Introduction
Serous microcystic adenomas (SMAs) are rare tumors
in the pancreas that are traditionally regarded as benign lesions
with no or little malignant potential (1). SMAs occur mainly in elderly women. The
majority of patients are asymptomatic and the tumors are only
detected incidentally (2). The
typical appearance of a SMA on computed tomography (CT) is a
well-demarcated, lobulated cystic lesion with multiple thin
septations. A central stellate scar is observed at the center of
the lesion in certain cases. Microscopic examination reveals that
the cysts are lined by a single layer of cuboidal or flattened
cells, and the cytoplasm is positive for Periodic acid-Schiff (PAS)
staining. The preoperative diagnosis is mainly based on a CT scan
or an ultrasound. The differential diagnosis between SMAs and other
non-neoplastic and neoplastic cysts is significant due to the
considerable difference in their management (3). Pancreatic pseudocysts occupy 80–90% of
pancreatic cystic lesions, and are associated with histories of
pancreatitis (4). Mucinous cystic
neoplasms (MCN), intraductal papillary-mucinous neoplasms (IPMN)
and solid pseudopapillary neoplasms (SPPN) are common cystic
neoplasms of the pancreas that have the potential to transform into
malignant tumors (1,5,6). Other
rare cystic lesions of the pancreas include pancreatic endocrine
neoplasms, acinar cell cystadenomas, enterogenous cysts, cystic
mesenchymal tumors, endometrial cysts and parasitic cysts (7,8). We
present two female cases of SMA and review the literature regarding
its etiology, clinical presentation, imaging characteristics,
histopathological features and management.
Case reports
Case 1
A 47-year-old woman was admitted to our hospital
with abdominal bloating and back pain, but without nausea,
vomiting, jaundice, melena or fever. She had undergone a left
radical mastectomy 10 years previously. No irregularities were
noted in the physical examination. The complete blood count,
biochemical parameters and tumor biomarkers were normal. A CT scan
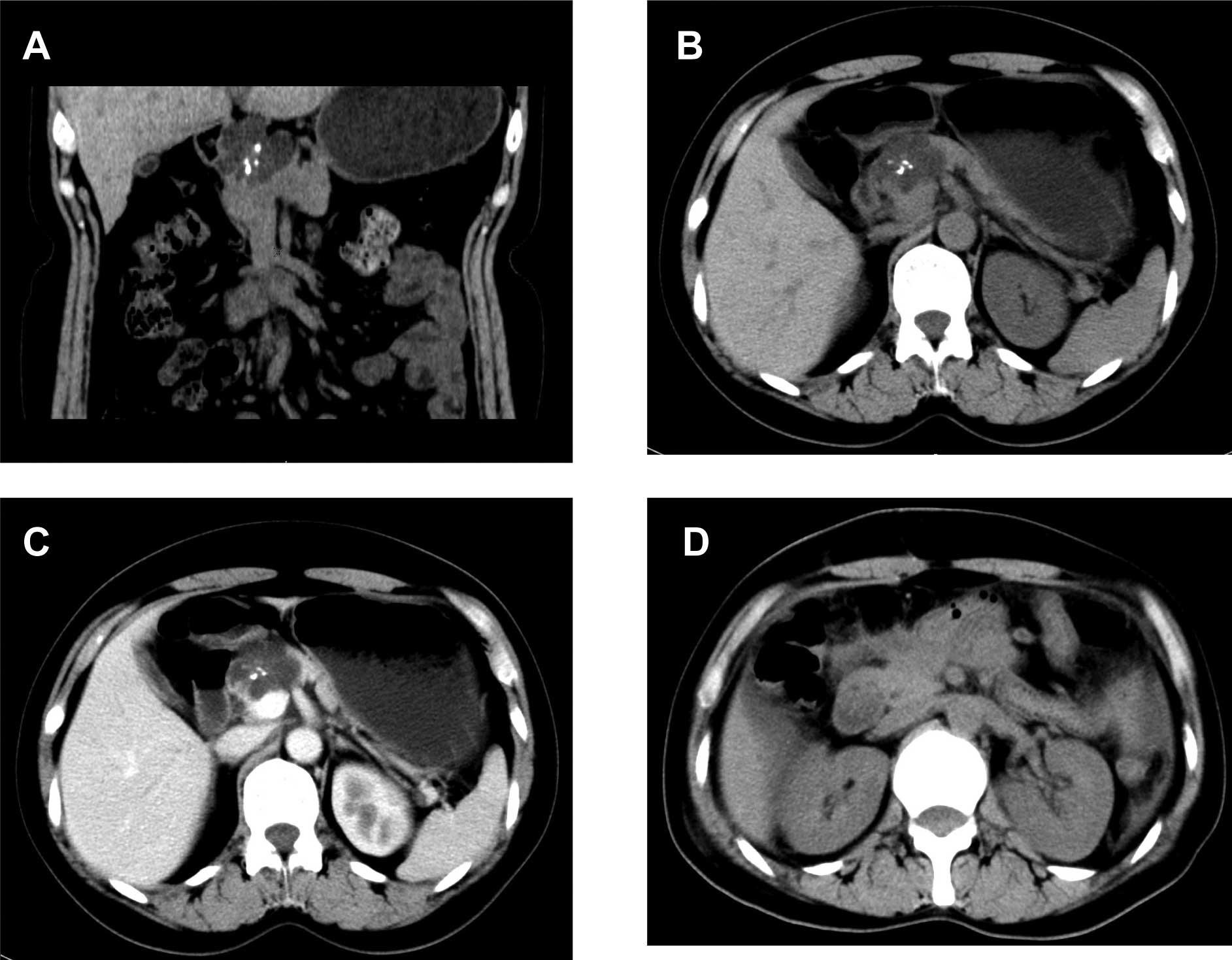
revealed a multiloculated cystic mass, 4.1×3.3 cm2 in
size, with a central scar and calcifications in the pancreatic head
(Fig. 1A and B). An enhanced CT
scan showed that the body and tail of the pancreas were normal,
without expansion of the pancreatic ducts or lymphadenopathy
(Fig. 1C). During an exploratory
laparotomy, a multiple cystic mass was located in the pancreatic
head. An intraoperative frozen section revealed a SMA. Segmental
resection of the pancreas and pancreatico-jejunal anastomosis were
performed. A post-operative CT scan indicated no tumor recurrence
(Fig. 1D). Grossly, the resected
mass had a central scar surrounded by tiny loculi filled with clear
fluid. Stellate calcifications were also noted. A microscopic
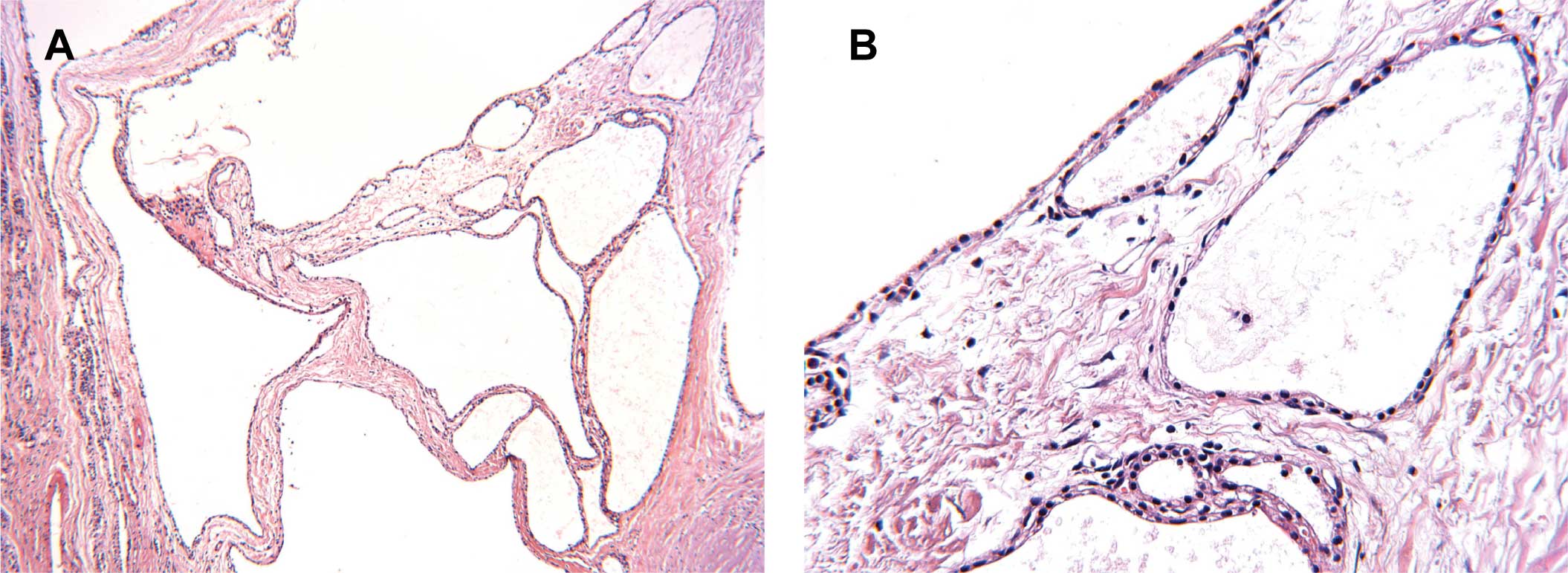
examination with hematoxylin and eosin (H&E) staining revealed
that the cysts were lined with a single layer of cuboidal cells
without atypia (Fig. 2), and the
cytoplasm was positive for PAS staining (data not shown). Two
pathologists confirmed the SMA diagnosis.
Case 2
A pancreatic space-occupying lesion was incidentally
discovered by ultrasound during a routine physical examination of a
71-year-old female. She was referred to our hospital for further
investigation and treatment. The patient was asymptomatic on
admission. The patient had exhibited a history of hypertension for
more than a decade, and her blood pressure was kept under control
by medication. The physical examination was negative. The complete
blood count, biochemical parameters, and tumor biomarkers were
normal. A CT scan showed an irregular and multiple cystic mass, 2×2
cm2 in size, in the head of pancreas (Fig. 3A, B and C). An
F-18-fluorodeoxyglucose positron emission tomography (FDG-PET)
showed a low-density area with a mildly increased metabolism in the
pancreatic head. During the segmental resection of pancreas, a
well-demarcated mass was found. An intraoperative frozen section
revealed a SMA. Subsequently, pancreatico-jejunal anastomosis was
performed. A postoperative CT scan indicated no recurrence of the
tumor (Fig. 3D). Grossly, the
resected mass had a central scar and multiple small cysts filled
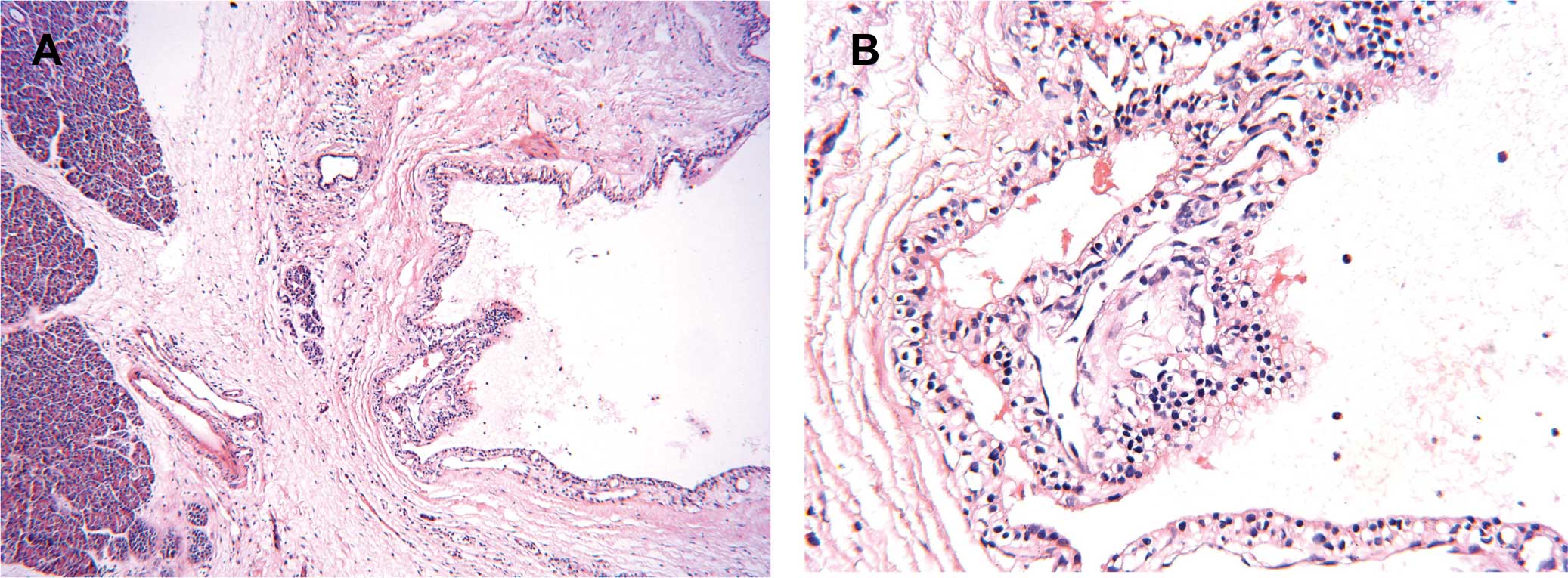
with clear fluid. A pathological examination with H&E staining
showed a SMA with the reactive hyperplasia of peripheral lymph
nodes (Fig. 4). The cytoplasm of
neoplastic cells was positive for PAS staining (data not shown).
Two pathologists confirmed the SMA diagnosis.
Immunohistochemical staining
Immunohistochemistry was performed using the
avidin-biotin immunoperoxidase method in both cases. As shown in
Fig. 5, the neoplastic cells were
positive for cytokeratin 7 (CK7, OV-TL 12/30 clone, Dako, Glostrup,
Denmark) and vimentin (Vim, V9 clone, Dako), but negative for
cytokeratin 20 (CK20, Ks20.8 clone, Dako) and synaptophysin (SYN,
SY38 clone, Dako).
Discussion
SMAs are uncommon cystic tumors of the pancreas and
constitute 1–2% of all exocrine pancreatic neoplasms (2,9). The
age range of patients was 24–91 years (mean age 66) (6). SMAs mainly occur in elderly women, and
the female-to-male ratio is 3:1 (2,6). As
shown in Table I, MCN and SPPN tend
to occur in females. In contrast, the incidence of IPMN is higher
in men (6). The etiology and
pathogenesis of SMAs remains largely unknown. SMAs are
traditionally considered to be benign; however, malignant
transformation has been noted (10). It has been reported that 29–47% of
patients are asymptomatic and that the tumors are detected
incidentally (1,11,12).
Patients with symptoms usually manifest abdominal pain, weight
loss, nausea, vomiting, fever and a palpable abdominal mass
(2,12). If the tumors are large, they may
result in obstructive symptoms such as jaundice. Portal
hypertension, hemoperitoneum, and acute gastrointestinal hemorrhage
are rare clinical manifestations (6). In this study, one patient presented
with abdominal bloating and back pain, while the other was
asymptomatic. We believe that the diagnosis of SMAs should be kept
in mind even though their clinical manifestations are
non-specific.
 | Table ICharacteristics of pancreatic cystic
lesions. |
Table I
Characteristics of pancreatic cystic
lesions.
| Type | SMA | MCN | IPMN | SPPN | Pseudocyst |
|---|
| Malignant
potential | Rarely | Yes | Yes | Yes | No |
| Clinical
features |
| Gender | F>M | F>M | M>F | F>M | M>F |
| Age | Elderly | Middle-aged | Elderly | Young | Variable |
| Pancreatitis
history | No | No | Yes | No | Yes |
| Symptoms | Asymptomatic
mostly | Abdominal pain,
weight loss | Vague abdominal
discomfort | Abdominal pain,
weight loss, palpable mass | Abdominal pain,
gastric outlet obstruction, nausea |
| Imaging findings | Well-delineated mass
with small cysts and central scar | Single cyst,
thickened wall | Thin capsule
communicated with the pancreatic duct | Solid mass without
septa | Single cyst, thin
wall |
| Histopathological
features | Cysts composed of
low-cuboidal, small cells with clear cytoplasm | Flat mucinous- type
epithelium | Flat/papillary
mucinous-type epithelium | Pseudopapillary
neoplasm with tumor cells arranged around capillaries | Necrotic fat and a
combination of necrotic cells |
| Management |
Resection/observation | Resection |
Resection/observation | Resection | Surgical
drainage/observation |
The radiological appearance of SMA is a
well-demarcated mass composed of multiple small cysts, usually
located in the body and tail of pancreas. However, in the two cases
studied herein, the cystic mass was located in the pancreatic head.
The small cysts are less than 2 cm in diameter. Central calcified
scars were observed in approximately 30% of cases. No communication
with the pancreatic duct was noted in SMAs (9). No enhancement of the tumor was
observed on enhanced CT scans of SMAs. In contrast, IPMN exhibited
a thin capsule and often communicated with the pancreatic ducts,
while SPPNs were considered to be solid and cystic lesions. It is
difficult to preoperatively distinguish between SMAs and MCNs using
radiologic methods (Table I). In
magnetic resonance imaging, SMAs exhibited a low signal intensity
on T1-weighted images and a high signal intensity on T2-weighted
images (7). Endoscopic
ultrasonography in conjunction with fine needle aspiration of cyst
fluid is feasible as well. The carcinoembryonic antigen values of
cyst fluid are universally low in SMAs, and are generally elevated
in mucinous lesions and cystadenocarcinomas (11). We believe that the preoperative
diagnosis of a SMA should be considered when ultrasounds and CT
scans indicate a well-delineated mass with multiple small
septations. However, it is difficult to distinguish a benign serous
cystadenoma from a malignant serous cystadenocarcinoma, since
imaging examinations and core needle biopsy are not entirely
credible (10). On the other hand,
the intraoperative frozen section is useful for the correct
diagnosis of SMAs.
Grossly, the cyst fluid of SMAs is clear, thin,
watery and straw-colored. In contrast, MCNs contain thick and
viscous fluid. In the center of SMAs, fibrous scars are surrounded
by many tiny cysts containing clear liquid (6). Histologically, the cysts of SMAs are
composed of simple cuboidal epithelium or flat epithelial cells
with clear cytoplasm, but without atypical cells (Table I). Glycogen in the cytoplasm can be
demonstrated with PAS staining. These characteristics are important
for the differential diagnosis from pancreatic pseudocysts that are
composed of inflammatory cells without epithelial cells (7). In addition, the cysts in SMAs are
lined by bland epithelial cells that are arranged in a single
layer, and MCNs are lined by mucinous-type epithelium (6). The immunohistochemical analysis showed
that SMAs are positive for cytokeratins (CKs), neuron-specific
enolase, α-inhibin and mucin 6, but negative for neuroendocrine
markers such as synaptophysin (SYN), chromogranin A and tyrosine
hydroxylase (8). This study showed
that the neoplastic cells were positive for CK7 and vimentin (Vim),
but negative for CK20 and SYN. However, we are in agreement with
the view that an immunohistochemical test is not necessary since
the histological appearance of the cells is characteristic
(13).
The management of SMAs depends on the symptoms,
tumor size and surgical risk (Table
I). Resections are reasonable for patients with symptoms or
tumors larger than 3 cm in maximal diameter (10,11).
Surgical excision of the mass should be performed when tumor growth
is rapid (14). Distal
pancreatectomy is nominated when tumors are located in the body or
tail of the pancreas. In addition, a Whipple resection is required
when the tumor is situated in the head or uncinate process of the
pancreas (13,15). It is widely accepted that close
follow-up is recommended in asymptomatic patients with tumors less
than 3 cm in maximal diameter, in elderly patients or in those who
have high surgical risks. Authors have suggested that follow-up
should be scheduled every 6 months for 2 years and annually
following that. The follow-up should be continued for at least 4
years, otherwise patients should no longer be considered as
candidates for surgical intervention (13,15).
Since SMAs have little or no potential of malignant transformation,
the prognosis is considered to be favorable. However, long-term
follow-up is required for better elucidation of the prognosis of
SMAs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 30200284, No. 30600278 and No.
30772359), the Program for New Century Excellent Talents in
University (NCET-06-0641), and the Scientific Research Foundation
for the Returned Overseas Chinese Scholars (2008-889).
References
|
1
|
Compagno J and Oertel JE: Mucinous cystic
neoplasms of the pancreas with overt and latent malignancy
(cystadenocarcinoma and cystadenoma). A clinicopathologic study of
41 cases. Am J Clin Pathol. 69:573–580. 1978.PubMed/NCBI
|
|
2
|
Morohoshi T, Held G and Kloppel G:
Exocrine pancreatic tumours and their histological classification.
A study based on 167 autopsy and 97 surgical cases. Histopathology.
7:645–661. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subramanian S, Marappagounder S, Selvaraj
DR and Elangovan B: A rare association of serous cystadenoma of the
pancreas with mediastinal lipoma: a case report. Cases J.
2:71652009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sand J and Nordback I: The differentiation
between pancreatic neoplastic cysts and pancreatic pseudocyst.
Scand J Surg. 94:161–164. 2005.PubMed/NCBI
|
|
5
|
Tanaka M, Chari S, Adsay V, et al:
International consensus guidelines for management of intraductal
papillary mucinous neoplasms and mucinous cystic neoplasms of the
pancreas. Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldsmith JD: Cystic neoplasms of the
pancreas. Am J Clin Pathol. 119(Suppl): 3–16. 2003.
|
|
7
|
Ng DZ, Goh BK, Tham EH, Young SM and Ooi
LL: Cystic neoplasms of the pancreas: current diagnostic modalities
and management. Ann Acad Med Singapore. 38:251–259. 2009.PubMed/NCBI
|
|
8
|
Kosmahl M, Wagner J, Peters K, Sipos B and
Kloppel G: Serous cystic neoplasms of the pancreas: an
immunohistochemical analysis revealing alpha-inhibin,
neuron-specific enolase, and muc6 as new markers. Am J Surg Pathol.
28:339–346. 2004. View Article : Google Scholar
|
|
9
|
Warshaw AL, Compton CC, Lewandrowski K,
Cardenosa G and Mueller PR: Cystic tumors of the pancreas. New
clinical, radiologic, and pathologic observations in 67 patients.
Ann Surg. 212:432–443; discussion 444–445. 1990.PubMed/NCBI
|
|
10
|
King JC, Ng TT, White SC, Cortina G, Reber
HA and Hines OJ: Pancreatic serous cystadenocarcinoma: a case
report and review of the literature. J Gastrointest Surg.
13:1864–1868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tseng JF, Warshaw AL, Sahani DV, Lauwers
GY, Rattner DW and Fernandez-del Castillo C: Serous cystadenoma of
the pancreas: tumor growth rates and recommendations for treatment.
Ann Surg. 242:413–419; discussion 419–421. 2005.PubMed/NCBI
|
|
12
|
Pyke CM, van Heerden JA, Colby TV, Sarr MG
and Weaver AL: The spectrum of serous cystadenoma of the pancreas.
Clinical, pathologic and surgical aspects. Ann Surg. 215:132–139.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcea G, Ong SL, Rajesh A, et al: Cystic
lesions of the pancreas. A diagnostic and management dilemma.
Pancreatology. 8:236–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vernadakis S, Kaiser GM, Christodoulou E,
et al: Enormous serous microcystic adenoma of the pancreas. JOP.
10:332–334. 2009.PubMed/NCBI
|
|
15
|
Sahani DV, Saokar A, Hahn PF, Brugge WR
and Fernandez-Del Castillo C: Pancreatic cysts 3 cm or smaller: how
aggressive should treatment be? Radiology. 238:912–919. 2006.
View Article : Google Scholar : PubMed/NCBI
|