Introduction
Measured by incidence, mortality and economic costs,
the global burden of breast cancer in women is substantial and on
the increase (1). It is estimated
that every year, more than one million women are diagnosed with
breast cancer worldwide, and more than 410,000 will die from the
disease, representing 14% of female cancer deaths (2–4). The
mortality from breast cancer is mainly due to metastatic disease.
While both tamoxifen and trastuzumab are very successful in
treating some breast cancer patients, others do not benefit from
these therapies, developing resistance to estrogen manipulation,
and suffer from progressive metastatic disease (5). The lymphatic system constitutes one of
the most important pathways of tumor dissemination. Studies of
tumor models in animals and clinicopathological data have indicated
that growth of lymphatic vessels (lymphangiogenesis) in the
vicinity of solid tumors may contribute to lymphatic metastasis.
The most extensively studied signaling system that promotes
lymphangiogenesis involves the secreted lymphangiogenic proteins
vascular endothelial growth factor-C (VEGF-C) and VEGF-D, and their
cognate receptor VEGF receptor-3 (VEGFR-3) (6).
Insulin-like growth factor-1 receptor (IGF-1R) is a
glycosylated heterotetramer composed of 2 extracellular α subunits
and β subunits that have intrinsic tyrosine kinase activity with
70% homology to the insulin receptor (7). IGF-1R mainly mediates the effect of
insulin-like growth factors (IGFs), which are potent mitogens that
regulate cell proliferation, differentiation, and protection from
apoptosis (8). IGF-1R expression
level in primary breast cancer was observed upregulated in 43.8% of
tumors detected by immunohistochemical analysis (9). Among node-negative patients, those
with high levels of IGF-1R were found to have significantly reduced
overall survival (10). The IGF-IR
pathway plays an important role in mediating resistance to both
general cytotoxic therapies, such as radiation and chemotherapy,
and targeted therapies, such as tamoxifen and trastuzumab (11). Therefore, targeting the IGF pathway
might be a novel approach to overcoming this resistance and
improving clinical outcome of breast cancer.
RNA interference (RNAi), a novel strategy of gene
silencing, has rapidly become a powerful tool for drug discovery
and target validation in cell culture (12). The natural role of RNAi is thought
to be a cellular defense against viral infection or potentially
harmful destabilizing genomic intruders such as transposons. RNAi
can also be induced in mammalian cells by the introduction of
synthetic small interfering RNA (siRNA) 21–23 base pairs in length,
or by plasmid and viral vector systems that express short hairpin
RNAs (shRNA) that are subsequently processed to siRNA by the
cellular machinery (13–15).
To investigate the potential value of targeting
IGF-1R in breast cancer, we utilised the lentivirus-based shRNA
expression plasmid pLL3.7 to knock down expression of endogenous
IGF-1R. We show that lentivirus (LV)-mediated shRNA that targets
IGF-1R can effectively inhibit the growth and migration of
MDA-MB-231 breast cancer cells both in vitro and in
vivo.
Materials and methods
Cell culture
The MDA-MB-231 breast cancer and the human embryonic
kidney 293T cell lines were purchased from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China). MDA-MB-231 cells and 293T cells were
cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) and Dulbecco’s
modified Eagle’s medium (DMEM; Gibco), respectively, and
supplemented with 10% fetal bovine serum (FBS; Gibco). Cells were
maintained in a humidified incubator with 5% CO2 at
37°C.
shRNA design and vector construction
The shRNA expression cassette contained 21
nucleotide (nt) of the target sequence followed by the loop
sequence (TTCAAGAGA), reverse complement to the 21 nt, stop codon
for U6 promoter and Xho1 site (sense strand:
5′-TGAGACCTGAAAGGAAGCGGAGA TTCAAGAGATCTCCGCTTCCTTTCAGGTCTCTTTTT
TC-3′; antisense strand:
5′-TCGAGAAAAAAGAGACCTGAAAGGAAGCGGAGATCTCTTGAATCTCCGCTTCCTTTCAGGTCTCA-3′).
The IGF-1R-shRNA contains the sense targeting sequence of
AGACCTGAAAGGAAGCGGAGA corresponding to the 2250–2270 nucleotide
positions of human IGF-1R coding sequence (GenBank accession
NM_000875.3). The shRNA cassettes and their complementary strands
were synthesized by Shanghai Sangon Biotech Co., and annealed by
heating to 95°C for 5 min followed by cooling to room temperature.
The resulting double-strand oligo DNA was cloned into the
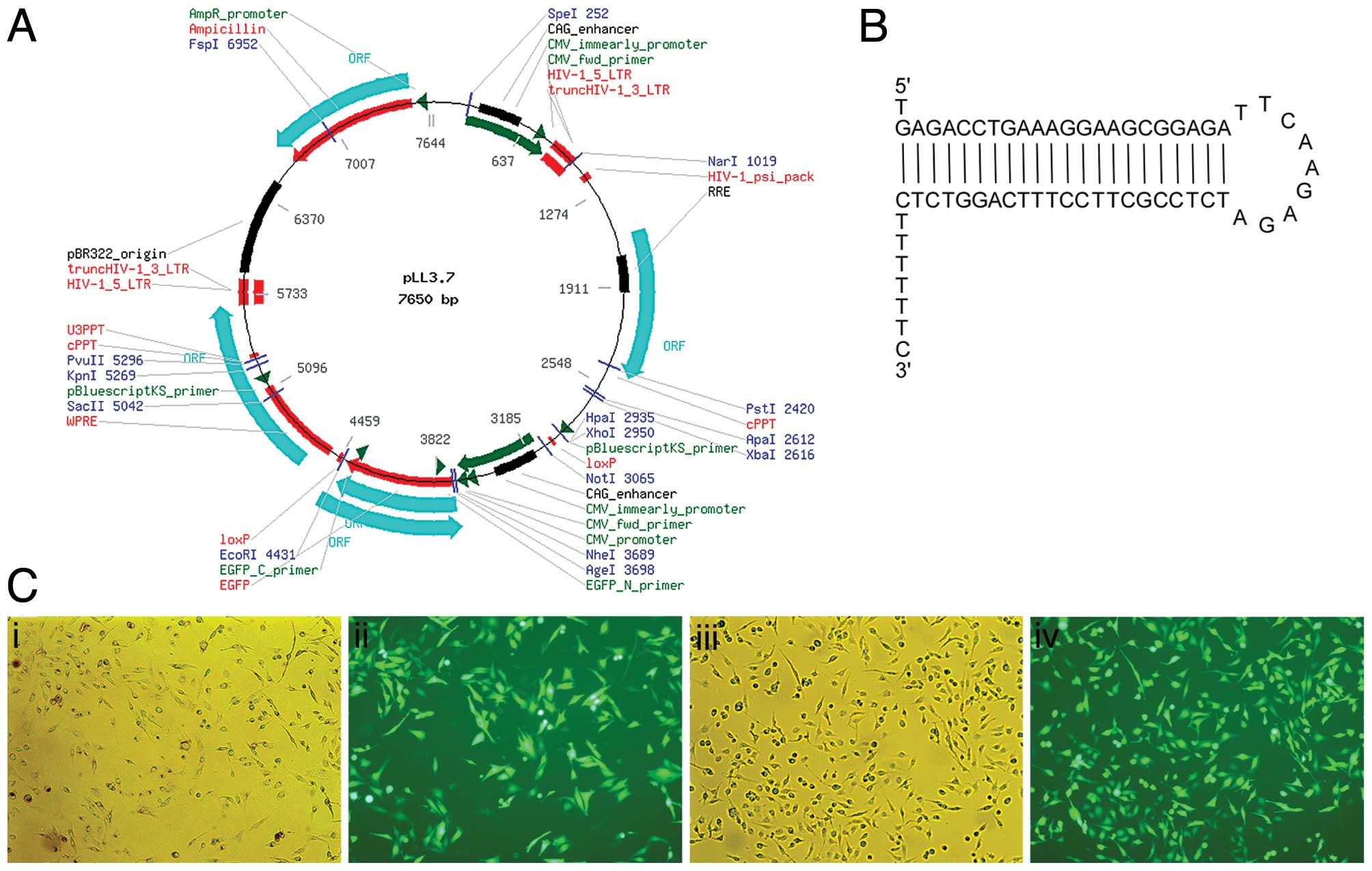
lentivirus-based shRNA expression plasmid pLL3.7 (available from
Shanghai Telebio Biomedical Co., Ltd., Shanghai, China), and
inserted between HpaI and XhoI sites (16). Plasmid pLL3.7 without any shRNA
inserted was used as negative control. The resulting plasmid was
confirmed by restriction enzyme digestion and DNA sequencing.
Plasmid pLL3.7 is designed to contain an EGFP reporter gene
controlled by the CMV promoter, enabling the monitoring of
lentivirus infection through EGFP expression (17).
Lentiviral vector production
A four-plasmid transfection system (available from
Shanghai Telebio Biomedical Co.) was used to produce high-titer
lentiviral vectors. Briefly, the packing plasmids and pLL3.7 were
amplified in Escherichia coli and purified using AxyPrep
Plasmid Maxiprep kit according to the manufacturer’s instructions.
The lentiviral vectors were then prepared by transfecting 293T
cells with the plasmids pLL3.7, using calcium phosphate
transfection method in the presence of the packaging plasmid pMD2G,
pMDLg/pRRE and pRSVrev. The viral supernatant was collected at 48 h
after transfection, concentrated, and passed through a 0.45 μm
filter. Titers were determined by infecting HeLa cells with serial
dilutions of concentrated lentivirus. For a typical preparation,
the titre was ~1×107 infectious units (IU) per ml. The
lentiviral stocks were stored in small aliquot at −70°C for future
use.
Cell infection
MDA-MB-231 cells were seeded in 6-well plates
(5×105/well) and were cultured overnight. Lentiviruses
(0.1 ml) were mixed with 1.5 ml complete medium and added to the
cells for incubation for 24 h at 37°C. After 24-h infection, the
medium was replaced with fresh 1640 medium. This procedure was
repeated for 3 days. The efficiency of transduction was assessed
and photomicrographs of EGFP expression were recorded using a Nikon
Eclipse TE2000U inverted microscope equipped with a charge-coupled
device (CCD) camera.
Real-time polymerase chain reaction
(qPCR)
Total RNA was isolated using TRIzol reagent
(ShineGene Molecular Biotech Co., Shanghai, China) according to the
manufacturer’s instructions. From total RNA, 1 μg was
reverse-transcribed into cDNA with EnergicScript First Strand cDNA
Synthesis kits (ShineGene Molecular Biotech Co.). Human β-actin RNA
was used as an internal control. Primers for IGF-1R were: forward,
5′-ACAAGTTGAGGATCAGCGAGAATG-3′; reverse, 5′-GGACAGCGACGGGCAGAG-3′.
Gene expression levels were evaluated by real-time quantitative PCR
kinetics with ShineSybr Real-Time qPCR MasterMix kits (ShineGene
Molecular Biotech Co.). Real-time PCR was performed with 2 μl of
appropriate diluted cDNA, 1 μl (25 pmol/μl) of forward and reverse
primers specific for human IGF-1R and β-actin, 25 μl of Hotstart
fluo-PCR mix, and 21 μl of ddH2O. Real-time PCR was
carried out using the iQ5 real-time PCR detection system (Bio-Rad
Laboratories, Hercules, CA, USA) with the following program:
pre-heating 94°C 4 min; then 40 cycles of 94 C 30 sec; 60°C 30 sec;
and 72°C 30 sec. The expression of target RNA relative to β actin
was calculated based on the threshold cycle (Ct) as R
=2−Δ(ΔCt), where ΔCt = CtIGF-1R −
Ctbeta actin; Δ (ΔCt) = ΔCtsample
− ΔCt control.
Western blotting
Cell lysates were prepared in RIPA buffer (Cell
Signaling Technology, Beverly, MA, USA); their protein
concentrations were determined using the BCA Protein Assay kit
(KeyGen Biotech). For electrophoresis, 30 μg of total protein in 5X
loading buffer was loaded to each well of a 10% (w/v) SDS-PAGE gel
and transferred to polyvinylidene fluoride (PVDF) membranes. After
electrotransferring, the blot was blocked and probed with primary
antibody at 4°C followed by incubation with horseradish peroxidase
(HRP)-conjugated secondary antibody. IGF-I receptor-β antibody
(Cell Signaling Technology, Beverly, MA, USA) was used at 1/1000
dilution, while a mouse monoclonal antibody against human GAPDH
(Abmart, Shanghai, China) at 1:5000 was used as control.
Immunoblots were developed using BeyoECL Plus (Beyotime Institute
of Biotechnology, Jiangsu Province, China) according to the
manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was determined by WST-8 assay
using Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology). MDA-MB-231 cells infected or uninfected with LVs
were trypsinized counted and seeded in a 96-well plate
(3×104/ml) for overnight incubation. Then cells were
inoculated with 10 μl CCK-8 solution at 37°C in a humid atmosphere
containing 5% CO2 for 2 h, and absorbance at 450 nm of
the supernatant was measured spectrophotometrically. This assay was
carried out at various time points (at 24, 48 and 72 h after
seeding). A total of three independent experiments were performed,
and the means were used to depict the growth curve.
Migration assay
A Transwell system (Corning, NY, USA) was used to
evaluate cell migration. The upper and lower chambers were
separated by a polycarbonate membrane with pores of 8 μm, which was
coated with fibronectin (BD Biosciences, San Jose, CA, USA) on the
lower surface. Approximately 5×103 cells suspended in
100 μl serum-free medium were seeded onto the upper chamber, and
500 μl of culture medium with 10% FBS was added to the lower
chamber. After 24 h of incubation at 37°C with 5% CO2,
the medium was removed from the upper chamber. The non-invaded
cells on the upper side of the chamber were scraped off with a
cotton swab. Cells on the underside of the membrane were fixed,
stained with crystal violet and mounted. The migration activity of
cancer cells was determined by counting the cells under a
microscope, in 4 different viewing fields, at ×200 magnification.
Each assay was repeated three times.
Animal experiments
Four-week-old female severe combined immunodeficient
(SCID) mice were purchased from Slac Laboratory Animal Co., Ltd.,
(Shanghai, China), and maintained in the specific pathogen-free
(SPF) facility. All animal protocols used for this study were
approved by the Institutional Animal Care and Use Committee.
MDA-MB-231 cells were infected with LVs as described above and
harvested. The infected cells were washed with PBS, counted and
resuspended in PBS at 1×107/ml. Female SCID mice (6
mice/group) were injected with 100 μl cell suspension
subcutaneously to the left inguinal mammary fat pads. The mice were
sacrificed on Day 60, and the tumors were measured and removed for
immunohistochemical analysis. The tumor volume was calculated as
length (mm) × the square of the width (mm2) × π/6.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed
using the Dako EnVision system. Briefly, serial 5-μm-thick sections
were cut from formalin-fixed and paraffin-embedded tumor blocks,
dewaxed in xylene, rehydrated through sequential changes of
alcohol, and then antigen retrieved in 0.01 M citrate buffer, pH
6.0, at 90°C for 20 min. After washing with phosphate-buffered
saline (PBS), the tissue sections were incubated with fresh 3%
hydrogen peroxide for 20 min at room temperature. Sections were
blocked with 20% goat serum for 30 min and incubated with IGF-1R
primary antibody (1:50 dilution; Abcam, Cambridge, UK), or
lymphatic vessel endothelial receptor 1 (LYVE-1) antibody (1:200
dilution; Abcam) for 2 h. Following this treatment, sections were
incubated with the EnVison complex at 37°C for additional 30 min
before incubation with substrate solution 3,3′-diaminobenzidine
(DAB; Beyotime Institute of Biotechnology). The sections were then
counterstained with hematoxylin, and pictures taken on Olympus BH2
microscope at ×200 magnification. Mean positive indices (MPI) of
IHC staining were analyzed semi-quantatively by information
management system (IMS) cell image analysis system, and calculated
as the pixel values of positive areas × optical density.
Statistical analysis
Data are expressed as means ± SD. The significance
of the data was determined by Student’s t-test (two-tailed) in two
groups and one-way ANOVA in multiple groups. Values of P<0.05
were considered statistically significant. All data were analyzed
with SPSS 16.0 software.
Results
Lentiviruses effectively transduced
MDA-MB-231 cells
Two LVs were produced: LV-IGF-1R shRNA carries shRNA
targeting IGF-1R and LV-con carries only pLL3.7. All LVs expressed
EGFP which allowed for titering in HeLa cells as well as measuring
their infection efficiency in MDA-MB-231 cells. To permit
high-efficiency transduction, we cultured the cells in the presence
of IGF-1 and subjected them to two more rounds of lentiviral
transduction with concentrated vector on 3 consecutive days. The
transfection rate was calculated by the percentage of fluorescent
cells in the total cells and was almost 100% in each visual field
(Fig. 1). When the transduced cells
were maintained in vitro for 30 days in the presence of
growth factors, >90% of the cells continued to express EGFP.
Effect of LV-IGF-1R shRNA on IGF-1R
expression
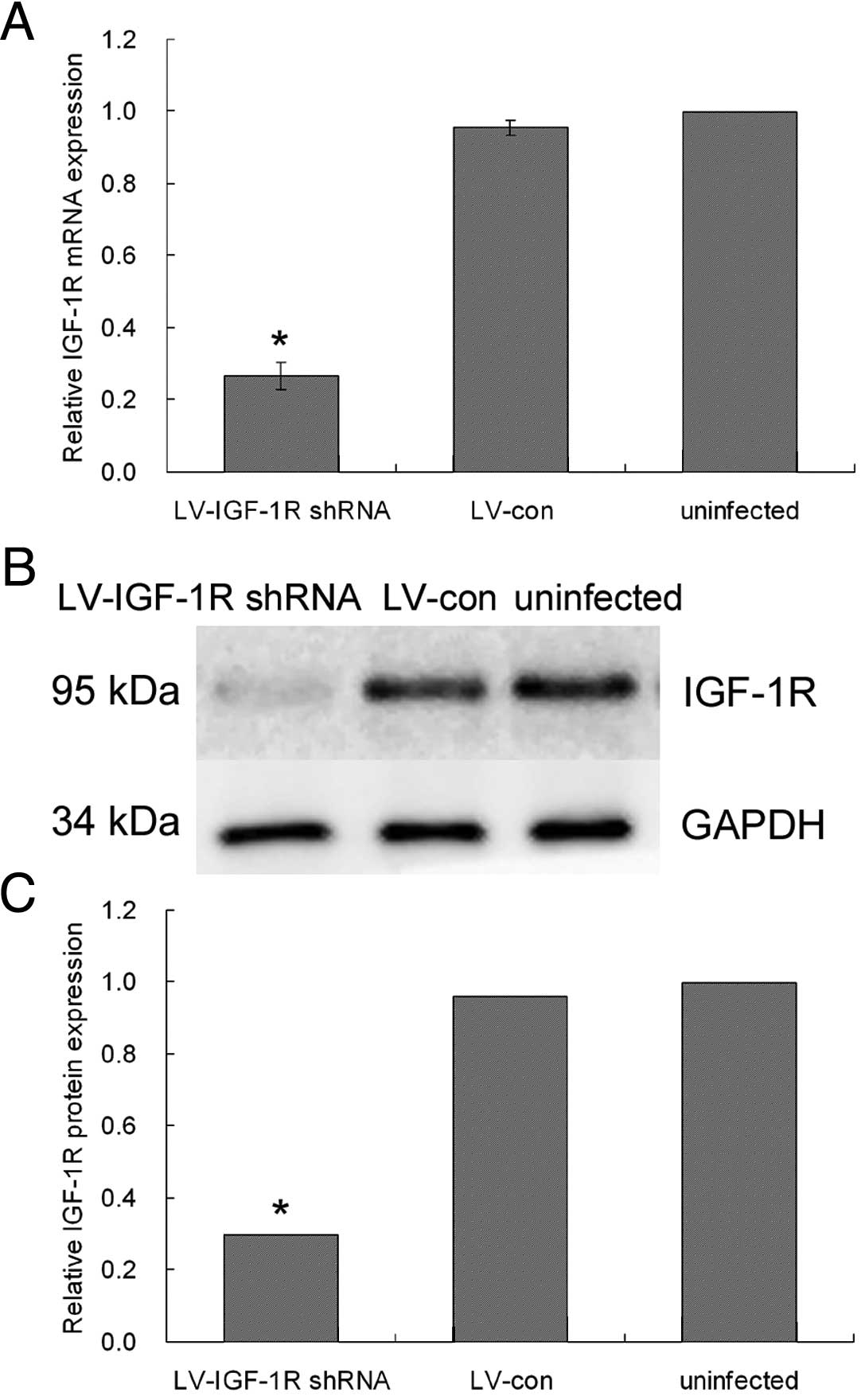
To evaluate silencing efficiency, infected cells
were characterized for IGF-1R mRNA by quantitative reverse
transcription-PCR (qRT-PCR) using specific primers against
endogenous IGF-1R; and for protein expression in immunoblots
obtained using the rabbit polyclonal antibody against IGF-1R. As
shown in Fig. 1B, qRT-PCR results
indicate that endogenous IGF-1R mRNA expression was significantly
inhibited at 24 h after infection in MDA-MB-231 cells. Compared
with the control group, the IGF-1R shRNA group showed lower
quantities of IGF-1R mRNA; mRNA expression was decreased by nearly
70% (Fig. 2A). In accordance with
this, western blot analysis showed that IGF-1R protein expression
was significantly suppressed in the IGF-1R shRNA group compared
with the control LV group in MDA-MB-231 cells (P<0.01) (Fig. 2B). IGF-1R expression was unaltered
in cells infected by vectors lacking the shRNA cassette.
LV-IGF-1R shRNA inhibits breast cancer
cell growth in vitro
To detect effects of suppression of IGF-IR
expression on cell proliferation, we carried out a CCK-8 assay
using equal number of cells infected with LV-IGF-1R shRNA or
LV-con. Results indicate that transfection with LV-IGF-1R shRNA
inhibited MDA-MB-231 cell proliferation by 37.3 and 43.5% compared
with the control group at 48 and 72 h, respectively (P<0.01)
(Fig. 3).
LV-IGF-1R shRNA inhibits breast cancer
cell migration in vitro
We next assessed effects of IGF-1R silencing on cell
motility using Transwell migration assays. Transfection of
MDA-MB-231 cells with anti-IGF-1R shRNA inhibited cell migration
through the polycarbonate membrane by 36.3%, whereas the control
shRNA had no effect (Fig. 4).
Transfection of LV-IGF-IR shRNA inhibits
MDA-MB-231 cell growth in vivo
To investigate the effects of IGF-1R silencing on
cell growth in vivo, we injected SICD mice with infected
MDA-MB-231 cells as described above. There was no evidence of
weight loss or physical distress resulting from the treatment
protocol. As shown in Fig. 5, the
tumor growth of LV-IGF-1R shRNA group was significantly inhibited
(66.8% decrease).
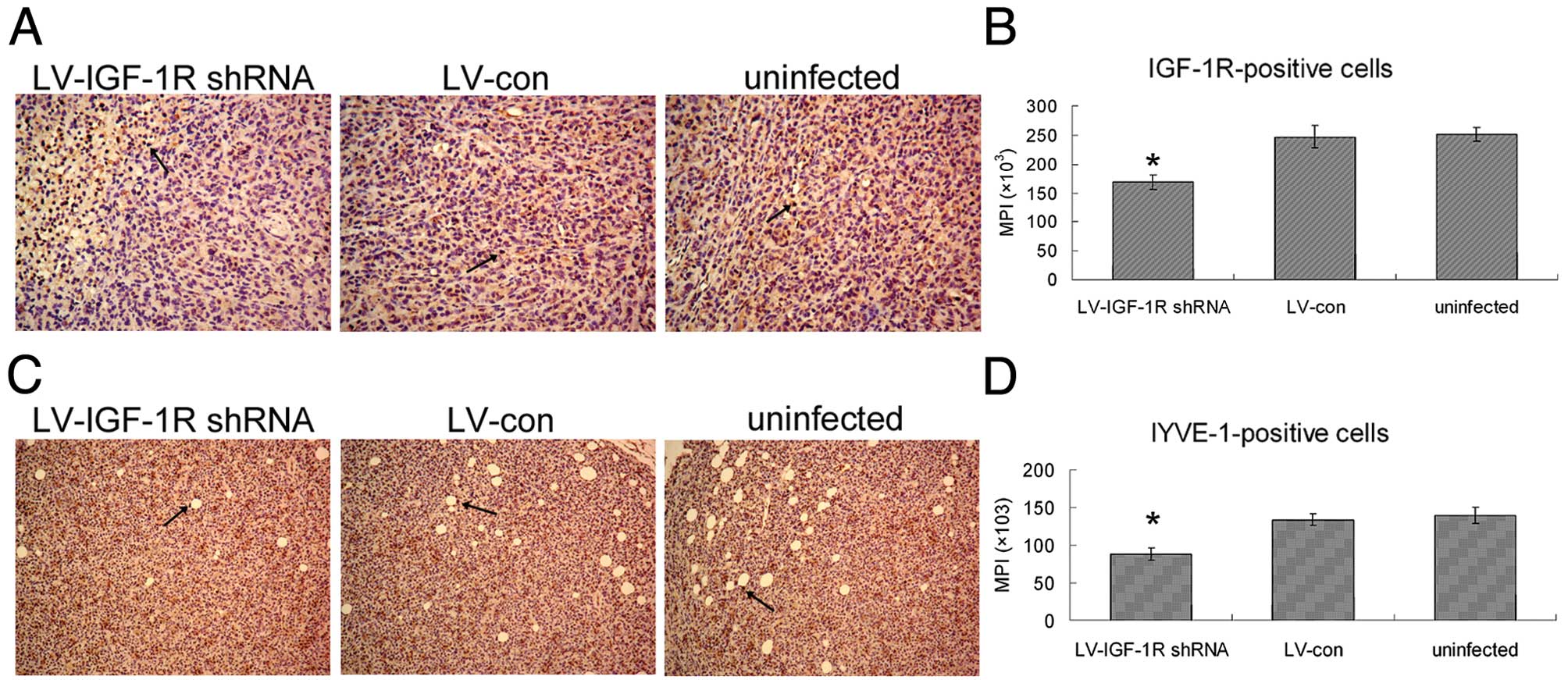
Effect of LV-IGF-IR shRNA on tumor
lymphangiogenesis
The immunohistochemical analysis for IGF-1R showed
the expression level of IGF-1R in xenograft was significantly
decreased in LV-IGF-1R shRNA goup, compared with the LV-con group
(Fig. 6A). The effect of LV-IGF-1R
shRNA on lymphangiogenesis was determined by immunohistochemical
analysis using LYVE-1 antibody. The semi-quantitative data from the
IMS cell image analysis is shown in Fig. 6D. The tumor lymphangiogenesis of
LV-IGF-1R shRNA group was inhibited by 34.2%, compared with control
tumors.
Discussion
We have demonstrated in this study that shRNA
delivered by a lentivirus was able to effectively suppress targeted
IGF-1R expression in breast cancer MDA-MB-231 cells leading to
significant suppression of cell growth and migration both in
vitro and in vivo. These results encourage further
exploration of RNAi as a potential method for breast cancer
treatment.
Although knocked-down IGF-1R expression in some
other cancer cells has been reported by a few groups using siRNA
(18–20), here we showed a stable silencing of
IGF-1R in MDA-MB-231 cells with LV-mediated shRNA. Other approaches
to abrogating IGF-IR signalling include dominant negative mutants,
kinase defective mutants, anti-sense oligonucleotides, soluble
receptors, antibodies against IGF-I and IGF-II, IGF-IR blocking
antibodies and, more recently, a family of IGF-IR kinase inhibitors
(21), but the stability and
delivery efficacy of these antibodies or inhibitors seems to be a
crucial limiting factor in exerting an inhibitory effect on the
targeted molecule in vivo. Current data support the notion
that in mammalian cells, due to the obvious amplification effect of
RNAi, it is superior to antisense approaches for downregulation of
gene expression though antisense has been widely used (22). Our study has indicated that the
suppressive effect of LV-mediated shRNA to IGF-IR can reach about
70%.
The role of IGF-1R in regulating tumor growth is
well understood (23). Several
studies have shown that IGF-IR plays an important role at critical
steps of the metastatic cascade, including cell adhesion,
migration, invasion, angiogenesis, and cell growth at distant organ
sites (24). It has been
demonstrated that IGF-1R regulates metastasis of colon cancer as
colon cancer cells expressing dominant negative IGF-1R failed to
form liver metastases following splenic injection or direct
injection into the livers of nude mice (25). Expression of antisense IGF-1R mRNA
inhibited Ewing’s sarcoma (ES) cell motility, and their ability to
form colonies in soft agar in vitro; the metastatic ability
of ES cells carrying antisense IGF-1R was significantly reduced
in vivo (26). Metastatic
uveal melanomas express higher levels of IGF-IRs than primary
tumors (27), and are sensitive to
IGF-IR targeting (28). These data
suggest IGF-1R is critical to metastasis of several types of cancer
cells. Our study has also demonstrated that LV-mediated shRNA
targeting IGF-1R significantly decreased breast cancer cell
MDA-MB-231 proliferation and migration both in vitro and
in vivo, indicating an important role of IGF-1R in breast
cancer growth and metastasis.
Besides facilitating migration, there are two other
ways in which IGF-1R can influence the spread of breast cancer to
distant sites. The first is by stimulating angiogenesis and the
second is by promoting lymphangiogenesis. Although angiogenesis is
important for the dissemination of many solid tumors, the major way
in which breast cancer cells metastasize is through the lymphatics
(29). Therefore, the processes
governing lymphangiogenesis may be of central importance to this
disease (30). Lymphangiogenesis is
another important mechanism by which tumor cells are disseminated
via the lymphatic system. VEGF-C has been identified as mediators
of this process (31). Lewis lung
carcinoma subline M-27 cells transfected with human IGF-IR cDNA
expressed VEGF-C and acquired a lymph node metastasizing potential
in vivo, implicating the role of IGF-1R in the control of
lymphatic metastasis (32). Our
previous study also showed that increased VEGF-C expression was
closely related to lymphangiogenesis in breast cancer invasion and
lymphatic metastasis (33). Since
IGF-1R is widely distributed in mammalian tissues, including blood
and lymphatic vessels (34),
intratumoral injections or tail vein injections of lentiviral
vectors targeting cancer cells may interfere with vessel formation.
In our study, breast cancer cells were infected with lentivirus
vectors in vitro, and then transplanted into SCID mice. The
results show that downregulation of IGF-1R inhibits
lymphangiogenesis and tumor metastasis in vivo. In another
previous study, we reported IGF-1 significantly increased VEGF-C
expression in MDA-MB-231 breast cancer cells in vitro
(35). Whether IGF-1R suppression
would interfere with VEGF-C secretion is in need of further
experimental demonstration. IGF-1R could be an important
therapeutic target to suppress breast cancer metastasis, but a
package of comprehensive and complementary research is
required.
Taken together, it can be concluded that RNAi is a
powerful genetic tool to reduce target gene expression. Our results
also indicate that LV-mediated shRNA targeting IGF-1R offers a
potent therapeutic strategy to inhibit lymphatic metastasis of
breast cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China, Grant Number 30872521.
References
|
1
|
Mackay J, Jemal A, Lee NC and Parkin DM:
The Cancer Atlas. American Cancer Society; Atlanta, GA: pp.
2006
|
|
2
|
Anderson BO, Yip CH, Ramsey SD, Bengoa R,
Braun S, Fitch M, Groot M, Sancho-Garnier H and Tsu VD: Breast
cancer in limited-resource countries: health care systems and
public policy. Breast J. 12:S54–S69. 2006.PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
4
|
Parkin DM and Fernández LM: Use of
statistics to assess the global burden of breast cancer. Breast J.
12:S70–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sachdev D: Regulation of breast cancer
metastasis by IGF signaling. J Mammary Gland Biol Neoplasia.
13:431–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Achen MG and Stacker SA: Tumor
lymphangiogenesis and metastatic spread - new players begin to
emerge. Int J Cancer. 119:1755–1760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ward CW, Garrett TP, McKern NM, Lou M,
Cosgrove LJ, Sparrow LG, Frenkel MJ, Hoyne PA, Elleman TC, Adams
TE, et al: The three dimensional structure of the type I
insulin-like growth factor receptor. Mol Pathol. 54:125–132. 2001.
View Article : Google Scholar
|
|
8
|
Baserga R, Peruzzi F and Reiss K: The
IGF-1 receptor in cancer biology. Int J Cancer. 107:873–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimizu C, Hasegawa T, Tani Y, Takahashi
F, Takeuchi M, Watanabe T, Ando M, Katsumata N and Fujiwara Y:
Expression of insulin-like growth factor 1 receptor in primary
breast cancer: immunohistochemical analysis. Hum Pathol.
35:1537–1542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taunk NK, Goyal S, Moran MS, Yang Q,
Parikh R and Haffty BG: Prognostic significance of IGF-1R
expression in patients treated with breast-conserving surgery and
radiation therapy. Radiother Oncol. 96:204–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casa AJ, Dearth RK, Litzenburger BC, Lee
AV and Cui X: The type I insulin-like growth factor receptor
pathway: a key player in cancer therapeutic resistance. Front
Biosci. 13:3273–3287. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeshita F and Ochiya T: Therapeutic
potential of RNA interference against cancer. Cancer Sci.
97:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brummelkamp TR, Bernards R and Agami R:
Stable suppression of tumorigenicity by virus-mediated RNA
interference. Cancer Cell. 2:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubinson DA, Dillon CP, Kwiatkowski AV,
Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus
MT, et al: A lentivirus-based system to functionally silence genes
in primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Addgene, Inc. http://www.addgene.org/11795/.
Accessed March 15, 2012
|
|
18
|
Fang J, Zhou Q, Shi XL and Jiang BH:
Luteolin inhibits insulin-like growth factor 1 receptor signaling
in prostate cancer cells. Carcinogenesis. 28:713–723. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel BB, Gupta D, Elliott AA, Sengupta V,
Yu Y and Majumdar AP: Curcumin targets FOLFOX-surviving colon
cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res.
30:319–325. 2010.PubMed/NCBI
|
|
20
|
Santen RJ, Fan P, Zhang Z, Bao Y, Song RX
and Yue W: Estrogen signals via an extra-nuclear pathway involving
IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer
cells. Steroids. 74:586–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Werner H and Bruchim I: The insulin-like
growth factor-I receptor as an oncogene. Arch Physiol Biochem.
115:58–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagy P, Arndt-Jovin DJ and Jovin TM: Small
interfering RNAs suppress the expression of endogenous and
GFP-fused epidermal growth factor receptor (erbB1) and induce
apoptosis in erbB1-overexpressing cells. Exp Cell Res. 285:39–49.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lann D and LeRoith D: The role of
endocrine insulin-like growth factor-I and insulin in breast
cancer. J Mammary Gland Biol Neoplasia. 13:371–379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reinmuth N, Fan F, Liu W, Parikh AA,
Stoeltzing O, Jung YD, Bucana CD, Radinsky R, Gallick GE and Ellis
LM: Impact of insulin-like growth factor receptor-I function on
angiogenesis, growth, and metastasis of colon cancer. Lab Invest.
82:1377–1389. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scotlandi K, Maini C, Manara MC, Benini S,
Serra M, Cerisano V, Strammiello R, Baldini N, Lollini PL, Nanni P,
et al: Effectiveness of insulin-like growth factor I receptor
antisense strategy against Ewing’s sarcoma cells. Cancer Gene Ther.
9:296–307. 2002.PubMed/NCBI
|
|
27
|
Mallikarjuna K, Pushparaj V, Biswas J and
Krishnakumar S: Expression of insulin-like growth factor receptor
(IGF-1R), c-Fos, and c-Jun in uveal melanoma: an
immunohistochemical study. Curr Eye Res. 31:875–883. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Girnita A, All-Ericsson C, Economou MA,
Aström K, Axelson M, Seregard S, Larsson O and Girnita L: The
insulin-like growth factor-I receptor inhibitor picropodophyllin
causes tumor regression and attenuates mechanisms involved in
invasion of uveal melanoma cells. Clin Cancer Res. 12:1383–1391.
2006. View Article : Google Scholar
|
|
29
|
Cunnick GH, Jiang WG, Gomez KF and Mansel
RE: Lymphangiogenesis and breast cancer metastasis. Histol
Histopathol. 17:863–870. 2002.PubMed/NCBI
|
|
30
|
Kucab JE and Dunn SE: Role of IGF-1R in
mediating breast cancer invasion and metastasis. Breast Dis.
17:41–47. 2003.PubMed/NCBI
|
|
31
|
Achen MG, McColl BK and Stacker SA: Focus
on lymphangiogenesis in tumor metastasis. Cancer Cell. 7:121–127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Zhang D, Fallavollita L and Brodt
P: Vascular endothelial growth factor C expression and lymph node
metastasis are regulated by the type I insulin-like growth factor
receptor. Cancer Res. 63:1166–1171. 2003.PubMed/NCBI
|
|
33
|
Gu Y, Qi X and Guo S: Lymphangiogenesis
induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome
in breast carcinoma: a retrospective study of 61 cases. Clin Exp
Metastasis. 25:717–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Björndahl M, Cao R, Nissen LJ, Clasper S,
Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ and Cao Y:
Insulin-like growth factors 1 and 2 induce lymphangiogenesis in
vivo. Proc Natl Acad Sci USA. 102:15593–15598. 2005.PubMed/NCBI
|
|
35
|
Zhu C, Qi X, Chen Y, Sun B, Dai Y and Gu
Y: PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in
IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res
Clin Oncol. 137:1587–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|