Introduction
RNA interference (RNAi) is a natural protective
mechanism that functions in various organisms (1–4).
Small-interfering RNAs (siRNAs) (21–23 nt) are thought to be
generated from stretches of double-stranded RNA by Dicer, a
conserved member of the RNase III gene family. siRNAs are then
incorporated into a large multiprotein RNA-inducing silencing
complex. In the siRNA-mediated mRNA degradation pathway, the
antisense strand of the siRNA molecule is used to target the
cognate mRNA for degradation. This process involves specific base
pairing between the antisense strand of the siRNA and the target
mRNA, endonucleolytic cleavage of the mRNA strand across the middle
of the siRNA strand and subsequent degradation of the unprotected
mRNA (5). RNAi provides a
revolutionary tool to identify gene functions (6,7) and
opens new possibilities for therapeutic interventions (8–10). In
early reports, 21 nt siRNA was thought to be too small to activate
PKR, a kinase that senses double-stranded RNA (11). However, other reports maintain that
the transfection or expression of even short duplexes or hairpins
is able to induce a subset of markers of innate immune response
(12,13).
The extracellular signal-regulated kinase (ERK)/MAPK
pathway is constitutively active in several human malignancies, and
it is critical for the induction of cell proliferation,
differentiation and cell survival. Four major groups of MAPKs in
mammalian cells are found: ERK, c-jun NH2-terminal
kinase/stress-activated protein kinase, p38 and extracellular
signal regulated kinase-5 (ERK5, also known as Big MAP kinase-1)
(14–17). ERK is mainly activated by mitogenic
stimuli such as growth factors and hormones to induce cell
proliferation. Previous studies showed that siRNA targeting for the
MAPK p42 gene partially inhibits proliferation and increases
apoptosis in human cervical carcinoma HeLa cells (18,19).
Results of a microarray analysis showed that MAPK p42 siRNA
inhibited cell growth through the regulation of cell cycle control
and apoptosis and induced an interferon-like response in HeLa cells
(20). In order to confirm the
specific effects of siRNA on MAPK p42 and the non-specific
interferon-like response effect, the roles of siRNA and U0126, an
inhibitor of MAPK p42, were compared in HeLa cells.
Materials and methods
siRNA synthesis
siRNAs were designed using RNAi target finder
(http://www.ambion.com/techlib/misc/siRNA_finder.html).
The two sets of siRNA sequences were: siRNA-1 (negative control)
sense CUCUACGUAAGAUCCAGCUUU and antisense AGCUGGAUCUUACGUAGAGUU,
bearing no homology with any known relevant human genes; and
siRNA-2 sense AGCAAAUAGUUCCUAGCUUUU and antisense AAG
CUAGGAACUAUUUGCUUU. siRNA were synthesized and purified by means of
the SilencerTM siRNA Construction kit (Ambion, Austin,
TX, USA).
Cell culture and transfection
Human HeLa cells (5.0×104 cells/ml) were
cultured in RPMI-1640 supplemented with 10% (v/v) fetal bovine
serum containing 2.0 mmol/l glutamine and 20 μg
penicillin-streptomycin/ml in 5% CO2 at 37°C, and
allowed to adhere for 24 h. HeLa cells were transfected by siRNA,
using Lipofectamine 2000 (Invitrogen) or treated with U0126 at
different concentrations. Two days after transfection and
treatment, cells were analyzed for MTT, cell cycle and real-time
quantitative PCR. All tests were repeated five times.
MTT assay for cell viability
Cells (1.0×104/well) were cultured in
96-well plates. After 24 h, the cells were incubated with siRNA for
the indicated times at 37°C in 5% CO2. Then, 20 μl/well
of MTT solution (5 mg/ml) was added, and cells were incubated for
another 4 h. The supernatants were removed, and formazan crystals
were solubilized in 200 μl of dimethylsulfoxide. Finally, optical
density was determined at 490 nm by a POLARstar+Optima
(BMG Labtechnologies).
Cell cycle analysis by flow
cytometry
DNA content per duplicate was analyzed using a
FACSCalibur FCM (Becton-Dickinson, Mountainview, CA, USA). The
adherent cells were harvested by brief trypsinization, washed with
PBS, fixed in 70% ethanol, stained with 20 μg/ml propidium iodide
containing 20 μg/ml RNase (DNase-free) for 30 min and analyzed by
flow cytometry. The populations of G0/G1, S and G2/M cells were
quantified.
Real-time quantitative PCR using
SYBR-Green I
The message RNA levels of seven genes, i.e.,
mitogen-activated protein kinase 1 (MAPK1), signal transducer and
activator of transcription 1/2 (STAT1/2), cyclin-dependent kinase
(PML), nucleoporin 188 (NUP188), 2′,5′-oligoadenylate synthetase
(OAS1) and p38 were measured in siRNA and control samples by
real-time quantitative PCR (Table
I). The reaction was performed using the ABI 7000 real-time PCR
detection system (ABI PRISM, USA) with SYBR-Green I.
Simultaneously, GAPDH was used in all of the specimens as the
reference, and the quantitative analysis of message RNA levels was
normalized by GAPDH. The fold change of the gene expression between
siRNA and the control group was calculated for each pair sample
using 2-ΔΔCt, where ΔΔCt = (Ctgene −
CtGAPDH)siRNA − (Ctgene −
CtGAPDH)control. 2− ΔΔCt >2 was
calculated to be gene overexpression (14).
 | Table IThe primer sequences of eight genes
for real-time quantitative PCR. |
Table I
The primer sequences of eight genes
for real-time quantitative PCR.
| Gene Bank no. | Gene | Primer sequence | PCR product length
(bp) | Temperature (°C) |
|---|
| NM_002534 | OAS1 | Forward:
5′-CCAGGAAATTAGGAGACAGC-3′
Reverse: 5′-GAGCGAACTCAGTACGAAGC-3′ | 165 | 88.0 |
| NM_002675 | PML | Forward:
5′-AGTCGGTGCGTGAGTTCCT-3′
Reverse: 5′-GGAACATCCTCGGCAGTAG-3′ | 110 | 88.9 |
| NM_015354 | NUP188 | Forward:
5′-GCACTCTGCTGCTTATCC-3′
Reverse: 5′-CTTGGCCTTGGTCTTCTC-3′ | 137 | 88.6 |
| NM_139014 | p38 | Forward:
5′-GCAGGAGCTGAACAAGACAATC-3′
Reverse: 5′-TTTCGCATGAATGATGGACTG-3′ | 169 | 88.4 |
| AA195999 | MAPK1 | Forward:
5′-CCCAAATGCTGACTCCAAAGC-3′
Reverse: 5′-GCTCGTCACTCGGGTCGTAAT-3′ | 131 | 84.8 |
| BC002704 | STAT1 | Forward:
5′-TGCTCCTTTGGTTGAATCCC-3′
Reverse: 5′-GGAATTTTGAGTCAAGCTGCTG-3′ | 92 | 90.7 |
| NM_005419 | STAT2 | Forward:
5′-GTGGTTCAGGAAAGGGCAG-3′
Reverse: 5′-GGAGGGTGTCCGTTTTCAG-3′ | 123 | 87.9 |
| NM_000094 | COL7A1 | Forward:
5′-GTGTTGCTGCGTGACTTGG-3′
Reverse: 5′-AACAGAAGCGTCAGTGCGAG-3′ | 114 | 90.3 |
| NM_002046 | GAPDH | Forward:
5′-AGTTAGCCGCATCTTCTTTTGC-3′
Reverse: 5′-CAATACGACCAAATCCGTTGACT-3′ | 100 | 87.8 |
Statistical analysis
Data were expressed as means ± SD and analyzed by
SPSS10.0 software. P<0.05 was considered to be statistically
significant.
Results
Effects of siRNA and U0126 on the
inhibition of HeLa cell growth
HeLa cells were exposed to siRNA or U0126 at
different concentrations at different times. U0126 significantly
inhibited the growth of HeLa cells at certain concentrations
(Fig. 1B), and the inhibition ratio
of U0126 reached 17.1% at 20 μmol/l for 48 h. In comparison with
the control group and negative siRNA-1 (Fig. 1A), siRNA-2 was found to inhibit the
proliferation of HeLa cells. The inhibition ratio of siRNA-2
reached the highest level of ~30.4% at 75 nmol/l for 48 h. The cell
cycle of HeLa cells was arrested at the G1 phase by U0126 (Fig. 1C) and at the S phase by siRNA-2
(Fig. 1D). The effects of siRNA-2
were greater than those of U0126 on the apoptosis of HeLa cells in
that U0126 induced early apoptosis (Fig. 1F), while siRNA-2 increased late
apoptosis (Fig. 1E).
Effects of siRNA and U0126 on the
expression of MAPK p42, NUP188 and p38
To evaluate the effects of siRNA-2 on MAPK p42
silencing, real-time quantitative PCR was used. The results showed
that the expression of MAPK p42 was inhibited ~60 and 70%,
respectively, by siRNA-2 in comparison with the control group and
negative siRNA-1 (Fig. 2A). By
contrast, U0126 induced MAPK p42 expression (Fig. 2B). The results showed that
inhibition of the MAPK p42 activity by U0126 led to an increase in
MAPK p42 expression in compensation.
NUP188 was down-regulated and p38 was up-regulated
by siRNA-2 and U0126. The consistency in the result between siRNA-2
and that of U0126 showed that the down-regulation or inhibition in
MAPK p42 activity led specifically to the response of the
expression in NUP188 (Fig. 2C and
D) and p38 (Fig. 2E and F).
Effects of siRNA and U0126 on the
expression of interferon-like response genes
The OAS1 gene is a member of the OAS family of
interferon-induced antiviral enzymes. siRNA-2 and negative siRNA-1
induced the overexpression of OAS1 in HeLa cells in comparison with
the control group (Fig. 3A); U0126
inhibited the expression of OAS1 (Fig.
3B). However, in addition to the treatment of siRNA-2 at 25
nmol/l, other siRNA-2 groups inhibited the expression of OAS1 in
comparison with negative siRNA-1. The mode of expression of the PML
gene was consistent with that of OAS1 induced by siRNA-2 (Fig. 3C). These results showed that the
up-regulation of the expression in OAS1 and PML was a non-specific
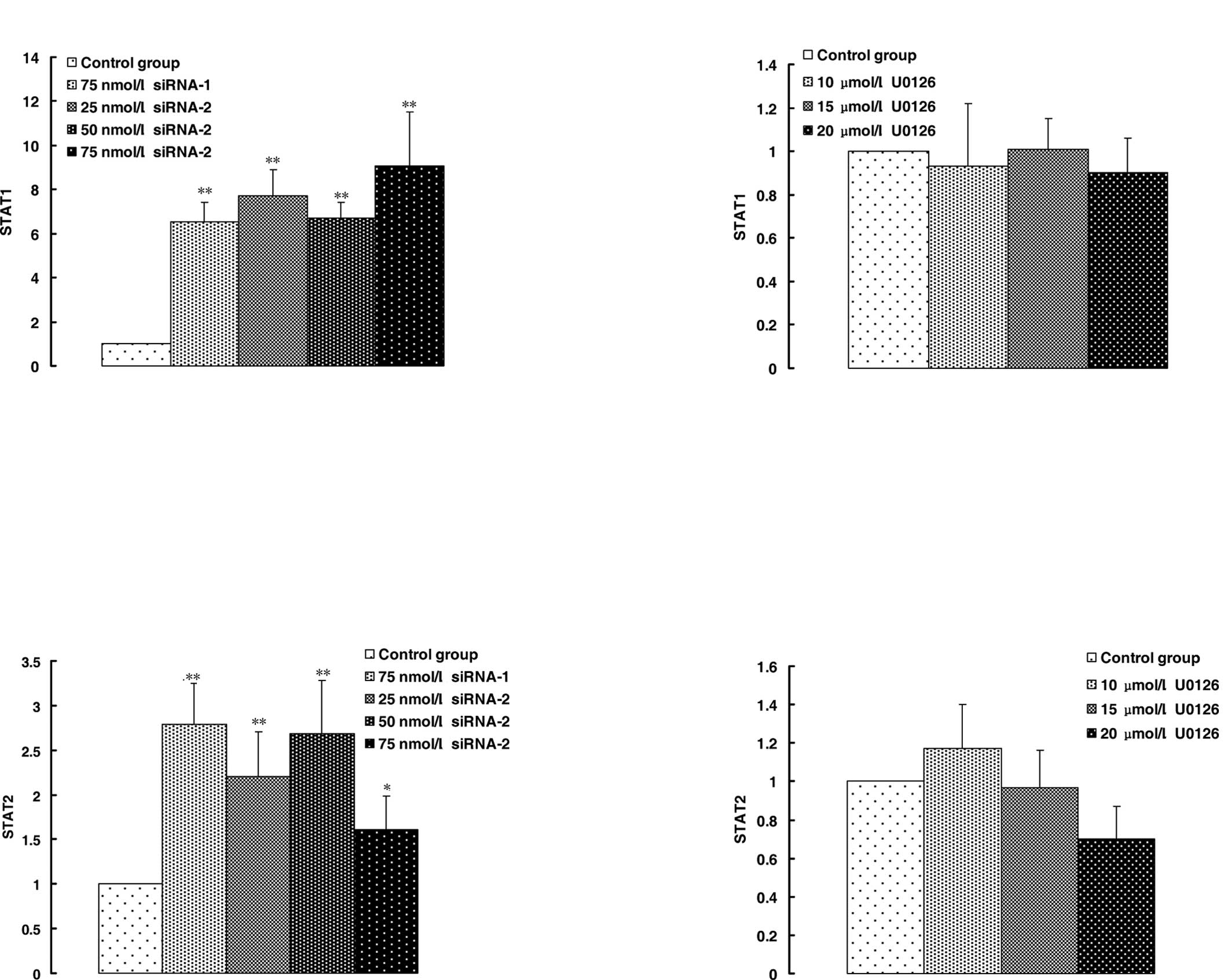
response to siRNA-2. STAT1 and STAT2 are involved in interferon
(IFN) signaling pathways and play a key role in promoting apoptosis
in a variety of cell types. STAT1 and STAT2 expression was slightly
down-regulated by U0126 (Fig. 4B and
D), but up-regulated significantly by siRNA-2 and negative
siRNA-1 (Fig. 4A and C).
Discussion
The overexpression and activation of ERK has been
documented in leukemia, renal cell carcinoma, breast cancer and
several ovarian cancer cell lines (21–23).
The suppression of MAPK p42 expression and activity by siRNA or
U0126 resulted in the inhibition of HeLa cell growth. Results
showed that MAPK p42 is involved in HeLa cell growth. Steinmetz
et al also demonstrated that silencing of the ERK1/2 protein
expression using RNAi led to the complete suppression of HeyC2 and
SKOV3 cell proliferation (24).
Tamemoto et al found that 44- and 42-kDa MAPKs exhibited
activities in the G1 through S and G2/M phases and were activated
biphasically in the G1 phase and around the M phase (25). Our results showed that the cell
cycle was arrested at the G1 phase by U0126 and at the S phase by
siRNA-2, suggesting different cell proliferation suppression
effects between U0126 and siRNA-2.
The 21 nt siRNA targeting MAPK p42 induced the
down-regulation of MAPK p42 in comparison with the control group
and negative siRNA-1, while U0126 induced MAPK p42 expression,
suggesting that siRNA-mediated silencing of the MAPK p42 gene was a
specific effect of siRNA. A decrease in MAPK p42 occurred along
with an increase in MAPK p38, another protein of the MAPK pathway
(26,27). This increase was thought to be
responsible for the progression of apoptosis. Our results were
similar in that the decrease in MAPK p42 expression induced by
siRNA or the decrease in MAPK p42 activity induced by U0126 caused
a slight increase in MAPK p38 expression (Fig. 2E and F). NUP188 is a type of
nucleoporin (Nup). Approximately 30 types of Nup family nucleoside
transporters can construct a nuclear pore complex in the membrane
of a cell nucleus. This complex is an important component involved
in the nucleocytoplasmic transport of biomacromolecules, but its
mechanism remains unknown. NUP188 was down-regulated by siRNA-2 and
U0126. The consistency between the result of siRNA-2 and U0126
showed that the down-regulation or inhibition of activity of MAPK
p42 led particularly to a response of expression of NUP188
(Fig. 2C and D).
dsRNA structures greater than 30 bp were found to
stimulate the IFN pathway mediated in part by the activation of the
dsRNA-dependent protein kinase R (PKR), which represented a host
response to viral infection (28,29).
Several genes were activated in the IFN pathway, including the
member of the OAS family, STAT1/2 and PML (30,31).
It was thought that 21 nt siRNAs were too short to induce
interferon expression (12). It was
possible to administer naked, synthetic siRNA to mice and
down-regulate an endogenous or exogenous target without inducing an
interferon response (32). However,
previous studies found that the transfection of siRNA resulted in
an interferon-mediated activation of the Janus kinase/STAT pathway
and the global up-regulation of interferon-stimulated genes
(12,13,33).
In order to confirm the interferon responses of siRNA-2, STAT1/2,
PML and OAS1 were detected by real-time PCR. Differential effects
of siRNA and U0126 on the expression of interferon-like response
genes were noted. The inhibition of MAPK p42 activity by U0126
induced the down-regulation of OAS1 and PML (Fig. 3B and D) in HeLa cells, but the
knockdown of MAPK p42 by MAPK p42 siRNA caused the up-regulation of
OAS1 and PML levels (Fig. 3A and
C), which were lower than those induced by negative siRNA. The
effects of U0126 on the expression of STAT1 and STAT2 were slight
(Fig. 4B and D) and MAPK p42 siRNA
promoted the expression of STAT1 and STAT2 (Fig. 4A and C). PML, STAT1 and STAT2 were
proven to be involved in the inhibition of cell growth (34–36).
In conclusion, MAPK p42 siRNA, not only specifically
knocked down MAPK p42 and increased p38 expression in comparison
with the small-molecule MEK inhibitor U0126, but also
non-specifically stimulated the interferon responses which
increased the expression of pro-apoptotic genes including PML,
STAT1 and STAT2, ultimately triggering HeLa cell apoptosis. Our
results suggest that siRNA-mediated down-regulation of MAPK p42 is
an attractive strategy for cancer gene therapy.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (30872481) and the Scientific and
Technological Planning Foundation of Shaanxi Province
(2006K09-G7-1).
References
|
1
|
Lohmann JU, Endl I and Bosch TC: Silencing
of developmental genes in Hydra. Dev Biol. 214:211–214. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang D, Lu H and Erickson JW: Evidence
that processed small dsRNAs may mediate sequence-specific mRNA
degradation during RNAi in Drosophila embryos. Curr Biol.
10:1191–1200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Z, Cao Y, Li M, et al: A
double-stranded RAN injection produces nonspecific defects in
zebrafish. Dev Biol. 229:215–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wianny F and Zernicka-Goetz M: Specific
interference with gene function by double-stranded RNA in early
mouse development. Nat Cell Biol. 2:70–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fire A, Xu S, Montgomery MK, et al: Potent
and specific genetic interference by double-stranded RNA in
Caenorhabditis elegans. Nature. 391:806–811. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quon K and Kassner PD: RNA interference
screening for the discovery of oncology targets. Expert Opin Ther
Targets. 13:1027–1035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, Rubin MA and Chinnaiyan AM: The polycomb group protein
EZH2 is involved in progression of prostate cancer. Nature.
419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez-Lebrón E, Gouvion CM, Moore SA,
Davidson BL and Paulson HL: Allele-specific RNAi mitigates
phenotypic progression in a transgenic model of Alzheimer’s
disease. Mol Ther. 17:1563–1573. 2009.PubMed/NCBI
|
|
9
|
Courties G, Presumey J, Duroux-Richard I,
Jorgensen C and Apparailly F: RNA interference-based gene therapy
for successful treatment of rheumatoid arthritis. Expert Opin Biol
Ther. 9:535–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pfister EL, Kennington L, Straubhaar J,
Wagh S, Liu W, DiFiglia M, Landwehrmeyer B, Vonsattel JP, Zamore PD
and Aronin N: Five siRNAs targeting three SNPs may provide therapy
for three-quarters of Huntington’s disease patients. Curr Biol.
19:774–778. 2009.PubMed/NCBI
|
|
11
|
Paddison PJ, Caudy AA and Hannon GJ:
Stable suppression of gene expression by RNAi in mammalian cells.
Proc Natl Acad Sci USA. 99:1443–1448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bridge AJ, Pebernard S, Ducraux A,
Nicoulaz AL and Iggo R: Induction of an interferon response by RNAi
vectors in mammalian cells. Nat Genet. 34:263–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sledz CA, Holko M, de Veer MJ, Silverman
RH and Williams BR: Activation of the interferon system by
short-interfering RNAs. Nat Cell Biol. 5:834–839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boulton TG, Nye SH, Robbins DJ, et al:
ERKs: a family of protein-serine/threonine kinases that are
activated and tyrosine phosphorylated in response to insulin and
NGF. Cell. 65:663–675. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Derijard B, Hibi M, Wu IH, et al: JNK1: a
protein kinase stimulated by UV light and Ha-Ras that binds and
phosphorylates the c-Jun activation domain. Cell. 76:1025–1037.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Lee JD, Bibbs L, et al: A MAP
kinase targeted by endotoxin and hyperosmolarity in mammalian
cells. Science. 265:808–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
18
|
Huang C, Liu LY, Song TS, et al: Small
interfering RNA-mediated MAPK p42 silencing induces apoptosis of
HeLa cells. Nan Fang Yi Ke Da Xue Xue Bao. 26:11–15.
2006.PubMed/NCBI
|
|
19
|
Liu L, Huang C, Li Z, et al: Related genes
for HeLa cell apoptosis induced by siRNA-mediated MAPK p42
silencing. Acta Med Univ Sci Technol Huazhong. 37:129–132.
2008.
|
|
20
|
Huang C, Liu L, Li Z, et al: Effects of
small interfering RNAs targeting MAPK1 on gene expression profile
in HeLa cells as revealed by microarray analysis. Cell Biol Int.
32:1081–1090. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Staber PB, Linkesch W, Zauner D,
Beham-Schmid C, Guelly C, Schauer S, Sill H and Hoefler G: Common
alterations in gene expression and increased proliferation in
recurrent acute myeloid leukemia. Oncogene. 23:894–904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, van den Beldt K, Duesbery NS, Resau JH and
Teh BT: Inhibition of MAPK kinase signaling pathways suppressed
renal cell carcinoma growth and angiogenesis in vivo. Cancer Res.
68:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salh B, Marotta A, Matthewson C, Ahluwalia
M, Flint J, Owen D and Pelech S: Investigation of the Mek-MAP
kinase-Rsk pathway in human breast cancer. Anticancer Res.
19:731–740. 1999.PubMed/NCBI
|
|
24
|
Steinmetz R, Wagoner HA, Zeng P, Hammond
JR, Hannon TS, Meyers JL and Pescovitz OH: Mechanisms regulating
the constitutive activation of the extracellular signal-regulated
kinase (ERK) signaling pathway in ovarian cancer and the effect of
ribonucleic acid interference for ERK1/2 on cancer cell
proliferation. Mol Endocrinol. 18:2570–2582. 2004. View Article : Google Scholar
|
|
25
|
Tamemoto H, Kadowaki T, Tobe K, Ueki K,
Izumi T, Chatani Y, Kohno M, Kasuga M, Yazaki Y and Akanuma Y:
Biphasic activation of two mitogen-activated protein kinases during
the cell cycle in mammalian cells. J Biol Chem. 267:20293–20297.
1992.PubMed/NCBI
|
|
26
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parameswaran N, Nambi P, Hall CS, Brooks
DP and Spielman WS: Adrenomedullin decreases extracellular
signal-regulated kinase activity through an increase in protein
phosphatase-2A activity in mesangial cells. Eur J Pharmacol.
388:133–138. 2000. View Article : Google Scholar
|
|
28
|
Sen GC: Viruses and interferons. Annu Rev
Microbiol. 55:255–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saunders LR and Barber GN: The dsRNA
binding protein family: critical roles, diverse cellular functions.
FASEB J. 17:961–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hovanessian AG and Justesen J: The human
2′-5′oligoadenylate synthetase family: unique interferon-inducible
enzymes catalyzing 2′-5′ instead of 3′-5′ phosphodiester bond
formation. Biochimie. 89:779–788. 2007.
|
|
31
|
Boese A, Sommer P, Gaussin A, Reimann A
and Nehrbass U: Ini1/hSNF5 is dispensable for retrovirus-induced
cytoplasmic accumulation of PML and does not interfere with
integration. FEBS Lett. 578:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heidel JD, Hu S, Liu XF, Triche TJ and
Davis ME: Lack of interferon response in animals to naked siRNAs.
Nat Biotechnol. 22:1579–1582. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sledz CA and Williams BR: RNA interference
and double-stranded-RNA-activated pathways. Biochem Soc Trans.
32:952–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lapi E, Di Agostino S, Donzelli S, Gal H,
Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X and
Blandino G: PML, YAP, and p73 are components of a proapoptotic
autoregulatory feedback loop. Mol Cell. 32:803–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Youlyouz-Marfak I, Gachard N, Le Clorennec
C, Najjar I, Baran-Marszak F, Reminieras L, May E, Bornkamm GW,
Fagard R and Feuillard J: Identification of a novel p53-dependent
activation pathway of STAT1 by antitumour genotoxic agents. Cell
Death Differ. 15:376–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Negoro S, Kunisada K, Tone E, et al:
Activation of JAK/STAT pathway transduces cytoprotective signal in
rat acute myocardial infarction. Cardiovasc Res. 47:797–805. 2000.
View Article : Google Scholar : PubMed/NCBI
|