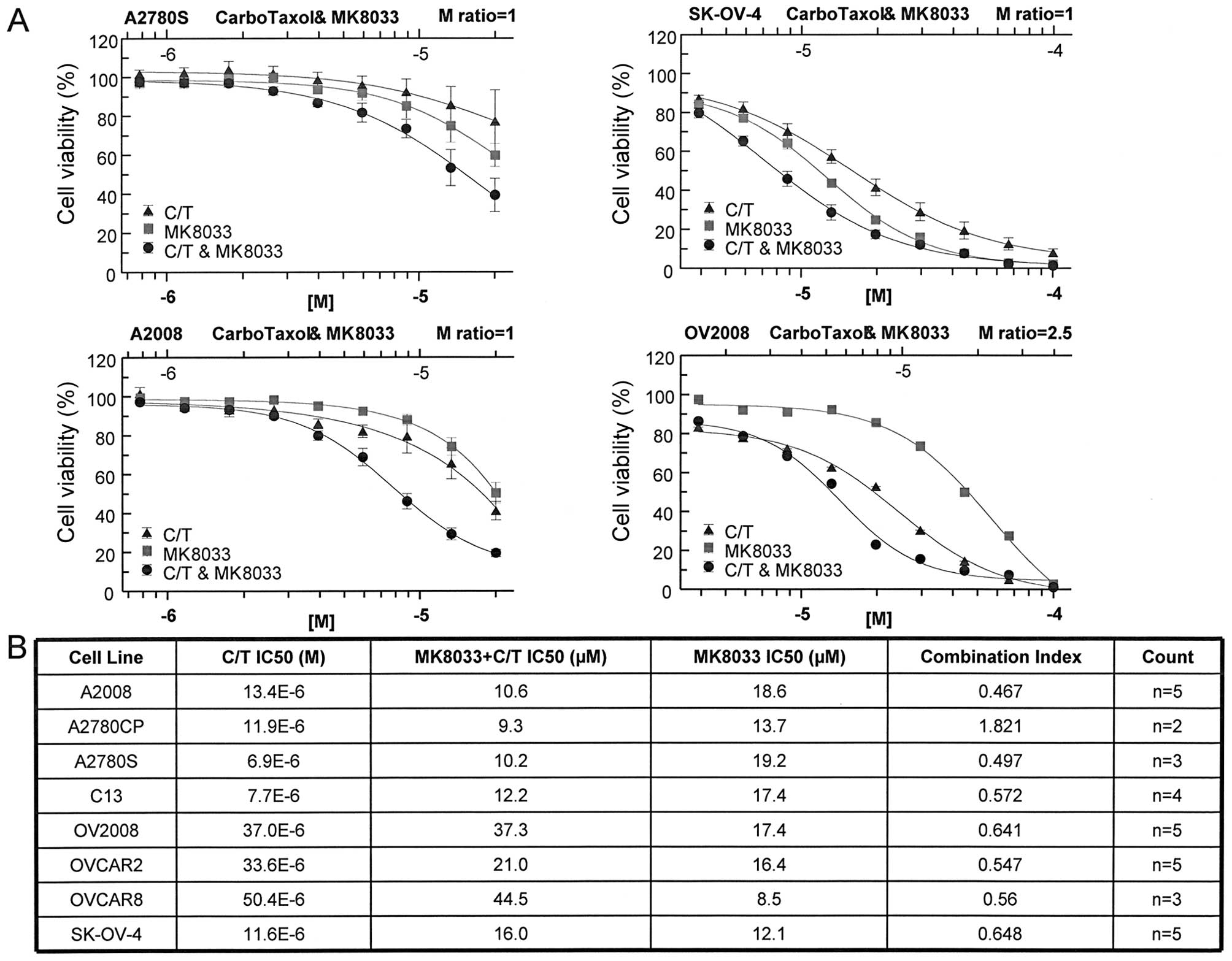
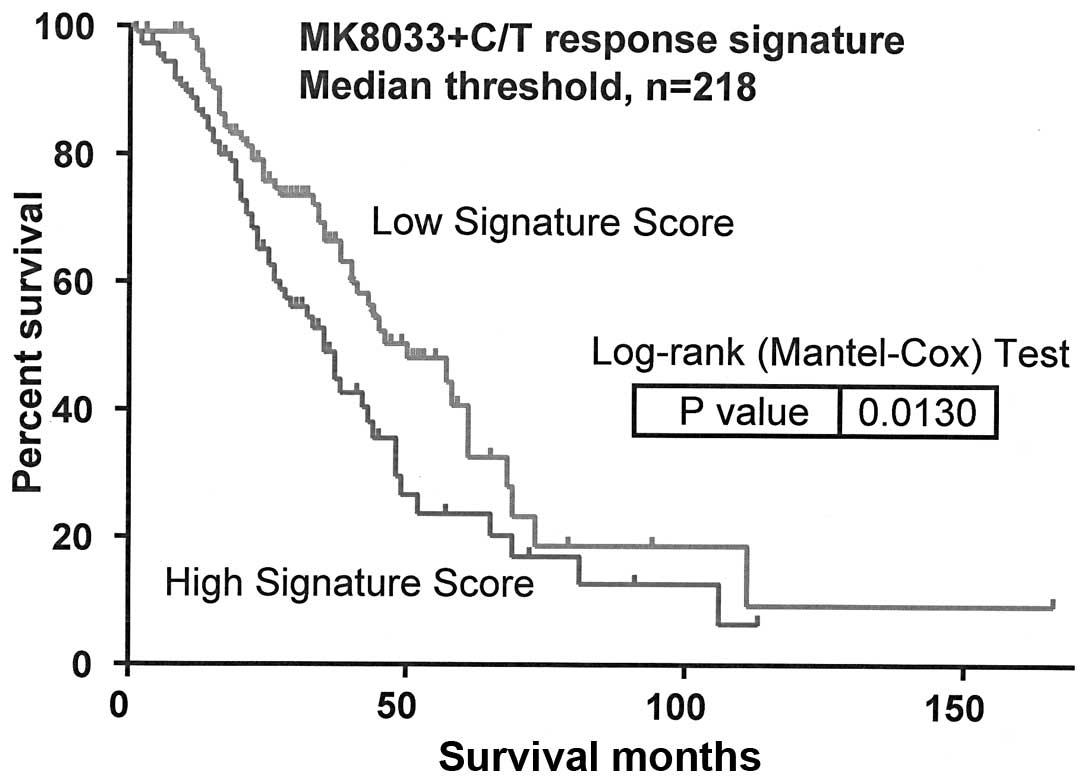
|
1
|
Baker VV: Salvage therapy for recurrent
epithelial ovarian cancer. Hematol Oncol Clin North Am. 17:977–988.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omura GA, Brady MF, Homesley HD, et al:
Long-term follow-up and prognostic factor analysis in advanced
ovarian carcinoma: the Gynecologic Oncology Group experience. J
Clin Oncol. 9:1138–1150. 1991.PubMed/NCBI
|
|
3
|
Di Renzo MF, Narsimhan RP, Olivero M, et
al: Expression of the Met/HGF receptor in normal and neoplastic
human tissues. Oncogene. 6:1997–2003. 1991.
|
|
4
|
Huntsman D, Resau JH, Klineberg E and
Auersperg N: Comparison of c-met expression in ovarian epithelial
tumors and normal epithelia of the female reproductive tract by
quantitative laser scan microscopy. Am J Pathol. 155:343–348. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aune G, Lian AM, Tingulstad S, et al:
Increased circulating hepatocyte growth factor (HGF): a marker of
epithelial ovarian cancer and an indicator of poor prognosis.
Gynecol Oncol. 121:402–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goode EL, Chenevix-Trench G, Hartmann LC,
et al: Assessment of hepatocyte growth factor in ovarian cancer
mortality. Cancer Epidemiol Biomarkers Prev. 20:1638–1648. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawada K, Radjabi AR, Shinomiya N, et al:
c-Met overexpression is a prognostic factor in ovarian cancer and
an effective target for inhibition of peritoneal dissemination and
invasion. Cancer Res. 67:1670–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Gene amplification and protein
overexpression of MET are common events in ovarian clear-cell
adenocarcinoma: their roles in tumor progression and
prognostication of the patient. Mod Pathol. 24:1146–1155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Accumulative copy number increase of MET
drives tumor development and histological progression in a subset
of ovarian clear-cell adenocarcinomas. Mod Pathol. 25:122–130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basha R, Ingersoll SB, Sankpal UT, et al:
Tolfenamic acid inhibits ovarian cancer cell growth and decreases
the expression of c-Met and survivin through suppressing
specificity protein transcription factors. Gynecol Oncol.
122:163–170. 2011. View Article : Google Scholar
|
|
11
|
Zillhardt M, Christensen JG and Lengyel E:
An orally available small-molecule inhibitor of c-Met, PF-2341066,
reduces tumor burden and metastasis in a preclinical model of
ovarian cancer metastasis. Neoplasia. 12:1–10. 2010.
|
|
12
|
Zillhardt M, Park SM, Romero IL, et al:
Foretinib (GSK1363089), an orally available multikinase inhibitor
of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and
impairs ovarian cancer metastasis. Clin Cancer Res. 17:4042–4051.
2011. View Article : Google Scholar
|
|
13
|
Marchion DC, Cottrill HM, Xiong Y, et al:
BAD phosphorylation determines ovarian cancer chemosensitivity and
patient survival. Clin Cancer Res. 17:6356–6366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bicaku E, Xiong Y, Marchion DC, et al: In
vitro analysis of ovarian cancer response to cisplatin,
carboplatin, and paclitaxel identifies common pathways that are
also associated with overall patient survival. Br J Cancer.
106:1967–1975. 2012. View Article : Google Scholar
|
|
15
|
Jolliffe IT: Principal Component Analysis.
2nd edition. Springer-Verlag; New York: 2002
|
|
16
|
Nakamura T, Nawa K and Ichihara A: Partial
purification and characterization of hepatocyte growth factor from
serum of hepatectomized rats. Biochem Biophys Res Commun.
122:1450–1459. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura T, Teramoto H and Ichihara A:
Purification and characterization of a growth factor from rat
platelets for mature parenchymal hepatocytes in primary cultures.
Proc Natl Acad Sci USA. 83:6489–6493. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Nawa K, Ichihara A, Kaise N
and Nishino T: Purification and subunit structure of hepatocyte
growth factor from rat platelets. FEBS Lett. 224:311–316. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeffers M, Rao MS, Rulong S, et al:
Hepatocyte growth factor/scatter factor-Met signaling induces
proliferation, migration, and morphogenesis of pancreatic oval
cells. Cell Growth Differ. 7:1805–1813. 1996.PubMed/NCBI
|
|
20
|
Montesano R, Matsumoto K, Nakamura T and
Orci L: Identification of a fibroblast-derived epithelial morphogen
as hepatocyte growth factor. Cell. 67:901–908. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidner KM, Di Cesare S, Sachs M,
Brinkmann V, Behrens J and Birchmeier W: Interaction between Gab1
and the c-Met receptor tyrosine kinase is responsible for
epithelial morphogenesis. Nature. 384:173–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bladt F, Riethmacher D, Isenmann S, Aguzzi
A and Birchmeier C: Essential role for the c-met receptor in the
migration of myogenic precursor cells into the limb bud. Nature.
376:768–771. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grant DS, Kleinman HK, Goldberg ID, et al:
Scatter factor induces blood vessel formation in vivo. Proc Natl
Acad Sci USA. 90:1937–1941. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar
|
|
26
|
Sonnenberg E, Meyer D, Weidner KM and
Birchmeier C: Scatter factor/hepatocyte growth factor and its
receptor, the c-met tyrosine kinase, can mediate a signal exchange
between mesenchyme and epithelia during mouse development. J Cell
Biol. 123:223–235. 1993. View Article : Google Scholar
|
|
27
|
Mizuno S and Nakamura T: Hepatocyte growth
factor: a regenerative drug for acute hepatitis and liver
cirrhosis. Regen Med. 2:161–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trusolino L, Bertotti A and Comoglio PM:
MET signalling: principles and functions in development, organ
regeneration and cancer. Nat Rev Mol Cell Biol. 11:834–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You WK and McDonald DM: The hepatocyte
growth factor/c-Met signaling pathway as a therapeutic target to
inhibit angiogenesis. BMB Rep. 41:833–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sattler M, Reddy MM, Hasina R, Gangadhar T
and Salgia R: The role of the c-Met pathway in lung cancer and the
potential for targeted therapy. Ther Adv Med Oncol. 3:171–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gumustekin M, Kargi A, Bulut G, et al:
HGF/c-Met overexpressions, but not met mutation, correlates with
progression of non-small cell lung cancer. Pathol Oncol Res.
18:209–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El-Attar HA and Sheta MI: Hepatocyte
growth factor profile with breast cancer. Indian J Pathol
Microbiol. 54:509–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Previdi S, Maroni P, Matteucci E, Broggini
M, Bendinelli P and Desiderio MA: Interaction between human-breast
cancer metastasis and bone microenvironment through activated
hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J
Cancer. 46:1679–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EJ, Eom SJ, Hong JE, Lee JY, Choi MS
and Park JH: Benzyl isothiocyanate inhibits basal and hepatocyte
growth factor-stimulated migration of breast cancer cells. Mol Cell
Biochem. 369:431–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Derman MP, Chen JY, Spokes KC, Songyang Z
and Cantley LG: An 11-amino acid sequence from c-met initiates
epithelial chemotaxis via phosphatidylinositol 3-kinase and
phospholipase C. J Biol Chem. 271:4251–4255. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li C, Wu JJ, Hynes M, et al: c-Met is a
marker of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YJ, Kim DH, Lee SH, Kim DW, Nam HS and
Cho MK: Expression of the c-Met proteins in malignant skin cancers.
Ann Dermatol. 23:33–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syed ZA, Yin W, Hughes K, Gill JN, Shi R
and Clifford JL: HGF/c-met/Stat3 signaling during skin tumor cell
invasion: indications for a positive feedback loop. BMC Cancer.
11:1802011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshizawa Y, Yamada Y, Kanayama S, et al:
Signaling pathway involved in cyclooxygenase-2 up-regulation by
hepatocyte growth factor in endometrial cancer cells. Oncol Rep.
26:957–964. 2011.PubMed/NCBI
|
|
40
|
You H, Ding W, Dang H, Jiang Y and
Rountree CB: c-Met represents a potential therapeutic target for
personalized treatment in hepatocellular carcinoma. Hepatology.
54:879–889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Osada S, Kanematsu M, Imai H and Goshima
S: Clinical significance of serum HGF and c-Met expression in tumor
tissue for evaluation of properties and treatment of hepatocellular
carcinoma. Hepatogastroenterology. 55:544–549. 2008.PubMed/NCBI
|
|
42
|
Eksioglu-Demiralp E, Akdeniz T and Bayik
M: Aberrant expression of c-met and HGF/c-met pathway provides
survival advantage in B-chronic lymphocytic leukemia. Cytometry B
Clin Cytom. 80:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Que W and Chen J: Knockdown of c-Met
inhibits cell proliferation and invasion and increases
chemosensitivity to doxorubicin in human multiple myeloma U266
cells in vitro. Mol Med Rep. 4:343–349. 2011.PubMed/NCBI
|
|
44
|
Inno A, Salvatore MD, Cenci T, et al: Is
there a role for IGF1R and c-MET pathways in resistance to
cetuximab in metastatic colorectal cancer? Clin Colorectal Cancer.
10:325–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bu R, Uddin S, Bavi P, et al: HGF/c-Met
pathway has a prominent role in mediating antiapoptotic signals
through AKT in epithelial ovarian carcinoma. Lab Invest.
91:124–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou XY, Tomatsu S, Fleming RE, et al: HFE
gene knockout produces mouse model of hereditary hemochromatosis.
Proc Natl Acad Sci USA. 95:2492–2497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xue X, Taylor M, Anderson E, et al:
Hypoxia-inducible factor-2alpha activation promotes colorectal
cancer progression by dysregulating iron homeostasis. Cancer Res.
72:2285–2293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ba Q, Hao M, Huang H, et al: Iron
deprivation suppresses hepatocellular carcinoma growth in
experimental studies. Clin Cancer Res. 17:7625–7633. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang C and Saunders AJ: An emerging role
for Ubiquilin 1 in regulating protein quality control system and in
disease pathogenesis. Discov Med. 8:18–22. 2009.PubMed/NCBI
|
|
50
|
Guidi F, Puglia M, Gabbiani C, et al:
2D-DIGE analysis of ovarian cancer cell responses to cytotoxic gold
compounds. Mol Biosyst. 8:985–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Menashe I, Figueroa JD, Garcia-Closas M,
et al: Large-scale pathway-based analysis of bladder cancer
genome-wide association data from five studies of European
background. PLoS One. 7:e293962012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y and Zhang B: TRAIL resistance of
breast cancer cells is associated with constitutive endocytosis of
death receptors 4 and 5. Mol Cancer Res. 6:1861–1871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bolden SW, Hambley JW, Johnston GA and
Rogers LJ: Neonatal stress and long-term modulation of GABA
receptors in rat brain. Neurosci Lett. 111:258–262. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burgett AW, Poulsen TB, Wangkanont K, et
al: Natural products reveal cancer cell dependence on
oxysterol-binding proteins. Nat Chem Biol. 7:639–647. 2011.
View Article : Google Scholar : PubMed/NCBI
|