Introduction
Lung cancer is the leading cause of cancer-related
mortality in both men and women worldwide (1). The strong invasive and metastatic
characteristics of lung tumor cells are responsible for their
relatively high malignancy. Heparins are glycosaminoglycans that
play a variety of cellular and plasmatic roles (2). Unfractionated heparin (UFH) and low
molecular weight heparin (LMWH) prevent the process of blood
coagulation in clinical therapy by activating antithrombin III and
inhibiting activated coagulation factors X and II (3). Recently, UFH was largely replaced by
LMWH in clinical use since LMWH has a longer half-life and less
bleeding (4).
Besides their anticoagulant effects, a wide variety
of biological activities of LMWH have been identified. Previous
clinical studies strongly suggest that LMWH, used to treat venous
thromboembolism in patients with cancer such as lung (5–7),
breast (8,9), brain (10) and other advanced cancers (11–13),
delays tumor progression and prolongs survival. Experimental
evidence from animal models strongly indicates that LMWH is an
efficient inhibitor of cancer metastasis (14–16).
The anticancer activity of LMWH may be correlated with
anti-metastasis activity. However, the effect and precise mechanism
of LMWH on the invasion and metastasis of lung cancer have yet to
be determined.
Fraxiparine (nadroparin calcium), a low molecular
weight heparin, is a heterogeneous combination of sulphated
polysaccharide glycosaminoglycan chains. It is used to treat deep
vein thrombosis in the clinic. In this study, intervention was
conducted with regards to the invasion and metastasis of A549 cells
with Fraxiparine and the alteration of cell invasion, migration and
adhesion status was observed. Concomitantly, the change in cell
viability, cell cycle progression and the apoptotic status of A549
cells treated with Fraxiparine was noted. Furthermore, the
expression of two tumor invasion- and metastasis-associated genes
(nm23-H1 and heparanase) was detected at the mRNA level. The
expression of four tumor invasion- and metastasis-associated genes,
i.e., integrin β1 and β3, as well as matrix metalloproteinase
(MMP)-2 and −9, was further examined at the protein and mRNA
levels.
Materials and methods
Materials
The A549 cell line was purchased from the American
Type Culture Collection (USA). The cell culture medium and reagents
were obtained from Gibco Laboratories (USA). Fraxiparine (LMWH) was
purchased from Glaxosmithkline (UK).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
and propidium iodide (PI) were products of Sigma (USA). Annexin
V-FITC Detection kit was purchased from Keygen (China). Matrigel
and transwell chambers were purchased from Becton-Dickinson (USA),
and the TRIzol reagent was obtained from Invitrogen (USA). The
reverse transcription kit was purchased from Tiangene (China), and
the PCR kit was purchased from Sinobio (China). The BCA Protein
assay kit was a product of Thermo (USA), and the PVDF membrane was
purchased from Millipore (USA). The rabbit polyclonal anti-GAPDH
was obtained from Trevigen (USA), and the mouse monoclonal
anti-integrin β1 and β3 were purchased from Santa Cruz
Biotechnology (USA). Rabbit monoclonal anti-MMP-2 and anti-MMP-9
were purchased from Abcam (USA). Enhanced chemiluminescence
reagents were obtained from Amersham Pharmacia Biotech (USA).
Cell viability assay
A549 cells were incubated in RPMI-1640 containing
10% FBS (fetal bovine serum) and 1% antibiotics at 37°C in a
humidified atmosphere containing 5% CO2. The effect of
Fraxiparine on the cell viability of A549 cells was determined
using the MTT assay. A549 cells (3,000/well) were seeded into a
96-well plate and incubated in a culture medium containing
Fraxiparine with a particular concentration for 24, 48 and 72 h,
respectively. Subsequently, the medium was replaced with 200 μl of
fresh medium, and 20 μl of sterile filtered MTT (5 mg/ml) stock
solution in phosphate-buffered saline (PBS) was added to each well.
After 4 h, unreacted dye was removed by aspiration. The formazan
crystals were dissolved in 150 μl DMSO per well and measured
spectrophotometrically in a microplate reader (Bio-Rad, USA) at a
wavelength of 490 nm. The cell survival was expressed as: cell
viability = (ODtreated/ODcontrol) × 100%.
Cell cycle distribution and cell
apoptosis ratio assay
A549 cells (1.5×105) in 2 ml were plated
in 6-well plates and incubated for 24 h. The following day, the
primary medium was replaced with medium containing the indicated
concentrations of Fraxiparine for a 24-h incubation. At the end of
the second 24-h incubation, both the adherent cell layer, which was
trypsinized, and the cells floating in the medium were collected.
Cells (1×106) were washed. For cell cycle analysis, the
cells were incubated with 2 μg/ml of RNase A in PBS (200 μl) and PI
(0.1 μg/ml) in 0.6% Nonidet P-40 on ice for 30 min. The DNA
contents of the samples were immediately measured using flow
cytometry (Becton-Dickinson). The apoptosis assay was carried out
as recommended by the manufacturer of the Annexin V-FITC Detection
kit. Data concerning the cell cycle phase distribution and
apoptosis were determined using CellQuest software
(Becton-Dickinson).
Determination of invasion and
migration
Cell invasion and migration were determined with or
without Matrigel-coated transwell chambers. Filters in the upper
compartment were loaded with 100 μl serum-free RPMI-1640 containing
5×104 cells incubated with Fraxiparine, and the lower
compartment was filled with RPMI-1640 containing 10% FBS. The
chamber was then cultivated in 5% CO2 at 37°C for 24 h.
The Matrigel and cells in the upper chamber were removed, and the
attached cells in the lower section were stained with 0.1% crystal
violet. These cells were then counted and photographed under a
light microscope.
Cell adhesion assay
Each well of 96-well plates was coated with Matrigel
for 1 h at 37°C. The plates were rinsed with PBS and blocked with
1% BSA for 1 h at 37°C. For the 24-h treatment with Fraxiparine,
cells were harvested with trypsin, added to the wells at
1×104 cells/well and allowed to adhere for 1 h at 37°C.
Non-adherent cells were removed by gentle washing, and the number
of attached cells was measured using the MTT assay as described for
the cell viability assay.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The mRNA expression levels of heparanase, nm23-H1,
integrin β1 and -β3, and MMP-2 and −9 in the A549 cells treated
with Fraxiparine were evaluated using RT-PCR analysis. The A549
cells were incubated in a culture medium containing Fraxiparine
with a particular concentration. Total RNA was extracted from the
A549 cells using TRIzol reagent according to the manufacturer’s
protocol. Total RNA (1 μg) was reverse-transcribed, and PCR was
performed with gene-specific primers. The PCR products were
electrophoresed in 1% agarose gel containing Golden View in 1X TAE
buffer.
Western blot analysis
The protein expression levels of integrin β1 and β3,
and MMP-2 and −9 in A549 cells treated with Fraxiparine were
examined by Western blot analysis. A549 cells were incubated in a
culture medium containing Fraxiparine with a particular
concentration for 24 h, harvested in RIPA buffer (150 mM NaCl, 50
mM Tris, pH 8.0, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1%
SDS supplemented with protease inhibitors and 1 mM
phenylmethylsulfonyl fluoride) and homogenized. The whole cell
lysates were centrifuged at 12,000 × g for 10 min at 4°C, and the
protein concentrations were determined by the BCA protein
quantification assay. Samples were boiled in SDS sample buffer, and
an equal amount of total proteins was loaded on each lane and
separated by SDS-PAGE. The separated proteins were transferred to a
polyvinylidene difluoride membrane and probed with primary
antibodies and horseradish peroxidase-conjugated secondary
antibodies. Immune complexes were visualized with enhanced
chemiluminescence reagents.
Statistics
The values yielded are the mean ± SEM. The
significance of difference between the experimental groups and
controls was assessed by the Student’s t-test. P<0.05 indicates
a significant difference.
Results
Effect of Fraxiparine on cell viability,
cell cycle progression and apoptosis
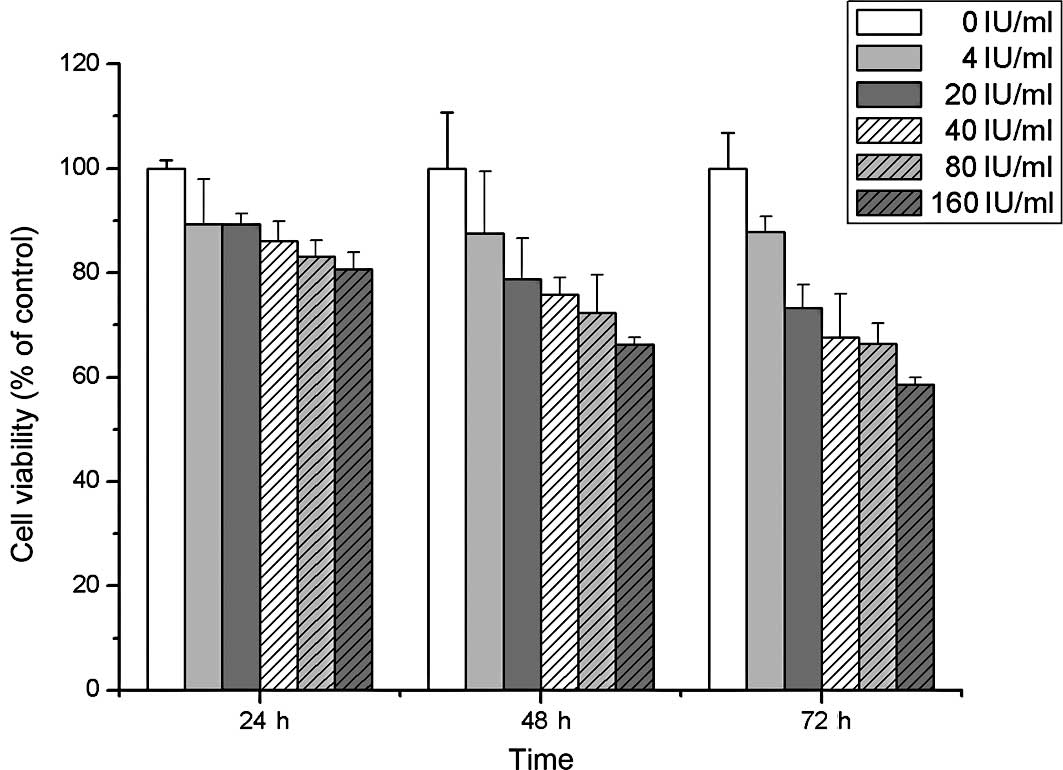
This study examined the cell viability of A549 cells
treated with Fraxiparine at different time points (24, 48 and 72 h)
using the MTT assay. A slight inhibition of cell viability was
observed following treatment with various concentrations of
Fraxiparine in a dose- and time-dependent manner (Fig. 1). Furthermore, the effects of
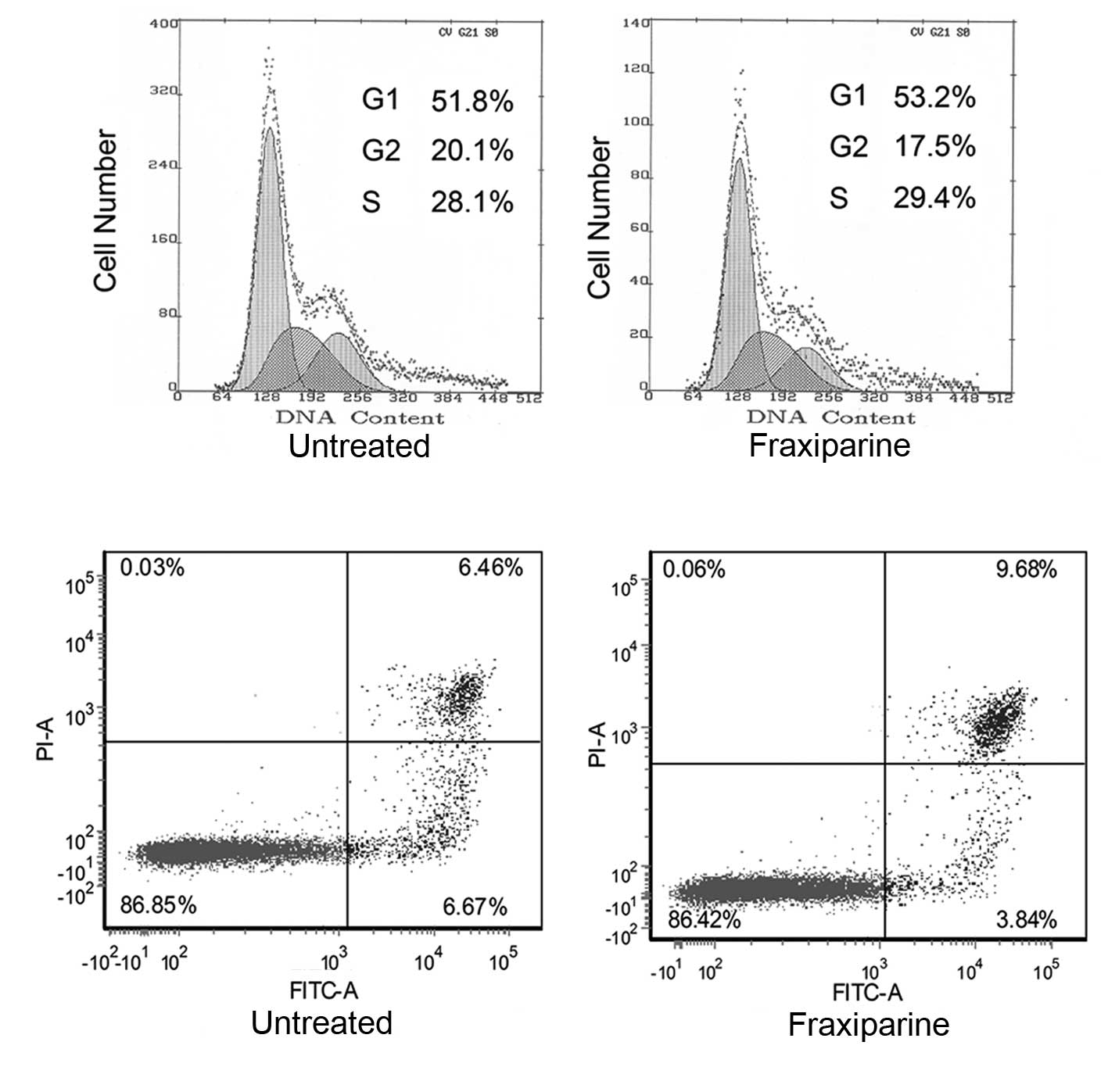
Fraxiparine on cell cycle progression and the apoptotic status of
A549 cells were investigated. Treatment of A549 cells with
Fraxiparine (20 IU/ml) for 24 h neither arrested cells in the G1
phase nor induced early apoptosis when compared to the control
(Fig. 2). Similar results were
obtained for longer (48 and 72 h) or higher concentration (80
IU/ml) treatments (data not shown).
Effect of Fraxiparine on A549 cell
invasion, migration and adhesion
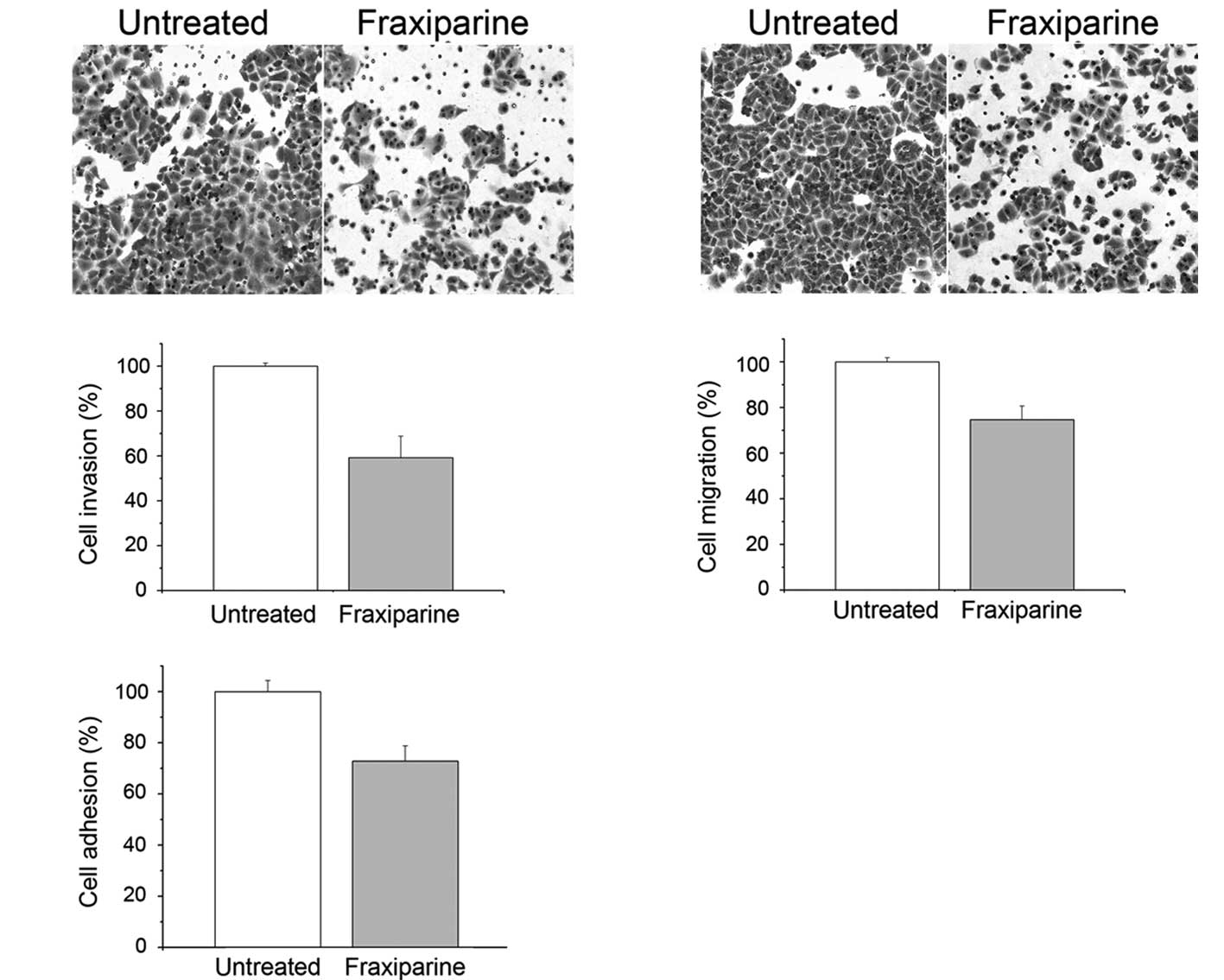
Invasion is critical for the formation of metastasis
and the spreading of cancer in vivo. To examine whether
Fraxiparine altered the invasion status of A549 cells,
Matrigel-coated transwell chambers were used. Cell invasion was
quantified as the number of cells that traversed the chamber in 24
h. The result showed that Fraxiparine caused a decrease in A549
cell invasion (Fig. 3A).
Quantification of the cells in the chamber showed a decrease of
~40% at 20 IU/ml of Fraxiparine (Fig.
3B). Moreover, less migration was observed in the
Fraxiparine-treated A549 cells (Fig.
3C). Quantitatively, this amounted to a 25% decrease in the
number of cells that traversed the transwell (Fig. 3D). Cell-matrix interaction is
crucial for cancer cell invasion since this interaction affects
tumor cell locomotion and the proteinase expression. Our result
showed that Fraxiparine inhibited the adhesion of the A549 cells to
Matrigel (Fig. 3E), and the
inhibition rate reached 27%. Additionally, the inhibition ratio of
Fraxiparine at a concentration of 80 IU/ml on the invasion,
migration and adhesion of A549 cells was found to be higher than
that at the concentration of 20 IU/ml (data not shown).
Effect of Fraxiparine on the expression
levels of nm23-H1, heparanase, integrin β1, integrin β3, MMP-2 and
MMP-9
To investigate the molecular mechanism of
Fraxiparine on the invasion and metastasis of A549 cells, we first
examined mRNA expression levels of heparanase, nm23-H1, integrin β1
and β3, and MMP-2 and −9 in A549 cells treated with Fraxiparine
using RT-PCR. The RT-PCR analysis showed that the expression level
of nm23-H1 increased and the levels of heparanase, integrin β1 and
β3, and MMP-2 and −9 decreased in the A549 cells treated with
Fraxiparine (20 IU/ml) for 24 h (Fig.
4A).
Furthermore, the protein expression levels of
integrin β1 and β3, and MMP-2 and −9 in the A549 cells treated with
Fraxiparine were analyzed by Western blotting. The results
indicated that the levels of integrin β1 and β3, as well as MMP-2
and −9 were apparently lower than those in the untreated groups
(Fig. 4B).
Discussion
In recent years, a number of pre-clinical research
studies have suggested that heparin and its pharmacokinetically
improved version, LMWH, possess an array of potential anticancer
properties in addition to their traditional anticoagulant
activities (17,18). Previous studies showed that heparin
and LMWH have effective anti-angiogenetic and anti-proliferative
activity (19). Apart from
anti-angiogenesis and anti-proliferation, the effect of LMWH on
tumor metastasis was discovered (20). The findings of certain studies
suggest that the action of anti-metastasis of LMWH is mediated by
inhibiting selectin, which is associated with platelet tumor cell
thrombus formation (21,22). However, the action of LMWH on the
invasion and metastasis of human A549 lung cancer cells and the
possible mechanism have yet to be addressed.
Results of our study showed that Fraxiparine did not
change cell viability, cell cycle progression and the apoptotic
status of A549 cells. However, it significantly inhibited the
invasion, migration and adhesion of A549 cells. It was also found
that Fraxiparine markedly caused the up-regulation of the
expression of nm23-H1 and the down-regulation of the expression of
the heparanase gene at the mRNA level. Furthermore, our results
demonstrated that Fraxiparine decreased the expression of integrin
β1 and β3, as well as MMP-2 and −9 of A549 cells at the mRNA and
protein levels. The data strongly suggest that the changes in cell
invasion, migration and adhesion status induced by Fraxiparine in
A549 cells are related to the expression levels of nm23-H1,
heparanase, integrin β1 and β3, and MMP-2 and −9.
Heparanase is an endoglycosidase that degrades
heparin sulfate on the cell surface and extracellular matrix (ECM).
Heparanase expression is rare in normal tissues, but becomes
evident in many human tumors where it significantly increases the
angiogenic and metastatic potential of tumor cells (23,24).
It has been reported that the inhibition of the transcription of
the heparanase gene or the enzyme activity suppresses metastasis
formation, indicating that heparanase is involved in the
extravasation and invasion processes of cancer (25,26).
As a highly specific anti-metastatic target of MMP-2 that plays a
significant role in tumor metastasis for anticancer therapy, the
change in the heparanase expression regulates MMP-2 expression
(27). In addition, it has been
reported that a number of LMWHs inhibit the enzymatic activity of
heparanase (19). Our result showed
that inhibition of the heparanase gene expression by Fraxiparine
was significant even with a concentration as low as 20 IU/ml. The
inhibition rate reached 81%. The result was consistent with
previous studies and demonstrated a direct effect of heparanase on
tumor cell invasion and metastasis in vitro.
nm23-H1, a tumor metastasis suppressor gene, was
first identified by the differential screening of melanoma cell
lines with a high and low metastatic potential (28). A low expression of nm23-H1 leads to
metastasis formation in lung cancer (29). Multiple transfection experiments
indicate that, when nm23-H1 expression is forcibly restored,
metastases to the lungs, lymph nodes and other organs significantly
decreased (30). On the other hand,
evidence indicates that the MMPs are down-regulated in cells
expressing relatively higher levels of nm23-H1 (31). The molecular mechanism of nm23-H1 in
suppressing lung cancer invasion and metastasis may be through the
regulation of the differential expression of a series of tumor
metastasis-associated genes. Findings of this study found that the
RNA expression level of nm23-H1 in A549 cells treated with
Fraxiparine increased approximately 2-fold compared to the
untreated cells. This result suggests that the mechanism of
Fraxiparine in the inhibition of the invasion and metastasis of
A549 cells occurs through the regulation of nm23-H1-mediated
signaling pathways or tumor metastasis-associated genes.
To further explore the potential mechanisms of
Fraxiparine on the invasion and metastasis of A549 cells, the
change in expression of various invasion and metastasis molecules
such as the integrins and members of the MMP family using RT-PCR
and Western blotting were examined. Integrins and MMPs play a
significant role in the invasion and metastasis of cancer.
Integrins are the primary receptors for cellular adhesion to ECM
molecules. They act as crucial transducers of bidirectional cell
signaling, regulate cell survival, adhesion, invasion,
differentiation, proliferation, migration and tissue remodeling
(32–34). However, the effect of Fraxiparine on
integrin expression in lung cancer cells has yet to be determined.
Fraxiparine was found to down-regulate the mRNA and protein
expression of integrin β1 and β3 in A549 cells and decrease the
migration rate of A549 cells.
MMPs are a multigene family of zinc-dependent
endopeptidases that play a significant role in ECM degradation for
tumor growth, invasion and tumor-induced angiogenesis. Among the
MMPs, MMP-2 and −9 play the most important role in basement
membrane type IV collagen degradation (35,36).
However, no report exists on the inhibition of LMWH on MMPs in lung
cancer cells. This study found that Fraxiparine decreased the
expression of MMP-2 and −9 in A549 cells at the mRNA and protein
levels. This decrease may account for the inhibitory effects of
Fraxiparine on the invasion and migration of A549 cells.
The group of LMWHs contains different molecules such
as dalteparin, enoxaparin, nadroparin, certoparin and tinzaparin.
Each molecule has a slight difference in its chemical structure
compared to the remaining molecules (37). Besides their similar anticoagulant
effects, they may exert differential biological activities
(38). To our knowledge, this is
the first report on the inhibitory effects and the mechanism of
Fraxiparine on the invasion and metastasis of A549 cells.
Fraxiparine did not significantly affect cell viability, cell cycle
progression and the apoptotic status of A549 cells. The molecular
mechanism of Fraxiparine against the invasion and metastasis of
A549 cells may be related to nm23-H1 and heparanase genes and
further mediated through the down-regulation of integrin β1 and β3,
as well as MMP-2 and −9. Our results therefore suggest the
potential of Fraxiparine for the treatment of lung cancer
metastasis. However, further studies are required to confirm these
results.
Acknowledgements
This study was supported by a grant from the
National Key Research Project for New Drug Development of China
(2009ZX09301-004).
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics. CA
Cancer J Clin. 55:10–30. 2005.
|
|
2
|
Rabenstein DL: Heparin and heparan
sulfate: structure and function. Nat Prod Rep. 19:312–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsh J, Warkentin TE, Shaughnessy SG,
Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM and Dalen JE:
Heparin and low-molecular-weight heparin: mechanisms of action,
pharmacokinetics, dosing, monitoring, efficacy and safety. Chest.
119:S64–S94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akl EA, Rohilla S, Barba M, Sperati F,
Terrenato I, Muti P, Bdair F and Schünemann HJ: Anticoagulation for
the initial treatment of venous thromboembolism in patients with
cancer: a systematic review. Cancer. 113:1685–1694. 2008.
View Article : Google Scholar
|
|
5
|
Robert F, Busby E, Marques MB, Reynolds RE
and Carey DE: Phase II study of docetaxel plus enoxaparin in
chemotherapy-naive patients with metastatic non-small cell lung
cancer: preliminary results. Lung Cancer. 42:237–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altinbas M, Coskun HS, Er M, Ozkan O, Eser
B, Unal A, Cetin M and Soyuer S: A randomized clinical trial of
combination chemotherapy with and without low-molecular-weight
heparin in small cell lung cancer. J Thromb Haemost. 2:1266–1271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akl EA, van Doormaal FF, Barba M, Kamath
G, Kim SY, Kuipers S, Middeldorp S, Yosuico V, Dickinson HO and
Schünemann HJ: Parenteral anticoagulation may prolong the survival
of patients with limited small cell lung cancer: a Cochrane
systematic review. J Exp Clin Cancer Res. 27:42008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Von Tempelhoff GF, Harenberg J, Niemann F,
Hommel G, Kirkpatrick CJ and Heilmann L: Effect of low molecular
weight heparin (Certoparin) versus unfractionated heparin on cancer
survival following breast and pelvic cancer surgery: A prospective
randomized double-blind trial. Int J Oncol. 16:815–824. 2000.
|
|
9
|
Nagy Z, Turcsik V and Blaskó G: The effect
of LMWH (Nadroparin) on tumor progression. Pathol Oncol Res.
15:689–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Constantini S, Kanner A, Friedman A,
Shoshan Y, Israel Z, Ashkenazi E, Gertel M, Even A, Shevach Y,
Shalit M, Umansky F and Rappaport ZH: Safety of perioperative
minidose heparin in patients undergoing brain tumor surgery: a
prospective, randomized double-blind study. J Neurosurg.
94:918–921. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kakkar AK, Levine MN, Kadziola Z, Lemoine
NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M and Williamson
RC: Low molecular weight heparin, therapy with dalteparin, and
survival in advanced cancer: the Fragmin Advanced Malignancy
Outcome Study (FAMOUS). J Clin Oncol. 22:1944–1948. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klerk CP, Smorenburg SM, Otten HM, Lensing
AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van
Tienhoven G and Büller HR: The effect of low molecular weight
heparin on survival in patients with advanced malignancy. J Clin
Oncol. 23:2130–2135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuderer NM, Ortel TL and Francis CW:
Impact of venous thromboembolism and anticoagulation on cancer and
cancer survival. J Clin Oncol. 27:4902–4911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kragh M, Binderup L, Vig Hjarnaa PJ, Bramm
E, Johansen KB and Frimundt Petersen C: Non-anti-coagulant heparin
inhibits metastasis but not primary tumor growth. Oncol Rep.
14:99–104. 2005.PubMed/NCBI
|
|
15
|
Ludwig RJ, Boehme B, Podda M, Henschler R,
Jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R and Gille J:
Endothelial p-selectin as a target of heparin action in
experimental melanoma lung metastasis. Cancer Res. 64:2743–2750.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mellor P, Harvey JR, Murphy KJ, Pye D,
O’Boyle G, Lennard TWJ, Kirby JA and Ali S: Modulatory effects of
heparin and short-length oligosaccharides of heparin on the
metastasis and growth of LMD MDA-MB 231 breast cancer cells in
vivo. Br J Cancer. 97:761–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smorenburg SM, Vink R, Otten HM, Swaneveld
F and Büller HR: The effects of vitamin K antagonists on survival
of patients with malignancy: a systematic analysis. Thromb Haemost.
86:1586–1587. 2001.PubMed/NCBI
|
|
18
|
Niers TM, Klerk CP, DiNisio M, van Noorden
CJ, Büller HR, Reitsma PH and Richel DJ: Mechanisms of
heparin-induced anti-cancer activity in experimental cancer models.
Crit Rev Oncol Hematol. 61:195–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi H, Ebihara S, Okazaki T and
Yamaya M: A comparison of the effects of unfractionated heparin,
dalteparin and danaparoid on vascular endothelial growth
factor-induced tumor angiogenesis and heparanase activity. Br J
Pharmacol. 146:333–343. 2005. View Article : Google Scholar
|
|
20
|
Mousa SA and Petersen LJ: Anti-cancer
properties of low-molecular-weight heparin: preclinical evidence.
Thromb Haemost. 102:258–267. 2009.PubMed/NCBI
|
|
21
|
Hostettler N, Naggi A, Torri G,
Ishai-Michaeli R, Casu B, Vlodavsky I and Borsig L: P-selectin- and
heparanase-dependent antimetastatic activity of non-anticoagulant
heparins. FASEB J. 21:3562–3572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stevenson JL, Choi SH and Varki A:
Differential metastasis inhibition by clinically relevant levels of
heparins – correlation with selectin inhibition, not antithrombotic
activity. Clin Cancer Res. 11:7003–7011. 2005.PubMed/NCBI
|
|
23
|
Vlodavsky I, Goldshmidt O, Zcharia E,
Atzmon R, Rangini-Guatta Z, Elkin M, Peretz T and Friedmann Y:
Mammalian heparanase: involvement in cancer metastasis,
angiogenesis and normal development. Semin Cancer Biol. 12:121–129.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasaki M, Ito T, Kashima M and Miura M:
Erythromycin and clarithromycin modulation of growth factor-induced
expression of heparanase mRNA on human lung cancer cells in vitro.
Mediators Inflamm. 10:259–267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edovitsky E, Elkin M, Zcharia E, Peretz T
and Vlodavsky I: Heparanase gene silencing, tumor invasiveness,
angiogenesis, and metastasis. J Natl Cancer Inst. 96:1219–1230.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joyce JA, Freeman C, Meyer-Morse N, Parish
CR and Hanahan D: A functional heparan sulfate mimetic implicates
both heparanase and heparan sulfate in tumor angiogenesis and
invasion in a mouse model of multistage cancer. Oncogene.
24:4037–4051. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uno F, Fujiwara T, Takata Y, Ohtani S,
Katsuda K, Takaoka M, Ohkawa T, Naomoto Y, Nakajima M and Tanaka N:
Antisense-mediated suppression of human heparanase gene expression
inhibits pleural dissemination of human cancer cells. Cancer Res.
61:7855–7860. 2001.PubMed/NCBI
|
|
28
|
Steeg PS, Bevilacqua G, Kopper L,
Thorgeirsson UP, Talmadge JE, Liotta LA and Sobel ME: Evidence for
a novel gene associated with low tumor metastatic potential. J Natl
Cancer Inst. 80:200–204. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakubo Y, Sato Y, Koh T, Kono H and
Kameya T: Expression of nm23 protein in pulmonary adenocarcinomas:
inverse correlation to tumor progression. Lung Cancer. 17:103–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steeg PS, Ouatas T, Halverson D, Palmieri
D and Salerno M: Metastasis suppressor genes: basic biology and
potential clinical use. Clin Breast Cancer. 4:51–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma W, Chen J, Xue X, Wang Z, Liu H, Wang
T, Bai Y, Tang SC and Zhou Q: Alteration in gene expression profile
and biological behavior in human lung cancer cell line NL9980 by
nm23-H1 gene silencing. Biochem Biophys Res Commun. 371:425–430.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar
|
|
33
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miranti CK and Brugge JS: Sensing the
environment: a historical perspective on integrin signal
transduction. Nat Cell Biol. 4:E83–E90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
36
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fareed J, Leong WL, Hoppensteadt DA, Jeske
WP, Walenga J, Wahi R and Bick RL: Generic low-molecular-weight
heparins: some practical considerations. Semin Thromb Hemost.
30:703–713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deepa PR and Varalakshmi P: The
cytoprotective role of a low-molecular-weight heparin fragment
studied in an experimental model of glomerulotoxicity. Eur J
Pharmacol. 478:199–205. 2003. View Article : Google Scholar : PubMed/NCBI
|