Introduction
Surgical resection is considered to be the only
effective treatment for localized renal cell carcinoma (RCC).
However, 20–40% of surgically treated patients experience
recurrence of the disease (1,2).
Moreover, up to 30% of patients with RCC present with metastatic
disease (2,3). Immunotherapies using interferons and
interleukin-2 have been used for advanced RCC since this tumor is
unresponsive to radiotherapy and also refractory to chemotherapy
(4). However, the two therapies,
alone and in combination, have demonstrated disappointing success
rates of 20% or lower (2,4). Up to 80% of clear cell RCC, the most
common histological subtype of RCC, show inactivation of the von
Hippel-Lindau gene (VHL) that regulates the expression of
hypoxia-inducible factor-1α (HIF-1α) (2,4,5).
Inactivation of VHL upregulates HIF-1α production, leading to the
activation of various genes containing hypoxia response elements,
including the vascular endothelial growth factor (VEGF),
platelet-derived growth factor (PDGF), epidermal growth factor
receptor type 1, transforming growth factor-α and glucose
transporter genes (5,6). Molecular targeting agents such as
sunitinib and sorafenib that target receptor protein-tyrosine
kinases, including VEGF and PDGF receptors, were shown to be
effective as first- or second-line treatment for metastatic RCC
(1,2,4,5). These
agents exhibit anti-tumor activities mainly by inhibiting
neovascular formation via the VEGF and PDGF pathways.
Hypoxia-inducible protein 2 (HIG2), a novel hypoxia
inducible gene, is expressed exclusively in RCC. Inhibition of the
HIG2 expression by small-interfering RNA significantly suppresses
the growth of human RCC cells (7).
The addition of polyclonal anti-HIG2 antibody to culture medium
induces apoptosis in RCC cell lines. By contrast, the addition of
HIG2 protein to culture medium enhances the growth of human RCC
cells. These findings indicate that HIG2 is an essential growth
factor for RCC. Moreover, HIG2 expression is not regulated by VHL.
In RCC, the absence of VHL mutation is associated with a more
advanced tumor and a poorer prognosis (8,9).
Molecular-targeting agents used for advanced RCC exhibit anti-tumor
activity mainly via suppression of the VHL/HIF-1α pathway (1,4,5,10).
Toxicities by these agents are caused by suppression of the signal
transduction that is essential for the maintenance of various organ
conditions. HIG2 expression is minimal or absent in normal human
organs including the liver, heart, kidney, lung prostate and spinal
cord (7). In addition, HIG2
expression is observed exclusively in RCC tissues. These
observations suggest that HIG2 is a candidate target for the
development of molecular-targeting therapy for advanced RCC.
However, few studies have examined the expression of HIG2 mRNA and
protein in RCC tissues, and the little available data are
restricted to clear cell RCC including the granular type (7). This study aimed to elucidate HIG2
expression in a variety of RCC histological types and analyze the
correlation between HIG2 expression and clinicopathological
findings or patient survival.
Materials and methods
Tissue samples and patients
This study was conducted after approval by the
institutional review board was obtained. A total of 93 surgical
specimens of primary RCC were obtained from 65 males and 28 females
who underwent radical nephrectomy between January 1991 and December
2001 at the Department of Urology, Iwate Medical University School
of Medicine, Japan. The median age of these patients was 62.5 years
(range 35–87). A total of 20 normal renal tissue samples were
obtained from nephrectomies for localized RCC (pT1) as controls.
For histological and immunohistochemical analysis, the renal tissue
samples were fixed in 20% buffered formalin, embedded in paraffin
and cut into 3-μm sections. The sections were stained with
hematoxylin and eosin for routine histological examination.
Table I shows patient
characteristics, including gender distribution, age, pathological
stage, nuclear grade, lymph node metastasis and distant metastasis.
Tumor staging was performed according to the TNM classification of
malignant tumors (11). Nuclear
grading was determined based on the General Rules for Clinical and
Pathological Studies on Renal Cell Carcinoma as proposed by Fuhrman
(11). The pathological stage was
pT1a in 22 tumors, pT1b in 27, pT2 in 19, pT3a in 8, pT3b in 16 and
pT4 in 1 tumor. Lymph node metastasis was found in 9 patients.
Distant metastases were found in 9 patients (lung, 7; bone, 1 and
liver, 1). The tumor grade was G1 in 25 cases, G2 in 62 and G3 in
6. The histological type was clear cell in 80 tumors including 6
tumors of the granular cell, papillary in 7, cyst-associated in 3,
chromophobe in 1 and spindle in 2. No patient received systemic
immunotherapy prior to the nephrectomy.
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| No. of patients | 93 |
| Male/female | 65/28 |
| Age (years) |
| Median (range) | 62.5 (35–87) |
| Tumor stage |
| pT1a | 22 |
| pT1b | 27 |
| pT2 | 19 |
| pT3a | 8 |
| pT3b | 16 |
| pT4 | 1 |
| Lymph node
status |
| N0 | 62 |
| N1 or pN2 | 9 |
| NX | 22 |
| Distant
metastasis |
| M0 | 84 |
| M1 | 9 |
| Site of
metastasis |
| Lung | 7 |
| Lung and brain | 1 |
| Bone | 1 |
| Nuclear grade |
| G1 | 25 |
| G2 | 62 |
| G3 | 6 |
Immunohistochemistry
Rabbit anti-human HIG2 antibody was used in the
immunohistochemical analysis (7).
Serial 3-μm sections cut from paraffin-embedded specimens were
deparaffinized in xylene, rehydrated in graded ethanol and immersed
in 100% methanol with 0.3% hydrogen peroxide to block endogenous
peroxidase activity. The sections were treated with 10% normal goat
serum, then incubated with primary antibodies overnight at 4°C. The
optimal dilution of the primary antibody for HIG2 was 1:200. The
sections were washed and incubated with peroxidase-conjugated goat
anti-rabbit immunoglobulin (Envision+, Dako) for 30 min at room
temperature. Peroxidase activity was detected by incubation in
3,3′-diaminobenzidine tetrahydrochloride solution (DAB+ liquid
system, Dako). Sections were then counterstained with hematoxylin.
No immunoreactivity was noted in the negative-control slides
stained with immunoglobulin fraction from normal rabbit serum used
instead of antibody.
Quantitative analysis of
immunohistochemical staining
For each tumor specimen, at least 20 high-power
fields were examined, and ~1,000 tumor cells per specimen were
counted. For semi-quantitative analysis, the amount of
HIG2-positive cells was calculated and expressed as labeling index
(LI). The distribution of immunostaining was graded as negative (no
staining or LI <10), focal (LI 10–25), regional (LI 26–50) or
diffuse (LI >50).
Statistical analysis
Cancer-specific survival was shown as Kaplan-Meier
survival curves. The differences between groups were analyzed using
Mann-Whitney U or Kruskal-Wallis tests. P<0.05 was considered to
be statistically significant. Statistical analysis was performed
using statistical software (StatView version 5.0, SAS Institute,
Inc. NC, USA).
Results
Immunohistochemical staining for
HIG2
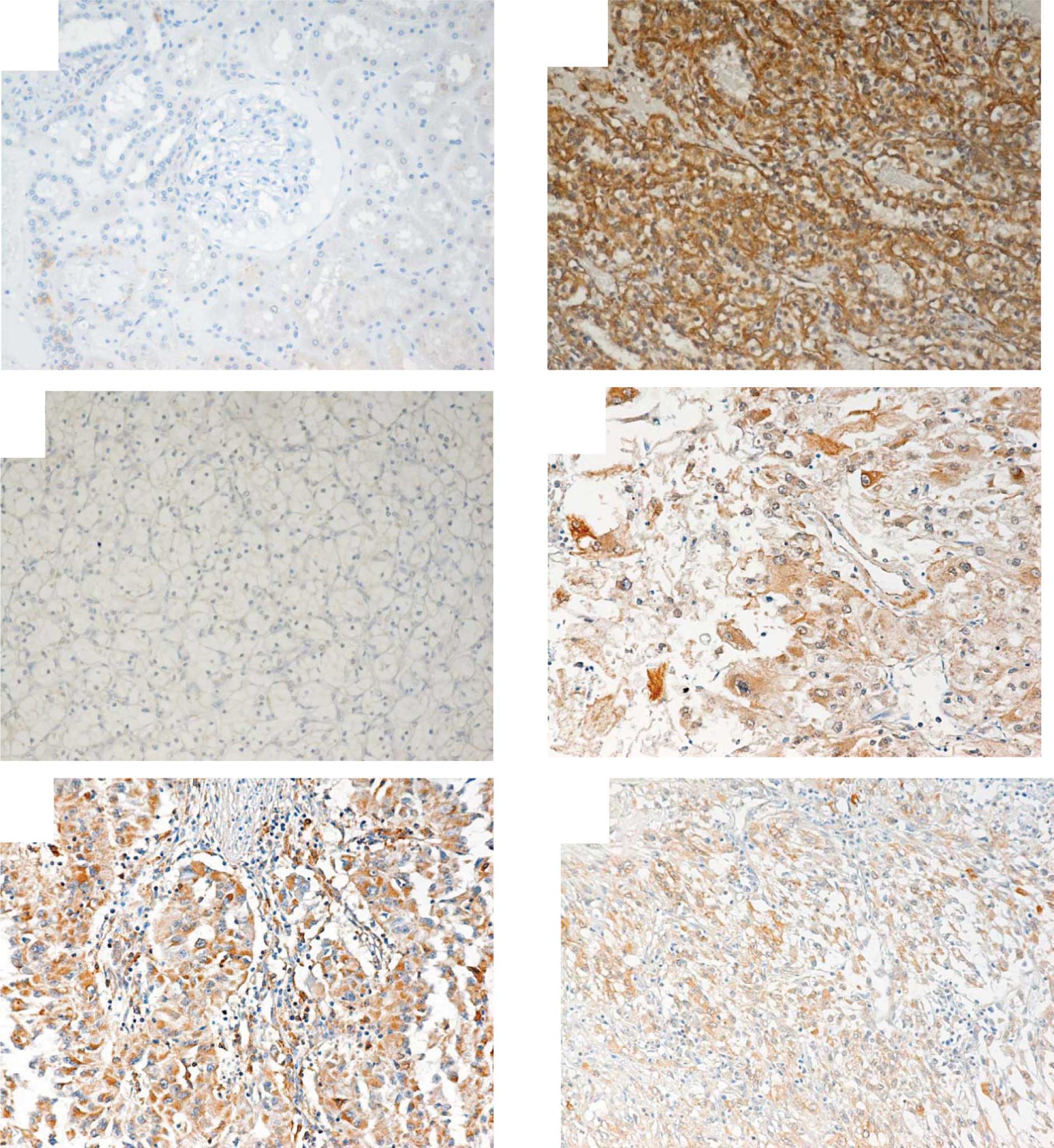
In normal kidney tissues only faint staining for
HIG2 was observed (Fig. 1A).
Positive staining for HIG2 was observed in 80/93 RCC tissues (86%)
(Fig. 1B-F). In clear cell RCC,
68/80 cases (85%) showed positive staining for HIG2. Among clear
cell RCC, 6 tumors were granular type. The granular cell RCC
expressed HIG2. The papillary, cyst-associated and chromophobe RCC
showed positive staining for HIG2 (Table II). The mean LI were 39, 66.8,
12.1, 45.4 and 24.8 in clear cell, papillary, chromophobe, spindle
cell and cyst-associated RCC, respectively (Table III). The mean LI in granular type
RCC was 39.
 | Table IIPositive staining for HIG2 according
to the histological type. |
Table II
Positive staining for HIG2 according
to the histological type.
| Histological
type | Percent
HIG2-positivity |
|---|
| Clear cell
carcinoma | 85 (68/80) |
| Papillary cell
carcinoma | 100 (7/7) |
| Chromophobe cell
carcinoma | 100 (1/1) |
| Spindle cell
carcinoma | 50 (1/2) |
| Cyst-associated
RCC | 100 (3/3) |
 | Table IIIHIG2 labeling indices (LI) according
to histological type. |
Table III
HIG2 labeling indices (LI) according
to histological type.
| Histological
type | LI % (mean ± SD) |
|---|
| Clear cell
carcinoma | 39.0 ± 2.98 |
| Papillary cell
carcinoma | 66.8 ± 11.0 |
| Chromophobe cell
carcinoma | 12.1 |
| Spindle cell
carcinoma | 45.4 |
| Cyst-associated
RCC | 24.8 ± 1.63 |
HIG2 expression and clinicopathological
data
No significant difference in positive staining for
HIG2 was detected between male and female patients (Table IV). Positive staining for HIG2
increased with increasing pathological stage or nuclear grade
(p<0.001 and p<0.006, respectively). Moreover, a
significantly higher percentage of HIG2-positive staining was
observed in RCC that metastasized to the lymph node compared to
that which did not metastasize (p<0.02).
 | Table IVHIG2 expression and clinopathological
data. |
Table IV
HIG2 expression and clinopathological
data.
| Total no. of
patients | No. of patients with
each grade of HIG2 expression |
|---|
|
|---|
| Negative | Weak | Moderate | Strong | p |
|---|
| Gender | | | | | | 0.49 |
| Male | 65 | 8 | 12 | 24 | 21 | |
| Female | 28 | 5 | 6 | 9 | 8 | |
| T stage | | | | | | <0.001 |
| pT1 | 49 | 7 | 9 | 22 | 11 | |
| pT2 | 19 | 2 | 6 | 4 | 7 | |
| pT3 | 24 | 4 | 3 | 7 | 10 | |
| pT4 | 1 | 0 | 0 | 0 | 1 | |
| LN status | | | | | | <0.02 |
| LN− | 62 | 11 | 16 | 19 | 16 | |
| LN+ | 9 | 0 | 0 | 3 | 6 | |
| LNx | 22 | 2 | 2 | 11 | 7 | |
| Nuclear grade | | | | | | <0.006 |
| G1 | 25 | 6 | 8 | 8 | 3 | |
| G2 | 62 | 6 | 10 | 25 | 21 | |
| G3 | 6 | 1 | 0 | 0 | 5 | |
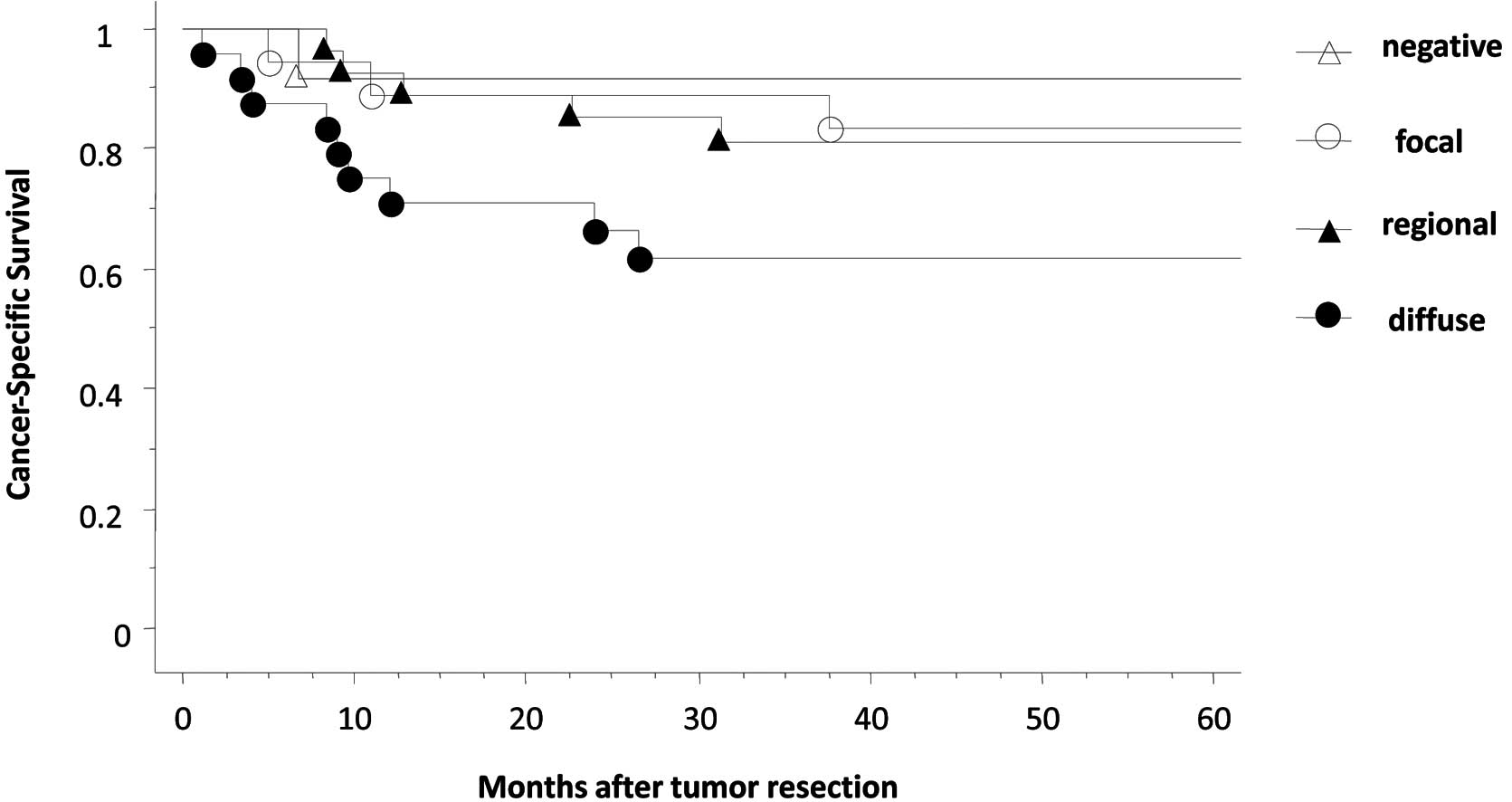
HIG2 expression and patient survival
The 5-year cancer-specific survival rate was 81%.
Patients with RCC showing negative HIG2 immunostaining exhibited an
apparently higher 5-year cancer-specific survival rate compared to
those with RCC showing positive staining for HIG2 (5-year
cancer-specific survival was 91.7% in HIG2-negative RCC vs. 93.3,
71.4 and 58.3% in focal, regional and diffuse HIG2-positive RCC,
respectively, although no significant difference was detected)
(Fig. 2).
Discussion
An understanding of the biology and genetics of RCC
is a key stage in the development of targeted therapeutic
approaches for advanced RCC (1,2,4,5,10).
The molecular targeted therapeutic agents sunitinib and sorafenib
have been shown to improve progression-free survival in patients
with metastatic RCC (1,2,4,5,10).
Recent reports described a survival benefit associated with other
targeted agents including bevacizumab, axitinib, temsirolimus and
everolimun in these patients (10).
The main targets of these agents are VHL/HIF-1α and related
pathways (1,2,4,5,10).
On the other hand, HIG2 is an essential growth factor for RCC, and
plays a critical role in the development and progression of RCC via
activation of the Wnt signaling pathway (7). Since over-expression of HIG2 has been
demonstrated in RCC tumors with and without VHL inactivation, HIG2
expression is considered to be indirectly regulated by the VHL
mutation or deletion (7). In this
study, the HIG2 protein was expressed in various subtypes of RCC
tumors. In addition, a significantly higher percentage of
HIG2-positive staining was associated with a high pathological
stage, advanced nuclear grade and lymph node metastasis, which
indicate a poor prognosis. In the present as well as previous
studies (7), HIG2 expression was
barely detectable in adult normal kidney tissues. In addition,
immunostaining for HIG2 in other adult human tissues is minimal or
absent (7). Using RT-PCR analysis,
a HIG2 mRNA expression is absent or scarcely detectable in tumors
of other organs, including colorectal, breast and hepatocellular
cancers (7). These findings suggest
that HIG2 is a promising candidate for the development of
molecular-targeted therapy for patients with advanced RCC. Since
HIG2 promotes carcinogenesis via pathways not involving VHL/HIF-1α
or its related signals, the combined use of a novel HIG2-targeting
agent with existing targeted therapeutic agents such as sunitinib,
sorafenib and temsirolimus is expected to have additive or
synergistic anti-tumor effects on RCC.
Carbonic anhydrase IX (CAIX) is a reliable
diagnostic biomarker of clear cell RCC. A low level of CAIX
expression in clear cell RCC (defined as ≤85% positivity in tumor
cells) has been shown to independently predict poor prognosis
(3,8,12).
CAIX is predominantly expressed in clear cell RCC since the
expression of this molecule is regulated by the VHL/HIF-1α pathway
which is disarrayed in clear cell RCC (8,13).
Clinical trials of vaccination with CA9-derived peptides and
administration of the cG250 monoclonal antibody, which identifies
the CAIX antigen, in patients with RCC have been conducted
(14,15). CAIX expression is observed in
various extrarenal organs including the stomach, pancreas and small
intestine (16). A high CAIX
expression is considered to be a favorable prognostic marker for
clear cell RCC (3,8,9). CAIX
expression is low in patients with clear cell RCC associated with
an aggressive clinicopathological phenotype and poor survival. In
such patients, targeted therapy is usually preferred as the
modality of treatment. By contrast, the HIG2 expression is rarely
observed in normal organs (7). The
present study showed that HIG2 expression was up-regulated in
patients with aggressive RCC and a poor prognosis. Moreover,
various histological subtypes of RCC showed a high HIG2 expression.
Compared with CAIX, we suggest that HIG2 is a more appropriate
target for molecular-targeted therapy, since there are fewer
adverse effects.
In conclusion, the present study demonstrated that
HIG2, an essential growth factor for RCC, was widely expressed in
various subtypes of RCC including granular type, which was
previously reported not to express HIG2. HIG2 expression was
up-regulated in RCC and associated with an aggressive
clinicopathological phenotype and poor cancer-specific survival.
The findings support a previous report that proposed HIG2 as a
potential target for the development of molecular-targeting therapy
for advanced RCC (7).
References
|
1
|
Weis RH and Lin PY: Kidney cancer:
identification of novel targets for therapy. Kidney Int.
69:224–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson D, Bukowski M, Rixe O, Oudard S, Negrier S, Szczylik C,
Kim ST, Chen I, Bycott PW, Baum CM and Figlin RA: Sunitinib vs.
interferon alpha in metastatic renal-cell carcinoma. N Engl J Med.
356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HL, Seligson D, Liu X, Janzen N, Bui
MHT, Yu H, Shi T, Figlin RA, Horvath S and Belldegrun AS: Using
protein expression to predict survival in clear cell renal
carcinoma. Clin Cancer Res. 10:5464–5471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ and Bukowski RM: Targeted
therapy for metastatic renal cell carcinoma. J Clin Oncol.
24:5601–5608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel PH, Chadalavada RSV, Chaganti RSK
and Motzer RJ: Targeting von Hippel-Lindau pathway in renal cell
carcinoma. Clin Cancer Res. 12:7215–7220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klatte T, Seligson DB, Riggs SB, Leppert
JT, Berkman MK, Kleid MD, Hong Yu, Kabbinavar FF, Pantuck AJ and
Belldegrun AS: Hypoxia-inducible factor α in clear cell renal cell
carcinoma. Clin Cancer Res. 13:7388–7393. 2007.
|
|
7
|
Togashi A, Katagiri T, Ashida S, Fujioka
T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T
and Nakamura Y: Hypoxia-inducible protein2 (HIG2), a novel
diagnostic marker for renal cell carcinoma and potential target for
molecular therapy. Cancer Res. 65:4817–4826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patard JJ, Fergelot P, Karakiewicz PI,
Klatte T, Trinh QD, Rioux-Leclecq N, Said JW, Belldegrun AS and
Pantuck AJ: Low CAIX expression and absence of VHL gene mutation
are associated with tumor aggressiveness and poor survival of clear
cell carcinoma. Int J Cancer. 123:395–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao M, Yoshida M, Kishida T, Nakaigawa N,
Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y,
Kubota Y and Kondo K: VHL tumor suppressor gene alterations
associated with good prognosis in sporadic clear-cell renal cell
carcinoma. J Natl Cancer Inst. 94:1569–1575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizutani Y: Recent advances in molecular
targeted therapy for metastatic renal cell carcinoma. Int J Urol.
16:444–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tozawa K, Okamoto T, Kawai N, Hashimoto Y,
Hayashi Y and Kohri K: Positive correlation between sialyl Lewis X
expression and pathologic findings in renal cell carcinoma. Kidney
Int. 67:1391–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao SY, Aurelio ON, Jan K, Zavada J and
Stanbridge EJ: Identification of the MN/CA9 protein as a reliable
diagnostic biomarker of clear cell carcinoma of the kidney. Cancer
Res. 57:2827–2831. 1997.PubMed/NCBI
|
|
13
|
Wykoff CC, Beasley NJP, Watson PH, Turner
KJ, Pastrek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, Pugh CW, Ratcliffe PJ and Harris AL: Hypoxia-inducible
expression of tumor-associated carbonic anhydrases. Cancer Res.
60:7075–7083. 2000.PubMed/NCBI
|
|
14
|
Uemura H, Fujumoto K, Tanaka M, Yoshikawa
M, Hirano Y, Uejima S, Yoshikawa K and Itoh K: A phase I trial of
vaccination of CA9-derived peptides for HLA-A24-positive patients
with cytokine-refractory metastatic renal cell carcinoma. Clin
Cancer Res. 12:1768–1775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis ID, Wiseman GA, Lee FT, Gansen DN,
Hopkins W, Papenfuss AT, Liu Z, Moynihan TJ, Croghan GA, Adjei AA,
Hoffman EW, Ingle JN, Old LJ and Scott AM: A phase 1 multiple dose,
dose escalation study of cG250 monoclonal antibody in patients with
advanced renal cell carcinoma. Cancer Immun. 7:132007.PubMed/NCBI
|
|
16
|
Leibovich BC, Sheinin Y, Lohse CM,
Thompson RH, Cheville JC, Zavada J and Kwon ED: Carbonic anhydrase
IX is not an independent predictor of outcome for patients with
clear cell renal cell carcinoma. J Clin Oncol. 25:4757–4764. 2007.
View Article : Google Scholar : PubMed/NCBI
|