Introduction
Malignant glioma is one of the most common brain
tumors, and its incidence rate tends to be increased worldwide,
especially in large cities (1). The
overall survival rates of malignant glioma remains very poor. Fewer
than 50% of glioma patients estimated to survive for longer than 5
years after diagnosis (2). Although
ionizing radiation and chemotherapy constitute the major treatment
for malignant gliomas (3,4), it is critical to identify potential
drugs for the disease.
β-elemene is one of the active components extracted
from the traditional Chinese medicinal herb Rhizoma
zedoariae (5). Due to its
effectiveness in retarding growth in various tumor cells both in
vitro and in vivo, β-elemene has been utilized in
treating certain types of tumors, including lung cancer, colorectal
cancer and glioblastoma (5,6). Previous studies showed that β-elemene
inhibited cell proliferation by inducing cell apoptosis as well as
arresting the cell cycle (7,8), and
Bcl-2, Bcl-X(L) and XIAP was involved in this process (3,9).
However, the mechanisms by which β-elemene induces cell apoptosis
are not completely understood, and further studies need to be done
to uncover the molecular mechanisms underlying its antitumor
activity.
Survivin (BIRC5), a protein of 16.5 kDa,
constitutes the smallest member of the inhibitor of apoptosis
(IAP) gene family (10).
Highly expressed during embryonic and fetal development, survivin
however is almost not or only barely detectable in most adult
tissues (11,12). Conversely, survivin was found to be
expressed in abundance in various cancer tissues and cancer cell
lines. In human gliomas, high levels of survivin expression
revealed to be correlated with a poor prognosis (13,14),
indicating its beneficial effects for glioma cells survival and/or
growth. Mechanically, survivin is able to inhibit the second
mitochondria-derived apoptosis-inducing factor Smac/DIABLO
(15) and other apoptosis-inducing
factors (16). Also there are data
supporting that survivin plays a unique role in regulating mitotic
events and TNF-related apoptosis-inducing ligand (TRAIL)-mediated
apoptosis (17–20). Survivin contains a single
baculoviral IAP repeat domain, the structure supposed to be able to
block caspase-3 and-9 activities. However, this molecule lacks the
RING domain, which can act as a ubiquitin ligase to facilitate the
proteasomal degradation of caspases and retard the apoptotic
process. Probably to complement to this, a novel complex formed
between survivin and hepatitis B X-interacting protein (HBXIP) was
identified, which was shown to be essential in regulating
caspase-9-mediated apoptotic signaling (21,22).
It suggested that survivin-HBXIP complexes, but neither survivin
nor HBXIP individually, are able to bind to pro-caspase-9, blocking
the recruitment of caspase-9 to Apaf1, and subsequently suppressing
the apoptosis initiated via the mitochondria/cytochrome c pathway
(13,23).
In this study, we report that β-elemene induced
apoptosis in human glioma cancer cells. Induction of the apoptosis
was associated with inhibition of survivin gene expression, and
overexpression of survivin gene reduced β-elemene-induced
apoptosis. Furthermore, β-elemene inhibited the direct interaction
between survivin and HBXIP, which enhanced the activation of
caspase-9 and promoted glioma apoptosis.
Materials and methods
Chemicals, reagents and antibodies
Q-VD-OPH and Q-LEHD-OPH were from MP Biomedicals
(Aurora, OH, USA), Ac-DEVD-CHO was purchased from Promega Co.
β-elemene was purchased from Zhejiang Institute for Food and Drug
Control (Hangzhou, China). Anti-actin antibodies were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Anti-capase-3,
anti-cleaved caspase-3, anti-caspase-7, anti-cleaved caspase-7,
anti-caspase-9, anti-cleaved caspase-9, anti-PARP, anti-cleaved
PARP, and anti-survivin antibodies were purchased from Cell
Signaling Technology (Beverly, MA, USA). HRP-conjugated goat
anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG secondary
antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA,
USA).
Cell culture
U251 and A172 cells were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and cultured in recommended growth media. U251 cells were
transfected with pMEG06-3HA-survivin (U251-survivin) or pMEG06-3HA
vector as control (U251-HA). Stable cell lines were established by
G418 selection (0.6 mg/ml, Sigma-Aldrich).
Plasmid construction
pMEG06-3HA-survivin plasmid, which contains the ORF
of survivin gene, a 3HA-Tag and the N-terminal, was purchased from
FuGENE Gene Co. Total cDNA was generated by reverse-transcription
from U251 mRNA using RT-kits (Takara), and the ORF of HBXIP was
amplified by PCR, the specific primers for PCR were as follows:
5′-GAC GAATTCATGGAGGCGACCTTGGAGCA-3′ (forward) and
5′-GATCTCGAGTCAAGAGGCCATTTTGTGCA-3′ (reverse) (23). The resultant cDNA fragments were
ligated into pCMV-Tag2B vector, which contains a FLAG-Tag and the
N-terminal, for mammalian cell expression.
Analysis of apoptosis by annexin V
staining
To determine apoptosis, U251 and A172 cells were
stained with annexin V and propidium iodide (PI) using a Vybrant
Apoptosis Assay kit (Invitrogen, Carlsbad, CA). In brief, cells
were collected by trypsinization and washed with ice-cold
phosphate-buffered saline (PBS). Then cells were resuspended in 500
μl binding buffer, and incubated with 5 μl of annexin V-FITC and 10
μl of the 100 μg/ml PI working solution at room temperature for 5
min in the dark. Stained cells were analyzed for apoptosis by flow
cytometry. FITC was detected at 518 nm and PI was detected at 620
nm.
Terminal
deoxynucleotidyltransferase-mediated deoxy-UTP-fluorescein nick
end-labeling (TUNEL) assay
For TUNEL assay, a DNA Fragmentation Detection kit,
Fluorescent-TdT Enzyme (Cabiochem, San Diego, CA), was used to
detect DNA fragmentation. In brief, cells were treated with
β-elemene, harvested, and resuspended in 80% ethanol at 4°C
overnight. The next day cells were washed with Tris-buffered saline
(TBS) and incubated with 20 μg/ml proteinase K for 5 min at room
temperature. Cells were then washed three times with PBS, incubated
with 100 μl TdT equilibration buffer at room temperature for 30
min, and incubated in the dark with 60 μl of TdT-labeling reaction
mixture at 37°C for 60 min. Finally, pellet cells were washed three
times with PBS and analyzed by a flow cytometer. The emission
wavelength of fluorescein is 517 nm.
Isolation of RNA and RT-PCR analysis
Total RNA was extracted using TRIzol (Invitrogen),
and after DNase I treatment, cDNAs were synthesized by using the
cDNA reverse-transcription kit (Takara). Survivin and GAPDH mRNA
was detected by RT-PCR, the specific primers for RT-PCR were as
follows: survivin: 5′-ACCAGG TGAGAAGTGAGGGA-3′ (forward) and
5′-AACAGTAGAGGAGCCAGGGA-3′ (reverse) (24); GAPDH: 5′-CATGG
GTTCAACATGCCAAGTGGT-3′ (forward) and 5′-TCCACGGCAGCATTAATCACAGGA-3′
(reverse).
Immunoprecipitation and western blot
analysis
For immunoprecipitation, after transfection, cells
were lysed in 1 ml of 1% Nonidet P-40, 25 mM Tris-HCl, 150 mM NaCl,
10 mM EDTA, pH 8.0, a 1:50 dilution of a protease inhibitor mixture
(Sigma) for 30 min on ice. Cell lysates were centrifuged at 14,000
g for 5 min to pellet cell debris and incubated with primary
antibodies overnight at 4°C with rotation followed by the addition
of protein A-agarose (60 μl of 50% slurry) for 1 h at 4°C with
rotation to capture antibody-antigen complex. The antibody-antigen
complex was washed, and samples were mixed with SDS-PAGE sample
buffer, boiled for 5 min, electrophoresed on an SDS-polyacrylamide
gel, and electro-blotted onto a nitrocellulose membrane (Millipore,
Bedford, MA). For western blot detection, proteins were resolved on
SDS-PAGE, transferred to a nitrocellulose membrane. The membrane
was blocked in PBS containing 5% skimmed milk powder and 0.02%
Tween-20, and then probed with specific antibodies at 4°C
overnight. After washing, the membrane was incubated with a
1:20,000 dilution of peroxidase-conjugated goat anti-mouse IgG or
goat anti-rabbit IgG in PBS containing 5% skimmed milk powder. The
blot was then developed using the ECL detection kit (Amersham
Biosciences) to produce a chemiluminescence signal, which was
captured on X-ray film.
Results
β-elemene induced glioma cell
apoptosis
In this experiment, we detected β-elemene-induced
apoptosis by annexin V staining and TUNEL assay in human glioma
cells. Human glioma U251 and A172 cells were treated with β-elemene
at concentrations of 0, 100, 200 and 300 μg/ml for 24 h. Flow
cytometry analysis showed a significant increase in the apoptotic
population of the cells treated with β-elemene for 24 h when
compared with that of the cells without treatment. The most
striking increase in the percentage of apoptotic cells occurred at
the concentration of 300 μg/ml (Fig.
1A). TUNEL assay further confirmed that apoptosis was induced
by β-elemene (Fig. 1B).
Next we tested the activities of caspase-9, -3 and
-7 in glioma cells when stimulated with β-elemene. As shown in
Fig. 2A and B, β-elemene clearly
induced the cleavage of pro-caspase-9, -3, -7 to their active forms
in human U251 and A172 glioma cells. Moreover, poly(ADP-ribose)
polymerase (PARP), a specific substrate of caspase-9, -3 and -7,
was also cleaved into its active form in a dose-dependent manner,
indicating that caspase-9, -3, -7-mediated pathways was involved in
β-elemene-induced glioma apoptosis. To further prove that, we
examined the effect of caspase inhibitors on the β-elemene-induced
apoptosis in U251 cells. The results showed that both of
pan-caspase inhibitors, Q-VD-OPH and Q-LEHD-OPH, markedly inhibited
β-elemene-induced apoptosis. Treatment with a specific caspase-3
inhibitor Ac-DEVD-CHO provided the protection for U251 cells
against apoptotic death (Fig. 2C).
These results suggested that β-elemene induced caspase-dependent
apoptosis of glioma cells.
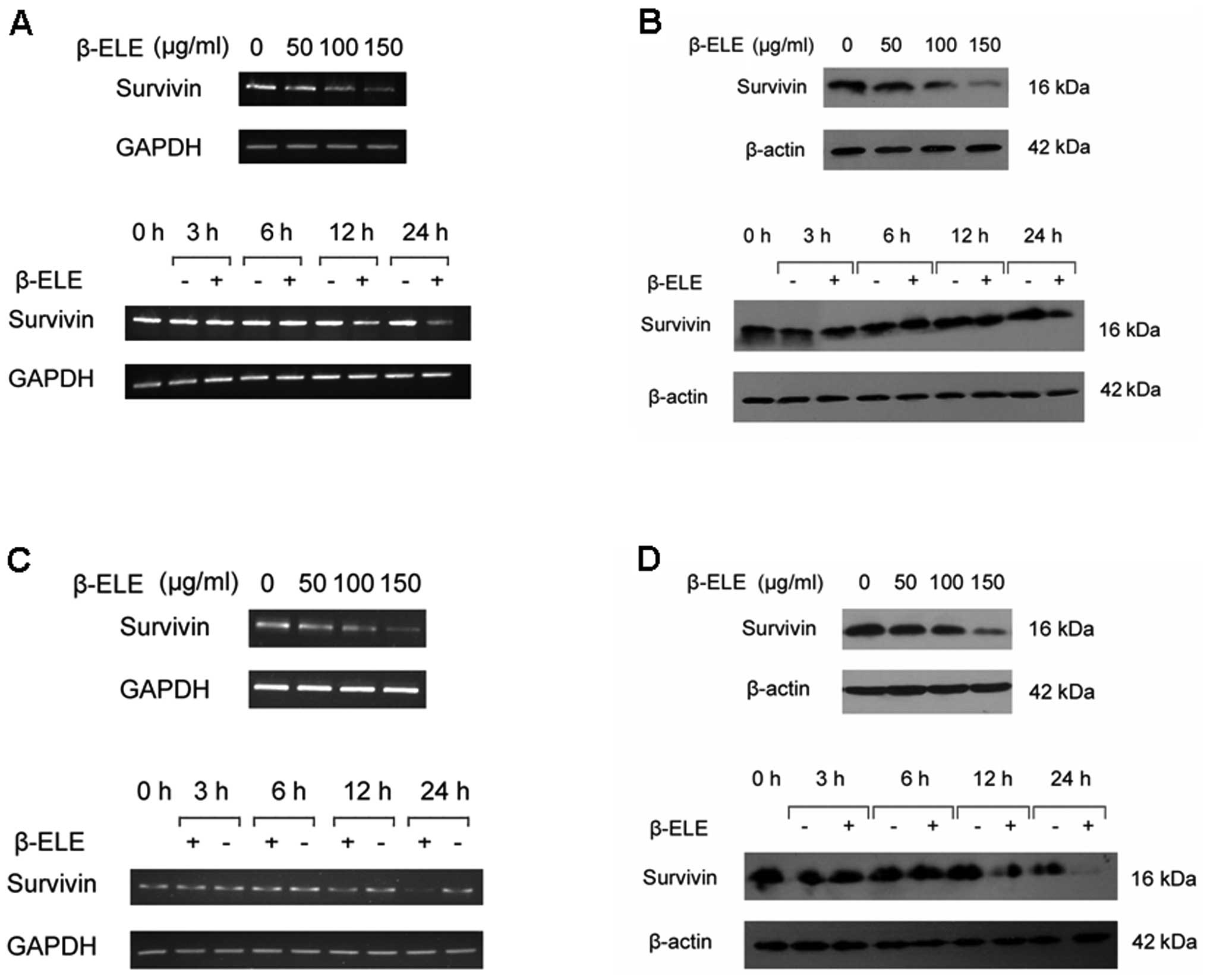
β-elemene inhibits the expression of
survivin gene in glioma cells
As anti-apoptotic protein survivin is expressed
abundantly in human gliomas and related with poor prognosis, we
investigated whether proapoptotic effect of β-elemene can be
mediated by regulation of survivin levels. We firstly determined
the expression of survivin in glioma cells with or without
β-elemene treatment. The results indicated that β-elemene induced a
dose- and time-dependent reduction of survivin expression either in
mRNA (Fig. 3A and C) or protein
levels (Fig. 3B and D), suggesting
a potential role for survivin in β-elemene-induced glioma cell
death.
β-elemene-induced glioma cell apoptosis
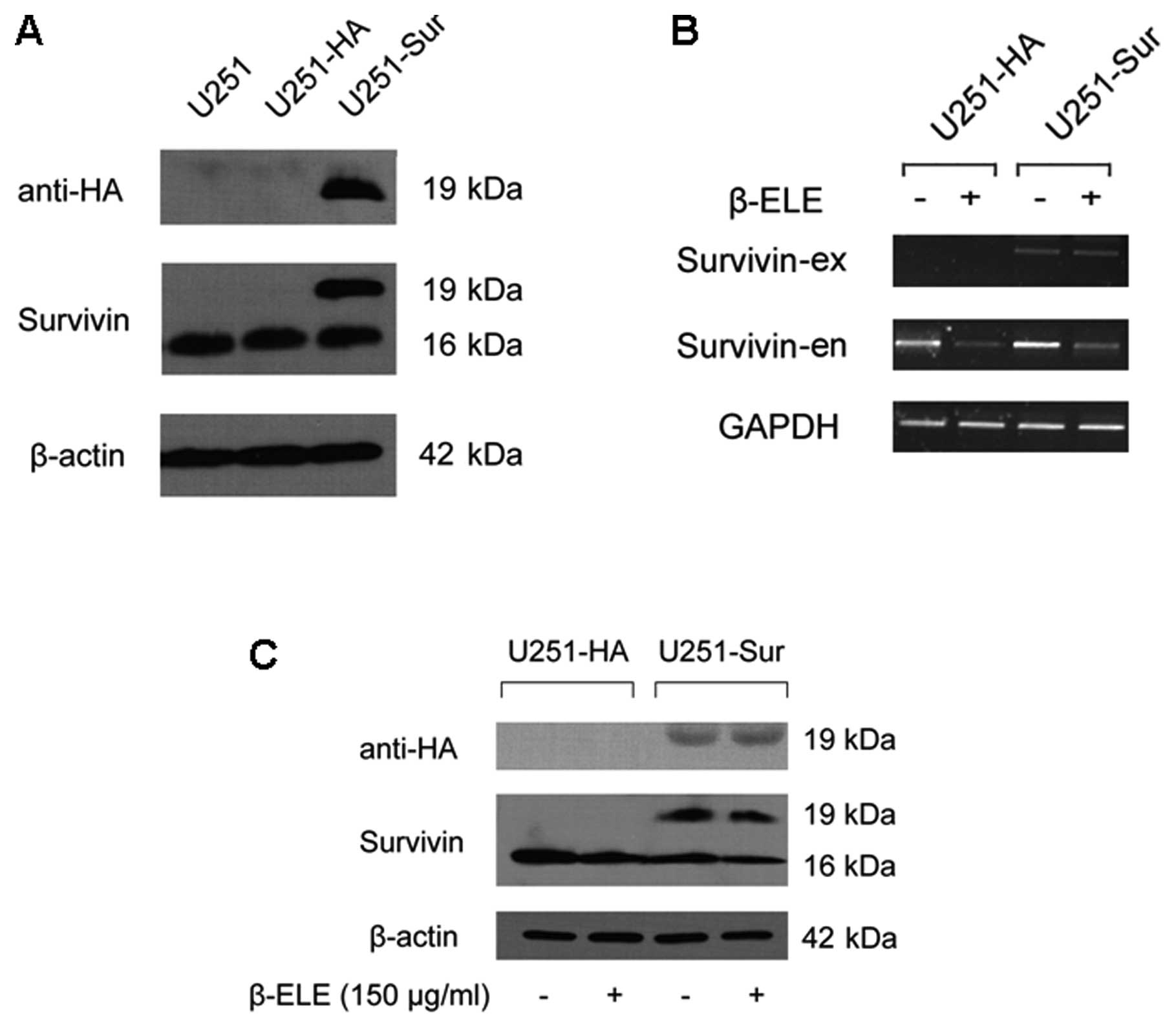
is blocked by restoring survivin expression
Next, we examined whether β-elemene-induced
downregulation of survivin was associated with apoptosis in glioma
cells. We established stable U251 cell lines with forced expression
of HA-tagged survivin and then compared the effect of β-elemene on
the U251-HA and U251-sur cells. Transfection efficiency of survivin
gene was proved by western blotting (Fig. 4A) and RT-PCR analysis showed that
β-elemene treatment did not affect the exogenous HA-tagged survivin
expression (Fig. 4B). As expected,
the treatment caused a marked decrease in endogenous survivin in
both U251-HA and U251-sur cells (Fig.
4B and C), and TUNEL assay showed apoptosis of U251 cells was
induced by β-elemene. Remarkably, forced expression of survivin
caused a reduction in apoptosis of U251-sur cells when compared
with U251-HA cells (Fig. 5A),
suggesting that apoptosis of U251 cells induced by β-elemene was
overridden by forced expression of survivin.
Since survivin exerts an anti-apoptotic effect
through caspase-9, -3 and -7-dependent pathway (25–27),
we further evaluated the apoptosis-related proteins in U251-HA and
U251-sur cells upon β-elemene stimulation. The cells were treated
with 150 μg/ml β-elemene for 24 h and the levels of caspase-9, -3,
-7 and PARP were detected by western blotting. Results showed that
the forced expression of survivin in U251 cells significantly
decreased β-elemene-induced activation of caspase-9, -3, -7, as
well as the cleavage of PARP (Fig.
5B).
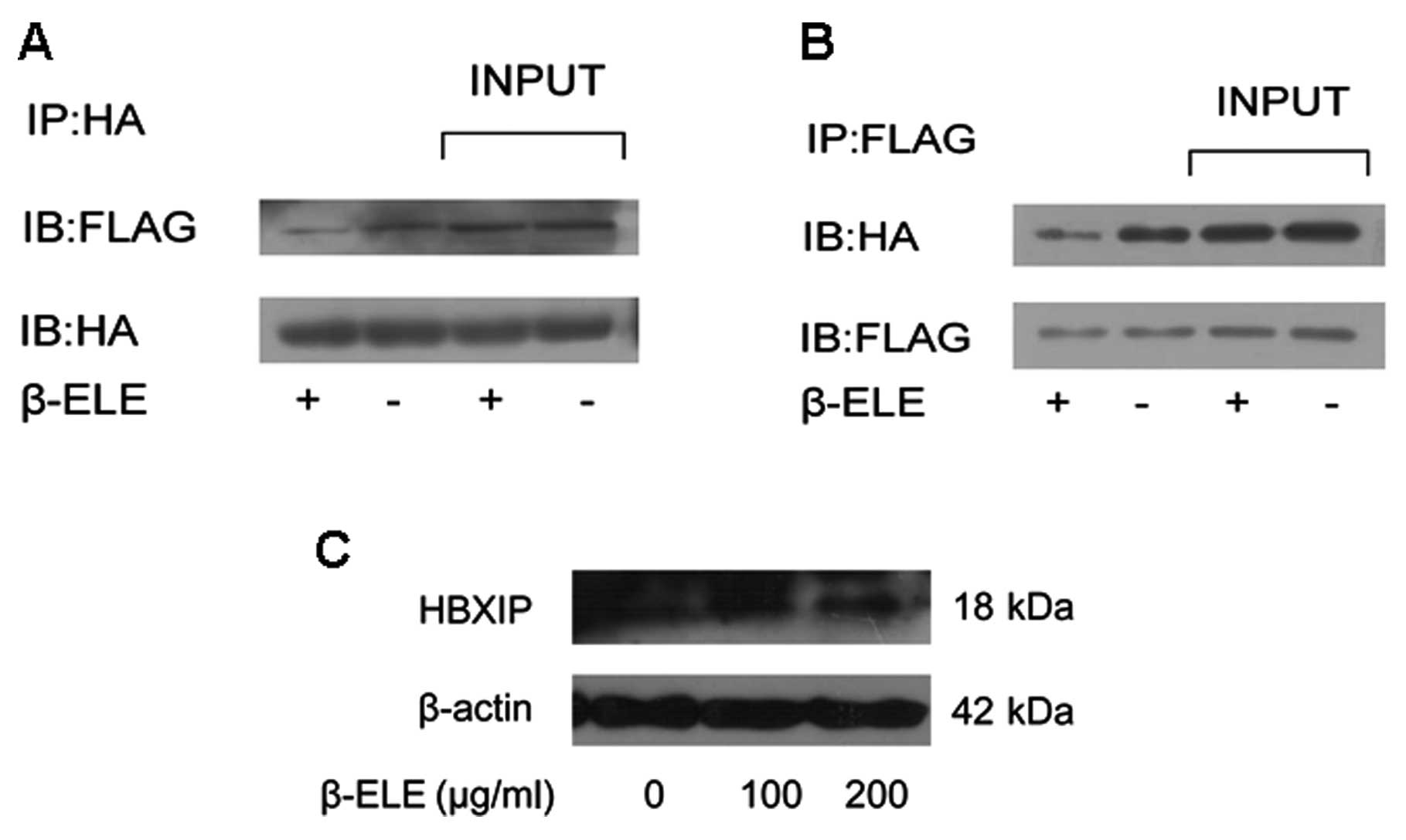
β-elemene inhibits the interaction of
survivin and HBXIP
It has been proved that survivin-HBXIP complexes,
but neither survivin nor HBXIP individually, have the capability to
bind pro-caspase-9 and inhibit the activation of caspase-9
(23), we hypothesized that in
human glioma cells, β-elemene can inhibit the interaction of
survivin and HBXIP. To test this hypothesis, we examined if the
binding of survivin to HBXIP was affected by β-elemene treatment.
Firstly, the ORF of HBXIP gene was cloned into pCMV-Tag2B plasmid
to construct pCMV-HBXIP plasmid which can express a FLAG-HBXIP
fusion protein in mammalian cells. pMEG-HA-survivin and pCMV-HBXIP
were then co-transfected into U251 cells and treated with β-elemene
(100 μg/ml). Using co-immunoprecipitation method we clearly showed
that survivin was bound to HBXIP protein in glioma cells. More
importantly, the interaction between the two molecules was
inhibited by β-elemene treatment (Fig.
6A and B). As survivin-HBXIP complex was assumed to be
responsible for the retaining and retarding of caspase-9 activity,
we suggested that β-elemene-induced impairment in survivin-HBXIP
interaction might promote cell apoptosis. Besides, HBXIP level was
not obviously affected by β-elemene as shown in Fig. 6C.
Discussion
β-elemene, an extract from the ginger plant R.
zeodaria, is a novel anti-cancer herbal medicine with broad
antitumor effects in vitro and in vivo (5,8,28,29).
It has been approved by the Chinese FDA (the State Food and Drug
Administration) for clinical treatment of malignant effusion and
some solid tumors. However, the effects of β-elemene on glioma
cancer cells have not been documented yet. In the present study, we
provide the first evidence that β-elemene could promote apoptosis
of human glioma cells and revealed a major pathway underlying this
effect.
Apoptotic process is mediated through various
pathways and regulated by multiple mechanisms. One of the pathways
is mitochondria-dependent apoptotic route which is essentially
dependent on the cleavage and activation of caspase-9 (30–32),
Caspases are therefore considered as central executors of the
apoptotic process, especially caspase-9, -3 and -7 (30,33,34).
We showed that β-elemene induced apoptosis of human glioma U251 and
A172 cells, as determined by TUNEL assay, annexin V staining and
western blot analysis. The cell apoptosis was closely associated
with activation of caspase-9, -3 and -7 and increased levels of
cleaved PARP. Caspase inhibitors substantially attenuated the
β-elemene-induced cell apoptosis. These data indicate that the
effect of β-elemene on cell apoptosis was mediated via a
mitochondrial-dependent apoptotic pathway.
One notable event associated with β-elemene
treatment was marked inhibition of survivin expression in glioma
cells. Survivin was found to be highly expressed in various human
cancers, including bladder cancer (35), gastric cancer (36), hepatocellular carcinomas (37), colon/colorectal cancer (38), neuroblastomas (39), and human gliomas (40). Survivin, a number of IAP protein
family, showed to be bound to caspase-3 and caspase-7 (41). Moreover, studies on transgenic mice
revealed that survivin was able to inhibit the intrinsic,
caspase-9-dependent apoptotic pathway (25). However, unlike XIAP, which can
inhibit caspases after activation, survivin does not have the
capability to directly interact with pro-caspase-9. HBXIP,
originally identified as HBV-interacting protein, was unexpectedly
found to act as an anti-apoptotic cofactor of survivin. HBXIP
allowed survivin to bind and suppress the activation of
pro-caspase-9, thus blocking the mitochondrial pathway for cell
death (23). In the present study,
we showed that the downregulation of survivin induce by β-elemene
is responsible for glioma cell apoptosis, as death of cells can be
rescued by forced expression of surviving shown in our test. More
importantly, our results revealed that β-elemene inhibited the
protein-protein interaction between survivin and HBXIP, which might
facilitate the release and activation of caspase-9, enhancing human
glioma cell apoptosis with β-elemene treatment. Our results merit
further investigation into the characteristic and kinetics of
survivin and HBXIP complex in tumorigenesis as well as in antitumor
activity.
In conclusion, we demonstrated that β-elemene exerts
an effective antitumor effect in human glioma cells by regulating
survivin gene expression, and also by suppressing the interaction
between survivin and HBXIP. These results establish β-elemene as a
promising therapeutic agent in the treatment of patients with
glioma.
Acknowledgments
We thank Dr D. Xu, Monash University, Australia and
Dr Z. Lu, East Carolina University, USA for their critical reading
of the manuscript. This study was supported by the National Natural
Science Foundation of China (81000956), Qianjiang Talent Project of
Science and Technology (2011R10027), and Program for New Century
Excellent Talents (NCET-08-0927).
Abbreviations:
|
β-ELE
|
β-elemene
|
|
HBXIP
|
hepatitis B X-interacting protein
|
|
IAP
|
inhibitor of apoptosis protein
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PI
|
propidium iodide
|
|
FITC
|
fluoresceine isothiocyanate
|
|
TUNEL
|
terminal
deoxynucleotidyltransferase-mediated deoxy-UTP-fluorescein nick
end-labeling
|
References
|
1
|
Fisher JL, Schwartzbaum JA, Wrensch M and
Wiemels JL: Epidemiology of brain tumors. Neurol Clin. 25:867–890.
vii2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Little MP, de Vathaire F, Shamsaldin A, et
al: Risks of brain tumour following treatment for cancer in
childhood: modification by genetic factors, radiotherapy and
chemotherapy. Int J Cancer. 78:269–275. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bondy ML, Wang LE, El-Zein R, et al:
Gamma-radiation sensitivity and risk of glioma. J Natl Cancer Inst.
93:1553–1557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Li X, Huang F, et al: Antitumor
effect of beta-elemene in non-small-cell lung cancer cells is
mediated via induction of cell cycle arrest and apoptotic cell
death. Cell Mol Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Deng Y, Mao S, Jin S, Wang J and
Bi D: Characterization and body distribution of beta-elemene solid
lipid nanoparticles (SLN). Drug Dev Ind Pharm. 31:769–778. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Wang G, Zhao J, et al:
Antiproliferative effect of beta-elemene in chemoresistant ovarian
carcinoma cells is mediated through arrest of the cell cycle at the
G2-M phase. Cell Mol Life Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie CY, Yang W, Li M, et al: Cell
apoptosis induced by delta-elemene in colorectal adenocarcinoma
cells via a mitochondrial-mediated pathway. Yakugaku Zasshi.
129:1403–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki K, Tsuno NH, Sunami E, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mesri M, Wall NR, Li J, Kim RW and Altieri
DC: Cancer gene therapy using a survivin mutant adenovirus. J Clin
Invest. 108:981–990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das A, Tan WL, Teo J and Smith DR:
Expression of survivin in primary glioblastomas. J Cancer Res Clin
Oncol. 128:302–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holcik M, Gibson H and Korneluk RG: XIAP:
apoptotic brake and promising therapeutic target. Apoptosis.
6:253–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holcik M and Korneluk RG: XIAP, the
guardian angel. Nat Rev Mol Cell Biol. 2:550–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Z, Yao X and Wu M: Direct interaction
between survivin and Smac/DIABLO is essential for the
anti-apoptotic activity of survivin during taxol-induced apoptosis.
J Biol Chem. 278:23130–23140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Brouha B and Grossman D: Rapid
induction of mitochondrial events and caspase-independent apoptosis
in Survivin-targeted melanoma cells. Oncogene. 23:39–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uren AG, Wong L, Pakusch M, et al:
Survivin and the inner centromere protein INCENP show similar
cell-cycle localization and gene knockout phenotype. Curr Biol.
10:1319–1328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fraser AG, James C, Evan GI and Hengartner
MO: Caenorhabditis elegans inhibitor of apoptosis protein (IAP)
homologue BIR-1 plays a conserved role in cytokinesis. Curr Biol.
9:292–301. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Speliotes EK, Uren A, Vaux D and Horvitz
HR: The survivin-like C. elegans BIR-1 protein acts with the
Aurora-like kinase AIR-2 to affect chromosomes and the spindle
midzone. Mol Cell. 6:211–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Temme A, Rieger M, Reber F, et al:
Localization, dynamics, and function of survivin revealed by
expression of functional survivinDsRed fusion proteins in the
living cell. Mol Biol Cell. 14:78–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liston P, Young SS, Mackenzie AE and
Korneluk RG: Life and death decisions: the role of the IAPs in
modulating programmed cell death. Apoptosis. 2:423–441. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melegari M, Scaglioni PP and Wands JR:
Cloning and characterization of a novel hepatitis B virus x binding
protein that inhibits viral replication. J Virol. 72:1737–1743.
1998.PubMed/NCBI
|
|
23
|
Marusawa H, Matsuzawa S, Welsh K, et al:
HBXIP functions as a cofactor of survivin in apoptosis suppression.
EMBO J. 22:2729–2740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paydas S, Tanriverdi K, Yavuz S, Disel U,
Sahin B and Burgut R: Survivin and aven: two distinct antiapoptotic
signals in acute leukemias. Ann Oncol. 14:1045–1050. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grossman D, Kim PJ, Blanc-Brude OP, et al:
Transgenic expression of survivin in keratinocytes counteracts
UVB-induced apoptosis and cooperates with loss of p53. J Clin
Invest. 108:991–999. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin S, Sung BJ, Cho YS, et al: An
anti-apoptotic protein human survivin is a direct inhibitor of
caspase-3 and -7. Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chandele A, Prasad V, Jagtap JC, Shukla R
and Shastry PR: Upregulation of survivin in G2/M cells and
inhibition of caspase 9 activity enhances resistance in
staurosporine-induced apoptosis. Neoplasia. 6:29–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li QQ, Wang G, Zhang M, Cuff CF, Huang L
and Reed E: Beta-Elemene, a novel plant-derived antineoplastic
agent, increases cisplatin chemosensitivity of lung tumor cells by
triggering apoptosis. Oncol Rep. 22:161–170. 2009.
|
|
29
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou H, Li Y, Liu X and Wang X: An APAF-1.
cytochrome c multimeric complex is a functional apoptosome that
activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Senderowicz AM: Cell cycle modulators for
the treatment of lung malignancies. Clin Lung Cancer. 5:158–168.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swana HS, Grossman D, Anthony JN, Weiss RM
and Altieri DC: Tumor content of the antiapoptosis molecule
survivin and recurrence of bladder cancer. N Engl J Med.
341:452–453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okada E, Murai Y, Matsui K, et al:
Survivin expression in tumor cell nuclei is predictive of a
favorable prognosis in gastric cancer patients. Cancer Lett.
163:109–116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito T, Shiraki K, Sugimoto K, et al:
Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawasaki H, Toyoda M, Shinohara H, et al:
Expression of survivin correlates with apoptosis, proliferation,
and angiogenesis during human colorectal tumorigenesis. Cancer.
91:2026–2032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Adida C, Berrebi D, Peuchmaur M,
Reyes-Mugica M and Altieri DC: Anti-apoptosis gene, survivin, and
prognosis of neuroblastoma. Lancet. 351:882–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chakravarti A, Noll E, Black PM, et al:
Quantitatively determined survivin expression levels are of
prognostic value in human gliomas. J Clin Oncol. 20:1063–1068.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|