5-FU and irinotecan based on
survival benefit (16), albeit that
this regimen has been found to cause serious gastrointestinal
toxicity. We previously conducted phase I (17) and phase II (18) clinical studies in a patient with
metastatic CRC to establish the feasibility and effectiveness of
employing a modified irinotecan and bolus 5-FU regimen. In these
studies, CPT-11 (100 mg/m2) and bolus 5-FU (500
mg/m2) plus l-LV (20 mg/m2) were administered
on days 1, 8 and 15 every 28 days, and a response rate of 53.3% for
patients without prior chemotherapy was achieved (18). As a result, we found that a modified
irinotecan-based regimen was promising for metastatic CRC, and we
selected this modified regimen for the treatment of the patient in
the present study. Six courses of this therapy were administered,
and the patient survived for 12 months. Her clinical response was
very similar to that noted previously, and the low level of adverse
events allowed the regimen to be administered safely (5,7,8,10,19,20).
The previous standard irinotecan-based regimen for
metastatic CRC, i.e., irinotecan and bolus 5-FU, has now been
replaced by FOLFIRI (5-FU, irinotecan and leucovorin) due to its
improved efficacy and safety profile (21,22).
Therefore, a modified irinotecan and bolus 5-FU regimen may also be
an option for patients who are not able to receive continuous
infusion of 5-FU. The results of the present study suggest that an
irinotecan-based regimen is effective against SIA. A prospective
phase II study of irinotecan combined with other appropriate agents
may lead to a more effective treatment for metastatic SIA.
References
|
1
|
Sager GF: Primary malignant tumors of the
small intestine. A twenty-two year experience with thirty patients.
Am J Surg. 135:601–603. 1978.PubMed/NCBI
|
|
2
|
Ciccarelli O, Welch JP and Kent GG:
Primary malignant tumors of the small bowel. The Hartford Hospital
experience, 1969–1983. Am J Surg. 153:350–354. 1987.PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics. CA Cancer J Clin. 55:2592005.
|
|
4
|
Kusumoto H, Takahashi I, Yoshida M, et al:
Primary malignant tumors of the small intestine: analysis of 40
Japanese patients. J Surg Oncol. 50:139–143. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: presentation,
prognostic factors and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polyzos A, Kouraklis G, Giannopoulos A,
Bramis J, Delladetsima JK and Sfikakis PP: Irinotecan as salvage
chemotherapy for advanced small bowel adenocarcinoma: a series of
three patients. J Chemother. 15:503–506. 2003.PubMed/NCBI
|
|
7
|
Jigyasu D, Bedikian AY and Stroehlein JR:
Chemotherapy for primary adenocarcinoma of the small bowel. Cancer.
53:23–25. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onodera H, Nishitai R, Shimizu K, Maetani
S and Imamura M: Small intestinal cancer with extensive lymph node
metastases showing complete remission by
methotrexate/5-fluorouracil sequential therapy: report of a case.
Surg Today. 27:60–63. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lioe TF and Biggart JD: Primary
adenocarcinoma of the jejunum and ileum: clinicopathological review
of 25 cases. J Clin Pathol. 43:533–536. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Talamonti MS, Goetz LH, Rao S and Joehl
RJ: Primary cancers of the small bowel: analysis of prognostic
factors and results of surgical management. Arch Surg. 137:564–571.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Overman MJ, Kopetz S, Wen S, et al:
Chemotherapy with 5-fluorouracil and a platinum compound improves
outcomes in metastatic small bowel adenocarcinoma. Cancer.
113:2038–2045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fishman PN, Pond GR, Moore MJ, et al:
Natural history and chemotherapy effectiveness for advanced
adenocarcinoma of the small bowel: a retrospective review of 113
cases. Am J Clin Oncol. 29:225–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibson MK, Holcroft CA, Kvols LK and
Haller D: Phase II study of 5-fluorouracil, doxorubicin and
mitomycin C for metastatic small bowel adenocarcinoma. Oncologist.
10:132–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Overman MJ, Varadhachary GR, Kopetz S, et
al: Phase II study of capecitabine and oxaliplatin for advanced
adenocarcinoma of the small bowel and ampulla of Vater. J Clin
Oncol. 27:2598–2603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ono M, Shirao K, Takashima A, et al:
Combination chemotherapy with cisplatin and irinotecan in patients
with adenocarcinoma of the small intestine. Gastric Cancer.
11:251–255. 2008.PubMed/NCBI
|
|
16
|
Saltz LB, Douillard JY, Pirotta N, Alakl
M, Gruia G, Awad L, Elfring GL, Locker PK and Miller LL: Irinotecan
plus fluorouracil/leucovorin for metastatic colorectal cancer: a
new survival standard. Oncologist. 6:81–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujishima H, Kikuchi I, Miyanaga O, Ueda
A, Baba E, Mitsugi K, Harada M and Nakano S: Phase I study of
CPT-11 and bolus 5-FU/l-leucovorin in patients with metastatic
colorectal cancer. Int J Clin Oncol. 9:92–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujishima H, Makiyama A, Miyanaga O, Ueda
A, Esaki T, Mitsugi K, Baba E, Kusaba H, Harada M and Nakano S: A
multicenter phaseIIstudy of irinotecan (CPT-11) and bolus
5-fluorouracil (5FU)/l-leucovorin(l-LV) in patients with metastatic
colorectal cancer. J Clin Oncol, 2005 ASCO Annual Meeting
Proceedings. 23(16): Part I of II. 37422005.
|
|
19
|
Di Marco L, Berghenti M and Felloni M:
Primary adenocarcinoma of the second portion of duodenum. Ann Ital
Chir. 74:573–577. 2003.PubMed/NCBI
|
|
20
|
Zhou Z, Wan D and Shi M: Diagnosis and
treatment of primary malignant tumors of the small bowel. Zhonghua
Zhong Liu Za Zhi. 19:297–299. 1997.PubMed/NCBI
|
|
21
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar
|
|
22
|
Tournigand C, Andre T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G,
Landi B, Colin P, Louvet C and de Gramont A: FOLFIRI followed by
FOLFOX6 or the reverse sequence in advanced colorectal cancer: a
randomized GERCOR study. J Clin Oncol. 22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|