Introduction
Gastric cancer, a leading cause of cancer-related
mortality worldwide, is the fourth most frequent malignancy.
Despite marked improvements in surgical, chemo-, radio- and other
adjuvant therapies, the 5-year survival rate of patients at the
advanced stage remains <20–25% (1,2). In
recent years, emerging evidence has revealed various genetic
changes involved in the progression of gastric cancer. It is
critical to investigate the precise molecular mechanism of gastric
cancer development for improved anticancer therapeutics.
Eukaryotic translation initiation factor 4E (eIF4E)
plays a crucial role in several human tumors, including breast,
head and neck, bladder, cervical, lung and prostate cancer
(2). Enhanced eIF4E function
resulting from eIF4E overexpression or activation of the Ras and
phosphatidylinositol 3-kinase/Akt pathways can selectively
upregulate the translation initiation of cancer-related mRNA, such
as c-Myc, cyclin D1 and Bcl-2 for tumor growth, angiogenesis and
cell survival (3–5). The overexpression or knockdown of
eIF4E provides insights into its functional significance in
tumorigenesis. Knockdown of eIF4E can suppress proliferation and
angiogenesis (1,6). Additionally, elevated eIF4E expression
confers resistance to multiple chemotherapy agents. A combination
of chemotherapy with eIF4E silencing was reported to increase
sensitivity to chemotherapeutic drugs such as cryptotanshinone,
cisplatin, adriamycin, paclitaxel and docetaxel (4,7,8).
Perifosine is a synthetic alkylphosphocholine
(9), exhibiting antitumor activity
through blocking cell membrane recruitment of the N-terminal Akt
pleckstrin homology (PH) domain. It also exerts an antitumor effect
through MAPK pathway inhibition while inducing c-Jun NH2-terminal
kinase (JNK) and upregulating death receptor 5 (DR5) (10–12).
The clinical efficacy of perifosine was evaluated in a phase I
clinical trial in patients with advanced tumor. Perifosine showed
potent anticancer efficacy in a various types of cancer, including
breast, prostate and renal cell cancer (13–15).
However, there has been no report on the effect of perifosine
against human gastric cancer cells, and its molecular mechanism has
yet to be fully elucidated.
In the present study, we first examined the
expression of total eIF4E (T-eIF4E) and phosphorylated eIF4E
(p-eIF4E) in human tissues and gastric cancer cell lines, and
analyzed the correlation between T-eIF4E and p-eIF4E levels and the
clinicopathological characteristics of gastric cancer. We then
discussed the proliferation of human gastric cancer SGC7901 and
MGC803 cells following eIF4E gene silencing. Furthermore, we
investigated the eIF4E signaling pathway regulated by perifosine
and the growth inhibitory effect of perifosine on gastric cancer
cells. We further identified a combined effect of eIF4E small
interfering RNA (siRNA) and perifosine on the growth of gastric
cancer cells. These findings identified the oncogenic function of
eIF4E and presented the possibility of eIF4E as an effective
antitumor target in gastric cancer.
Materials and methods
Reagents
Perifosine was purchased from Selleck Chemicals LLC
(Houston, TX, USA) and was dissolved in PBS to make a 50 mmol/l
stock solution and stored at −20°C. RPMI-1640 culture medium was
purchased from Gibco-BRL (Grand Island, NY, USA). Sulforhodamine B
(SRB) was purchased from Sigma Chemical Co. The Lipofectamine 2000
transfection reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Rabbit polyclonal antibodies
against p-Akt (s473) (9271) and eIF4E (9742), and rabbit monoclonal
antibody against Akt (9272) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Rabbit monoclonal antibody
against actin (sc-130300) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit polyclonal
anti-GAPDH (AP0063) was purchased from Bioworld Technology Inc.
(Louis Park, MN, USA). Rabbit monoclonal antibody against p-eIF4E
(s209) (2227-1) was purchased from Epitomics Inc. (Burlingame, CA,
USA).
Human tissue samples and
immunohistochemical assay
The tissues used in this study were obtained from 42
gastric cancer samples and 42 adjacent normal mucosal tissues from
patients (32 men and 10 women; age range, 31–77 years) who
underwent curative gastrectomy at the First Affiliated Hospital of
Nanjing Medical University since 2012. The histologic types and
staging of the gastric cancer samples were recorded in accordance
with the Lauren and TNM classifications (proposed by the American
Joint Committee, 2010 version) respectively. None of the patients
had conducted routine treatment including chemotherapy and
radiotherapy. Immunohistochemical staining was conducted on serial
sections for formalin-fixed and paraffin-embedded tissues. The
T-eIF4E and p-eIF4E were detected using a labelled streptavidin
biotin (LSAB) method following autoclave antigen retrieval. The
slides were then incubated with 3% hydrogen peroxide for 15 min to
block endogenous peroxidase activity. Immunohistochemical staining
was performed on a BenchMark XT (Ventana Medical Systems, Inc.,
Tucson, AZ, USA) according to the manufacturer's instructions.
Finally the slides were lightly counterstained with hematoxylin.
Immunohistochemical staining was assessed according to the
immunoreactive score (IRS) that evaluated the staining intensity
and the proportion of positive tumor cells. The staining intensity
was graded as 0 (no staining), 1 (light yellow), 2 (yellow) and 3
(dark yellow). The proportion of positive tumor cells was scored as
0 (negative), 1 (<10%), 2 (10–50%), and 3 (>50%). The two
scores were multiplied and the IRS was determined: values ≥3 were
defined as cytoplasmic expression positive, and values <3 were
regarded as negative.
Cells and cell culture
The gastric cancer cell lines SGC7901, MGC803, AGS
and MKN45 were obtained from the Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences, China. The cells were
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(Gibco-BRL; FBS) at 37°C in an incubator containing 95% air and 5%
CO2.
RNA interference
The oligonucleotides of siRNA targeting eIF4e
(5′-AAGGACGATGGCTAATTACAT-3′) and a non-targeting control were
chemically designed and synthesized by Invitrogen Life
Technologies. MGC803 and SGC7901 cells were seeded in 6-well plates
at a density of 3.6×105 cells/well and transfected with
100 nmol/l eIF4e or control siRNAs for 24 h using Lipofectamine
2000 (Invitrogen Life Technologies) according to the manufacturer's
instructions. The cells were reseeded to 96-well plates and then
treated with perifosine for a further 3 days for SRB assay. The
cells transfected with the above siRNAs for 48 h were harvested for
total RNA, the purification of whole protein lysates, quantitative
real-time PCR (qRT-PCR) assay and western blot analysis.
SRB assay
Gastric cancer cells (3×103) were plated
in 96-well plates for 24 h and then treated with perifosine
(0.125–15 μmol/l). The cells following RNA interference were seeded
in 96-well plates (1.5×103 cells/well) and treated with
different concentrations of perifosine for 72 h. Monolayer cells
were fixed and stained with 0.4% (w/v) SRB. Finally, the dye was
dissolved in 10 mM Tris base solution for 5 min at room temperature
with agitation. Absorbance was measured at 500 nm using a μQuant
Universal Microplate Spectrophotometer (BioTek Instruments,
Inc.).
Colony formation assay
MGC803 and SGC7901 cells were seeded in 24-well
plates for 24 h and then exposed to different concentrations of
perifosine (0.25–15 μmol/l). After cultivating in RPMI-1640 medium
containing 10% FBS for 12 days, the resulting cell colonies were
fixed and stained with 0.5% crystal violet for 10 min. All colonies
visible with the naked eye (>50 cells) were counted individually
and their colony formation rates were evaluated (clone formation
rate = the number of clones/well/100). Each clone was repeated in
triplicate.
Two days after siRNA transfection, 100 cells from
each of the siRNA, negative control and blank groups were seeded in
24-well plates and cultured in 3 ml RPMI-1640 medium with 10% FBS
for 12 days. All colonies visible with the naked eye were counted
individually and the clone formation rates were evaluated.
qRT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer's
instructions and treated with RNase-free DNase. Then, cDNA was
prepared using M-MLV reverse transcriptase (Promega, Madison, WI,
USA). qRT-PCR analysis was performed using a Power SYBR-Green PCR
Master Mix from Applied Biosystems, Inc. (Foster City, CA, USA)
under the following conditions: 10 min at 95°C followed by 40
cycles at 95°C for 15 sec, 60°C for 1 min using the ABI PRISM 7300
sequence detection system of Applied Biosystems Inc. The relative
expression levels of target genes were calculated through
normalizing to the expression of the control gene GAPDH in each
experiment.
The sequences of specific primers were: eIF4E,
5′-CCTACAGAACAGATGGGCACTC-3′ (forward) and
5′-GCCCAAAAGTCTTCAACAGTATCA-3′ (reverse); GAPDH,
5′-ATGGGGAAGGTGAAGGTCG-3′ (forward) and
5′-GGGGTCATTGATGGCAACAATA-3′ (reverse) and were designed and
synthesized by Invitrogen Life Technologies, Inc.
Western blot analysis
RIPA lysis buffer was purchased from Cell Signaling
Technology, Inc. and was stored at 4°C. Protease inhibitors were
added prior to use. Cells were harvested after treatment and
subjected to western blot analysis as previously described
(16).
Statistical analysis
All data from three independent experiments are
expressed as the means ± SD. Differences between the groups were
assessed by two-sided unpaired Student's t-test. The Fisher's exact
test or the χ2 test was used to test the correlation
between eIF4E expression and clinicopathological parameters.
P<0.05 was considered to indicate a statistically significant
difference. The IC50 value of perifosine was calculated
by Bliss software.
Results
Expression of T-eIF4E and p-eIF4E
correlates with the clinicopathological parameters of gastric
cancer
Due to the frequently elevated level of eIF4E in the
progression of human cancer (17),
we examined the expression of T-eIF4E and p-eIF4E in gastric cancer
samples (Fig. 1). T-eIF4E and
p-eIF4E were overexpressed in gastric tumor tissues compared with
adjacent non-cancerous tissues. The positive rate of T-eIF4E and
p-eIF4E was 78.57% (33/42) and 76.19% (32/42), respectively.
T-eIF4E and p-eIF4E were mainly seen in the cytoplasm of primary
cancer cells. Table I shows the
correlation between T-eIF4E and p-eIF4E expression and
clinicopathological parameters in gastric cancer. A significant
correlation was found between T-eIF4E overexpression and distance
metastasis in patients (P=0.026) (Table
I), which was consistent with previous studies (2). The results indicated eIF4E gene
expression may be involved in the development and could be used as
an independent prognosis marker in patients with gastric
cancer.
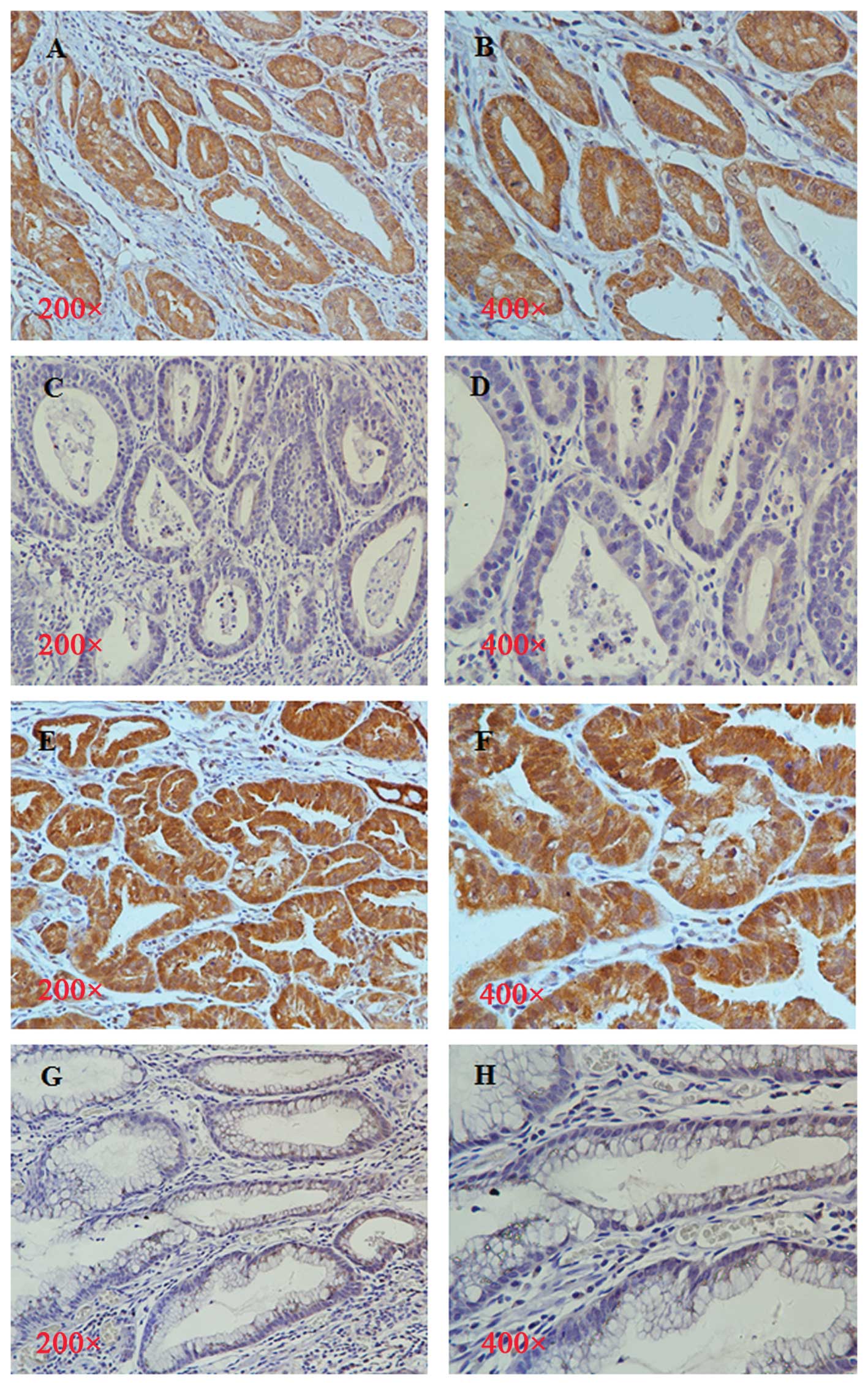 | Figure 1Immunohistochemical staining of
T-eIF4E and p-eIF4E in the non-tumorous mucosal and tumor tissues
of gastric cancer patients. T-eIF4E was overexpressed in gastric
cancer samples, original magnification, (A) ×200, (B) ×400. The
staining of T-eIF4E was weak in normal gastric mucosa, original
magnification, (C) ×200, (D) ×400. The overexpression of p-eIF4E
was found in gastric cancer tissues, original magnification, (E)
×200, (F) ×400. The staining of p-eIF4E was weak in normal gastric
mucosa, original magnification, (G) ×200, (H) ×400. |
 | Table IThe correlation between T-eIF4E and
p-eIF4E expression and the clinicopathological characteristics in
gastric cancer patients. |
Table I
The correlation between T-eIF4E and
p-eIF4E expression and the clinicopathological characteristics in
gastric cancer patients.
| | T-eIF4E | | p-eIF4E | |
|---|
| |
| |
| |
|---|
| Characteristics | Total | − | + | P-value | − | + | P-value |
|---|
| Age |
| <60 | 20 | 4 | 16 | 1.000 | 4 | 16 | 0.849 |
| ≥60 | 22 | 5 | 17 | | 6 | 16 | |
| Gender |
| Male | 32 | 6 | 26 | 0.753 | 9 | 23 | 0.454 |
| Female | 10 | 3 | 7 | | 1 | 9 | |
| Tumor size |
| ≤5 cm | 27 | 6 | 21 | 1.000 | 8 | 19 | 0.418 |
| >5 cm | 15 | 3 | 12 | | 2 | 13 | |
| TNM stagea |
| I, II | 15 | 4 | 11 | 0.823 | 6 | 9 | 0.145 |
| III, IV | 27 | 5 | 22 | | 4 | 23 | |
| Depth of invasion
(T) |
| T1, T2 | 15 | 4 | 11 | 0.823 | 6 | 9 | 0.145 |
| T3, T4 | 27 | 5 | 22 | | 4 | 23 | |
| Lymph node
metastasis (N) |
| N0 | 15 | 4 | 11 | 0.823 | 5 | 10 | 0.483 |
| N1–3 | 27 | 5 | 22 | | 5 | 22 | |
| Distant metastasis
(M) |
| Yes | 4 | 3 | 1 | 0.026 | 1 | 3 | 1.000 |
| No | 38 | 6 | 32 | | 9 | 29 | |
Expression of eIF4E is higher in gastric
cancer cells than in human gastric epithelial cells
To further investigate the relationship between
elevated eIF4E levels and gastric cancer, we examined the mRNA and
protein levels of eIF4E in gastric cancer SGC7901 and MGC803 cells,
as well as human gastric epithelial cells (GES-1) using qRT-PCR
assay and western blot analysis. The expression of eIF4E mRNA was
higher in gastric cancer cells than in GES-1 cells (Fig. 2A). Western blot analysis showed that
T-eIF4E and p-eIF4E expression levels were considerably higher in
gastric cancer cells than in GES-1 (Fig. 2B). These results indicated that
eIF4E functions as an oncogene in gastric cancer.
Downregulation of eIF4E expression
reduces the growth of gastric cancer cells
To investigate the potential functions of eIF4E gene
in gastric cancer, we first explored the effect of eIF4E silencing
on the growth of MGC803 and SGC7901 cells. In the qRT-PCR assay, a
significantly decreased level of eIF4E mRNA was observed after 48 h
transfection of 100 nmol/l eIF4E siRNA in comparison with a control
siRNA in MGC803 and SGC7901 cells (Fig.
3A). Western blot analysis confirmed a significantly decreased
level of T-eIF4E and p-eIF4E (s209), indicating a successful eIF4E
knockdown (Fig. 3B). Our results
showed that the gastric cancer cells with eIF4E siRNA exhibited
markedly higher growth inhibition rates compared with the cells
with a control siRNA (P<0.01) in a 5-day SRB assay (Fig. 3C and D), which was consistent with
previous reports on breast cancer, primary central nervous system
lymphoma cells (18,19). Moreover, such inhibitory effects
were also seen when the cells were transfected with eIF4E siRNA in
a 10-day colony formation assay (Fig.
3E and F) and its colony formation rate was significantly lower
in comparison with the control group. The result indicated that
downregulation of eIF4E expression reduced the possibility of
colony formation.
Perifosine decreases eIF4E mRNA level and
inhibits the Akt/eIF4E signaling pathway while downregulating
T-eIF4E and p-eIF4E protein expression
Due to the crucial role of eIF4E in the growth of
gastric cancer, we further examined the levels of eIF4E following
perifosine (an Akt inhibitor) treatment. We explored whether
perifosine reduced the proliferation of gastric cancer cells
through regulating the Akt signaling pathway. After 24 h of
treatment, perifosine significantly downregulated the level of
eIF4E mRNA in SGC7901 cells (P<0.05), but showed a mild change
and not in a time- and dose-dependent manner (Fig. 4A and B); perifosine (0.25, 0.5, 0.75
and 1.0 μmol/l) produced the dose-dependently decreased expression
of T-eIF4E and p-eIF4E in SGC7901 cells (Fig. 4D and E). In MGC803 cells, the
expression of eIF4E mRNA was significantly downregulated by
perifosine in a dose-dependent manner (Fig. 4C). Furthermore, we also examined the
expression of total Akt and p-Akt (s473), and the T-eIF4E and
p-eIF4E (s209) were also decreased in a dose- and time-dependent
manner in MGC803 cells (Fig. 4F and
G). These results indicated that eIF4E could be a target of
perifosine in gastric cancer, and the Akt/eIF4E signaling pathway
was involved in the regulation mechanism of perifosine.
Perifosine inhibits the growth of gastric
cancer cells in a dose-dependent manner
Perifosine dose-dependently decreased the viability
of gastric cancer cells at the concentrations ranging from 0.625 to
15 μmol/l in a 3-day SRB assay (Fig.
5A). Among these cell lines, SGC7901 was the most sensitive to
perifosine treatment with an IC50 of 0.36 μmol/l. The
IC50 of MGC803, MKN45 and AGS cells was 5.18, 11.65 and
4.0 μmol/l respectively, which indicated that perifosine inhibited
the growth of gastric cancer cells with different sensitivity. In
addition, perifosine dose-dependently substantially decreased the
ability of MGC803 (Fig. 5B) and
SGC7901 (Fig. 5C) cells to form
colonies in a dose-dependent manner.
Co-targeting eIF4E enhances the
inhibitory effect on gastric cancer SGC7901 and MGC803 cells
Since perifosine inhibited the growth of gastric
cancer cells, we used RNA interference to block eIF4E expression
and then evaluated the combined effect of eIF4E siRNA and
perifosine on gastric cancer cell lines. The results of qRT-PCR
assay showed eIF4E siRNA had a very high inhibitory effect on eIF4e
in SGC7901 and MGC803 cells (Fig.
3A). Western blotting also confirmed the knockdown rate of
eIF4E (Fig. 3B). The combined
treatment of eIF4E siRNA and perifosine enhanced the inhibitory
effect on gastric cancer cell growth (Fig. 6A and B). We further examined the
eIF4E signaling pathway regulated by perifosine. After SGC7901 and
MGC803 cells transfected with eIF4E siRNA or a control siRNA were
treated with perifosine, eIF4E expression was further downregulated
(Fig. 6C and D). The results
suggested the combination of eIF4E siRNA and perifosine may
represent an effective therapy against gastric cancer.
Discussion
Abnormal eIF4E activity is present in a large
spectrum of human malignancies. eIF4E overexpression has been
commonly seen in the prostate, breast, stomach, colon, lung, skin
and hematopoietic system. p-eIF4E has oncogenic potential in human
cancer (20,21). However, little is known about the
relationship between the eIF4E gene and gastric cancer progression.
In the current study, we found that the expression levels of
T-eIF4E and p-eIF4E were upregulated in gastric cancer samples.
Further analysis showed that T-eIF4E expression was related to
cancer vascular invasion, suggesting T-eIF4E promoted the
progression of gastric cancer. However, we did not find a
relationship between p-eIF4E expression and any clinical
characteristics of the patients. Meanwhile, due to limited samples,
the relationship between T-eIF4E and p-eIF4E expression and the
malignancy of human tumors remains to be further determined. These
data indicate that the eIF4E gene may be an attractive target for
gastric cancer therapy.
Translation activation is critical for cancer cell
growth and survival. Therefore, translation is a rational target
for novel cancer therapeutics. Translation initiation is largely
dependent on eIF4E activity. According to research on different
types of tumors, eIF4E can be regulated by the PI3K/mTOR, MAPK/MNK
signaling pathways at multiple levels, such as transcription,
serine 209 phosphorylation, as well as the inhibitory interaction
between binding proteins (4EBP) (22,23).
Furthermore, the difference between eIF4E activation and response
to eIF4E siRNA suggests varying influence of the Akt pathway
depending on cell types. In this study, we found that eIF4E was
overexpressed in gastric cell lines and knockdown of eIF4E in
MGC803 and SGC7901 cells through RNA interference significantly
inhibited in vitro proliferation. Therefore, eIF4E may play
an important role in gastric cancer, and clarification of the eIF4E
signaling pathway is urgently required.
Perifosine, an alkylphospholipid with antitumor
activity in both preclinical and clinical studies, inhibits Akt
through targeting its pleckstrin homology (PH) domain and
interferes with its recruitment to the plasma member and subsequent
phosphorylation and activation (24). In the present study, we investigated
the effect of perifosine on human gastric cancer SGC7901, MGC803,
AGS and MKN45 cells. Our results indicate that perifosine treatment
has a significant cytotoxic effect on SGC7901, MGC803, AGS and
MKN45 cells in a dose-dependent manner, which is associated with
rapidly decreased Akt activation as assessed by the quantification
of Ser473 phosphorylation and the decreased T-eIF4E and p-eIF4E
expression. Previous studies have indicated that eIF4E is one of
the proposed downstream mediators in the Akt signaling pathway and
regulated by Akt. These findings indicate that perifosine may
downregulate the expression of eIF4E through Akt inhibition
(25).
We first observed that perifosine downregulated
T-eIF4E and p-eIF4E expression and inhibited the Akt/eIF4E
signaling pathway in gastric cancer. We also noted less changed
eIF4E amounts at the mRNA level in SGC7901 cells than at the
protein level, which suggested the involvement of other mechanisms
in addition to transcriptional regulation. Furthermore, enhanced
eIF4E level has been demonstrated to increase the expression of
proteins that contribute to tumor development, including growth
factors such as c-Myc and cyclin D1, and angiogenesis factors such
as VEGF and FGF-2. Perifosine inhibited the proliferation of
gastric cancer cells through decreasing eIF4E levels. Further
studies on whether the inhibition of eIF4E by perifosine also
inhibits the expression of other downstream targets that are
necessary for oncogenesis and cancer progression are warranted.
After identifying that the eIF4E signaling pathway
was regulated by perifosine, we also found that the combination of
eIF4E gene silencing and perifosine treatment could significantly
increase the growth inhibition efficacy in human gastric cancer
cells in comparison with eIF4E gene silencing or perifosine
treatment alone. Cancer cells tend to be more highly dependent on
translation than normal tissues. Targeting eIF4E appears to be an
attractive anticancer strategy for chronic myeloid leukemia and
endometrial adenocarcinoma (5,7,18).
Increasing evidence supports that inhibited eIF4E can cause more
inhibitory effects on drug-induced growth (8). Therefore, suppressing translation by
targeting eIF4E with RNA interference may play a more important
role in perifosine-induced growth repression. The result may
suggest a possible strategy of combined eIF4E knockdown.
In conclusion, the current results showed that the
expression of T-eIF4E and p-eIF4E was increased in gastric cancer
tissues and cell lines. Downregulation of eIF4E significantly
suppressed the proliferation of gastric cancer cells. Moreover,
perifosine exerted its inhibitory effect on the regulation of
growth and survival through the eIF4E signaling pathway and the
combination of eIF4E gene silencing and perifosine was more
effective than eIF4E gene silencing or perifosine alone. Further
research might focus on a specific mechanism through which eIF4E
regulates the growth of gastric cancer and may confirm the
potential effectiveness of eIF4E as a therapeutic target of gastric
cancer in clinical practice.
Acknowledgements
This study was supported by the Jiangsu Province's
Key Provincial Talents Program, China (RC2011059) (L.Y.), and the
‘Six Talents Peak’ Project of Jiangsu Province (2012) (L.Y.), the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), and the National Natural Science Foundation of
China (nos. 30873099, 81102458 and 81172004) (X.W.). The authors
also thank Professor Xuerong Wang of Nanjing Medical University for
the valuable comments on this study.
Abbreviations:
|
eIF4E
|
eukaryotic translation initiation
factor 4E
|
|
p-eIF4E
|
phosphorylated eIF4E
|
|
siRNA
|
small interfering RNA
|
|
SRB
|
sulforhodamine B
|
References
|
1
|
Avdulov S, Li S, Michalek V, et al:
Activation of translation complex eIF4F is essential for the
genesis and maintenance of the malignant phenotype in human mammary
epithelial cells. Cancer Cell. 5:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen CN, Hsieh FJ, Cheng YM, Lee PH and
Chang KJ: Expression of eukaryotic initiation factor 4E in gastric
adenocarcinoma and its association with clinical outcome. J Surg
Oncol. 86:22–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004.PubMed/NCBI
|
|
4
|
Dong K, Wang R, Wang X, et al:
Tumor-specific RNAi targeting eIF4E suppresses tumor growth,
induces apoptosis and enhances cisplatin cytotoxicity in human
breast carcinoma cells. Breast Cancer Res Treat. 113:443–456.
2009.PubMed/NCBI
|
|
5
|
Choi CH, Lee JS, Kim SR, et al: Direct
inhibition of eIF4E reduced cell growth in endometrial
adenocarcinoma. J Cancer Res Clin Oncol. 137:463–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbert TP, Fahraeus R, Prescott A, Lane
DP and Proud CG: Rapid induction of apoptosis mediated by peptides
that bind initiation factor eIF4E. Curr Biol. 10:793–796. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge Y, Cheng R, Zhou Y, et al:
Cryptotanshinone induces cell cycle arrest and apoptosis of
multidrug resistant human chronic myeloid leukemia cells by
inhibiting the activity of eukaryotic initiation factor 4E. Mol
Cell Biochem. 368:17–25. 2012. View Article : Google Scholar
|
|
8
|
Zhou FF, Yan M, Guo GF, et al: Knockdown
of eIF4E suppresses cell growth and migration, enhances
chemosensitivity and correlates with increase in Bax/Bcl-2 ratio in
triple-negative breast cancer cells. Med Oncol. 28:1302–1307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gills JJ and Dennis PA: Perifosine: update
on a novel Akt inhibitor. Curr Oncol Rep. 11:102–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elrod HA, Lin YD, Yue P, et al: The
alkylphospholipid perifosine induces apoptosis of human lung cancer
cells requiring inhibition of Akt and activation of the extrinsic
apoptotic pathway. Mol Cancer Ther. 6:2029–2038. 2007. View Article : Google Scholar
|
|
11
|
Papa V, Tazzari PL, Chiarini F, et al:
Proapoptotic activity and chemosensitizing effect of the novel Akt
inhibitor perifosine in acute myelogenous leukemia cells. Leukemia.
22:147–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tazzari PL, Tabellini G, Ricci F, et al:
Synergistic proapoptotic activity of recombinant TRAIL plus the Akt
inhibitor perifosine in acute myelogenous leukemia cells. Cancer
Res. 68:9394–9403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leighl NB, Dent S, Clemons M, et al: A
Phase 2 study of perifosine in advanced or metastatic breast
cancer. Breast Cancer Res Treat. 108:87–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghobrial IM, Roccaro A, Hong F, et al:
Clinical and translational studies of a phase II trial of the novel
oral Akt inhibitor perifosine in relapsed or relapsed/refractory
Waldenstrom's macroglobulinemia. Clin Cancer Res. 16:1033–1041.
2010. View Article : Google Scholar
|
|
15
|
Cho DC, Hutson TE, Samlowski W, et al: Two
phase 2 trials of the novel Akt inhibitor perifosine in patients
with advanced renal cell carcinoma after progression on vascular
endothelial growth factor-targeted therapy. Cancer. 118:6055–6062.
2012. View Article : Google Scholar
|
|
16
|
Ma Z, Zhu L, Luo X, Zhai S, Li P and Wang
X: Perifosine enhances mTORC1-targeted cancer therapy by activation
of GSK3β in NSCLC cells. Cancer Biol Ther. 13:1009–1017.
2012.PubMed/NCBI
|
|
17
|
Wang S, Rosenwald IB, Hutzler MJ, et al:
Expression of the eukaryotic translation initiation factors 4E and
2alpha in non-Hodgkin's lymphomas. Am J Pathol. 155:247–255. 1999.
View Article : Google Scholar
|
|
18
|
Muta D, Makino K, Nakamura H, Yano S, Kudo
M and Kuratsu J: Inhibition of eIF4E phosphorylation reduces cell
growth and proliferation in primary central nervous system lymphoma
cells. J Neurooncol. 101:33–39. 2011.
|
|
19
|
Soni A, Akcakanat A, Singh G, et al: eIF4E
knockdown decreases breast cancer cell growth without activating
Akt signaling. Mol Cancer Ther. 7:1782–1788. 2008. View Article : Google Scholar
|
|
20
|
Fan SQ, Ramalingam SS, Kauh J, Xu ZH,
Khuri FR and Sun SY: Phosphorylated eukaryotic translation
initiation factor 4 (eIF4E) is elevated in human cancer tissues.
Cancer Biol Ther. 8:1463–1469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wheater MJ, Johnson PW and Blaydes JP: The
role of MNK proteins and eIF4E phosphorylation in breast cancer
cell proliferation and survival. Cancer Biol Ther. 10:728–735.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fischer PM: Cap in hand: targeting eIF4E.
Cell Cycle. 8:2535–2541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia Y, Polunovsky V, Bitterman PB and
Wagner CR: Cap-dependent translation initiation factor eIF4E: an
emerging anticancer drug target. Med Res Rev. 32:786–814. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hideshima T, Catley L, Yasui H, et al:
Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt
and induces in vitro and in vivo cytotoxicity in human multiple
myeloma cells. Blood. 107:4053–4062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshizawa A, Fukuoka J, Shimizu S, et al:
Overexpression of phospho-eIF4E is associated with survival through
AKT pathway in non-small cell lung cancer. Clin Cancer Res.
16:240–248. 2010. View Article : Google Scholar
|




















