Introduction
Malignant pleural mesothelioma (MPM) is a highly
aggressive and conventional treatment-resistant tumor with a dismal
prognosis. Its incidence has been increasing over the past two
decades in industrialized countries and is expected to peak during
the period 2010–2020 (1,2). There are three distinct histologic
subtypes of MPM and the epithelioid type is the most common one
which comprises 50–70% of all cases. Asbestos exposure,
non-asbestos mineral fibers, organic chemicals, radiation and
simian virus 40 (SV40) have been suggested as risk factors for
mesothelioma (3). These etiologic
agents can cause chronic tissue injury and tissue repair in the
long latency period. The misappropriating homeostatic mechanisms
that govern tissue repair and stem cell self-renewal may lead to
carcinogenesis (4). Targeting the
genes which play a part in the determination and maintenance of the
stem cell compartment may contribute to identifying more accurate
prognostic factors and developing effective therapies.
Numb is an evolutionarily conserved protein that
plays a critical role in cell fate determination. Numb was
originally identified in Drosophila(5). It has been reported to be involved in
the control of asymmetric cell division (6), maintenance of stem cell compartments
(7), regulation of cell polarity
and adhesion (8), migration
(9) and ubiquitination of specific
substrates (10–12). Subversion of Numb has been linked to
many pathological mechanisms, including cancer. Numb plays an
important role in the transformation target and the plasticity of
the stem cell compartment (13),
which are closely related to tumor formation. In particular, the
plasticity of the stem cell compartment is related to
epithelial-mesenchymal transition (EMT), which contributes to the
acquirement of invasive properties as well as resistance to cell
apoptosis and chemotherapy (14,15).
Subversion of Numb is also predicted to have a major impact on the
homeostasis of endocytosis (16,17)
and the alteration of many polarity functions through connecting
with the PAR complex (18,19), which both facilitate transformation
events of tumors. In the absence of Numb, both Notch (10,20,21)
and Hedgehog (Hh) signaling (11)
are augmented, with pro-proliferative and anti-differentiation
effects, while the suppressor TP53 signaling is attenuated
(12).
Experimental evidence indicates that Numb has the
potential to function as a tumor suppressor. Studies of mutant Numb
in Drosophila have shown neuroblast overproliferation and
tumor formation, which strongly supports the hypothesis that
impairment of asymmetric cell division in stem cells results in an
imbalance in self-renewal and differentiation which leads to cancer
(22). In addition, in vivo
RNA interference of Numb in a model of mouse lymphomagenesis was
found to accelerate the onset of lymphomas (23). Loss of Numb expression has also been
reported in some types of human cancers, such as breast cancers,
non-small cell lung carcinomas, salivary gland carcinomas and
medulloblastomas (20,21,24,25).
In human astrocytomas, there is a trend of higher Numb expression
in more malignant tumors (26).
Whether Numb functions as a tumor suppressor in MPM or not has not
been clarified.
In the present study, we analyzed the expression of
Numb in epithelioid MPM and investigated the potential role of Numb
in MPM cells. The aim of our study was to clarify the role of Numb
in epithelioid MPM and to explore novel strategies for
drug-resistant MPM therapy.
Materials and methods
Patients and specimens
Tissue samples of 39 MPM patients who underwent
medical thoracoscopy procedures (Department of Respiratory
Medicine, Provincial Hospital, Shandong University, China), from
2007 to 2010, were analyzed and 22 normal pleural samples as a
control group were recruited. The MPM diagnosis was based on WHO
criteria (27) and confirmed in all
instances by clinical, morphologic and immunohistochemical data.
Specimens were reviewed by two pathologists, and the histological
type was confirmed as epithelioid cell type according to the 2004
WHO classification of pleural tumors (27). All procedures were approved by the
Ethics Committee of Shandong University.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections
(4 μm) were processed for immunohistochemical investigation. Tissue
sections were first deparaffinized, followed by rehydration with
serially decreased concentrations of ethanol. The following antigen
retrieval was carried out in 0.01 M citrate buffer (pH 6.0) at 96°C
for 15 min in a thermostat controlled water bath. The tissue
sections were then immersed in 3% H2O2 for 30
min and were blocked with normal serum at 37°C for 30 min. The
primary antibodies anti-Numb antibody (1:200, rabbit polyclonal;
Abcam, Cambridge, UK) and anti-Ki-67 antibody (1:150, rabbit
polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were
incubated at 4°C overnight. The following biotinylated secondary
antibodies (Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) were added at 37°C for 30 min. For detection
of the binding sites, a streptavidin-biotin system (Beijing
Zhongshan Golden Bridge Biotechnology) was used. Diaminobenzidine
(DAB) was used as the enzyme substrate to observe the specific
antibody localization, and nuclei were counterstained using
hematoxylin. Negative controls were performed in all cases by
omitting the primary antibodies. Observation was carried out under
a light microscope (Leica DM4000, Wetzlar, Germany) and the images
were processed with image processing software (Leica IM45).
Histological evaluation
Two independent observers blinded to the clinical
data were assigned to evaluate all samples. Quantitation of cells
staining positivity for Numb in the cytoplasm or membrane was
recorded. Sections with staining in ≥10% of cells were considered
as positive. Immunohistochemical reactivity was graded on a scale
of 0–3 according to the percentage of immunopositive cells as
follows: grade 0, no staining or <10%; grade 1, 10–30%; grade 2,
30–50%; grade 3, >50% positive cells. For Ki-67, the percentage
of positively stained nuclei out of the total cancer cells was
calculated, and we used 25% as the cut-off point for
categorizing.
Cell culture and plasmid
transfection
Human MPM cell line NCI-H2452 was stemed from the
American Type Culture Collection (ATCC, Manassas, VA, USA),
cultured in RPMI-1640 medium supplemented with 10% fetal calf
serum, 1.5 g/l NaHCO3, 2.5g/l glucose, 0.11 g/l sodium
pyruvate and maintained at 37°C in a humidified atmosphere with 5%
CO2. We selected the HEK-293T cell line which has been
proven to express Numb as a control (11). HEK-293T cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal
bovine serum (FBS). Plasmid pcDNA3.1-Numb was synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). First,
1×106 cells in a 60-mm dish were transfected with either
the empty vector (mock) or pcDNA3.1-Numb (Numb) using
Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s
guidelines. After 24 h, the cells were split into three sets: one
set was used for cell viability assay; one was used to analyze
protein expression by western blot analysis; and one was used for
cell apoptosis analysis.
Real-time quantitative reverse
transcription-polymerase chain reaction
Primer sequences used for Numb were:
5′-CAATCTCCTACCTTCCAAGGG-3′ and 5′-CGGACGC TCTTAGACACCTC-3′; and
for GAPDH, 5′-TGGTCACCA GGGCTGCTT-3′ and
5′-AGCTTCCCGTTCTCAGCCTT-3′. All primers were synthesized by Takara
Co., Ltd. (Dalian, China).
Total RNA from cells was isolated using TRIzol
(Invitrogen) and reverse-transcribed by the ReverTra Ace qPCR RT
kit (Toyobo, Osaka, Japan). qRT-PCR was performed using SYBR Green
Real-Time PCR Master Mix (Toyobo) following the manufacturer’s
instructions and on the LightCycler 480 System (Roche, Germany).
Each reaction was performed in triplicate and analysis was
performed by the 2−ΔΔCt method.
Western blot analysis
At 48–72 h after transfection, cells were harvested
for western blot analysis. Total protein was extracted by RIPA
lysis buffer. To analyze cytochrome c expression, cytosolic
and mitochondrial fractions were prepared using the Mitochondria
Extraction kit (Nanjing KeyGen Biotech., Co., Ltd., Nanjing,
China). Proteins were transferred to a PVDF membrane and subjected
to the antibodies including Numb (1:1,000; Abcam), caspase-3,
caspase-9, cytochrome c, XIAP and survivin (1:500; Santa
Cruz Biotechnology). Peroxidase-labeled anti-mouse or anti-rabbit
antibodies were used as secondary antibodies (Zhongshan
Goldenbridge Biotechnology). Blots were developed using enhanced
chemiluminescence detection reagent (Applygen Technologies Inc.,
Beijing, China).
Cell viability assay
We used an MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide)
assay to evaluate cell viability. Cells (5×103/well)
were grown in a 96-well plate and were tested at 24, 48 and 72 h
after transfection. The cells (1×104/well) were grown in
a 96-well plate and incubated for another 24 h, and these cells
were then treated with different concentrations of cisplatin
ranging from 1 to 10 μmol/l for 24 h in triplicate. Twenty
microliters of 5 mg/ml MTT was added into each well and incubated
for 4 h at 37°C in a culture hood. Media were carefully removed,
and 150 μl DMSO was added. The absorbance was measured at a
wavelength of 490 nm from which the background was subtracted. The
cell survival value index was calculated by the formula: [A490
(+cisplatin)/A490 (−cisplatin)] × 100%.
Cell apoptosis analysis
The morphological changes in apoptotic cells were
investigated using Hoechst 33258 staining (Solarbio, Shanghai,
China) and fluorescence microscopy. The apoptosis rate was examined
by two-color analysis of Annexin V/FITC binding and propidium
iodide (PI) uptake using flow cytometry. Cells (1×105)
were seeded into 6-well plates and were tested at 72 h after
transfection. Cells (1×105) were then seeded into 6-well
plates and incubated for another 24 h, and these cells were then
treated with 5 μmol/l cisplatin for 24 h. FITC-conjugated Annexin V
was added at a concentration of 0.5 μg/ml. After incubation for 20
min at room temperature in the dark, PI was added at 1 μg/ml, and
the samples were immediately analyzed by fluorescence-activated
cell sorting (FACS).
Statistical analysis
All analyses were performed using SPSS 17.0 software
package (SPSS Inc., Chicago, IL, USA). Chi-square test was used to
compare the expression of Numb in the MPM and control groups.
Correlations of protein expression (grade scores) with clinical and
pathological parameters of the tumors were evaluated with the
Mann-Whitney test for ordinal variables. Overall survival (OS) was
defined as the time of diagnosis to the time of death from
cancer-related cause or to the date the patient was last known to
be alive. The univariate analysis of OS was carried out by the
Kaplan-Meier method using the log-rank test. All cell culture
experiments were conducted at least three times. Data are shown as
means ± SD and were analyzed using the Student’s t-test for two
samples. All statistical tests were two-sided, and probability
values <0.05 and <0.01 were defined as being statistically
significant.
Results
Expression of Numb in MPM tissues
The characteristics of the 39 epithelioid MPM
patients enrolled in the present study were as follows. The median
age for the patients was 55 years (range, 32–77). Nineteen patients
were males and 20 were females, with a male to female ratio of
1:1.05. Patients were divided into two groups according to the
Eastern Cooperative Oncology Group (ECOG) performance status.
Twenty-nine patients were in the grade <2 group and 10 patients
were in the grade ≥2 group (Table
I).
 | Table IExpression of Numb in epithelioid MPM
and its correlation with clinical features. |
Table I
Expression of Numb in epithelioid MPM
and its correlation with clinical features.
| | Numb
expression | |
|---|
| |
| |
|---|
| Variables | N | Grade 0/1/2/3 | P-value |
|---|
| MPM (epithelioid)
total | 39 | 20/10/6/3 | |
| Age (years) | | | 0.783 |
| ≤55 | 20 | 10/5/3/2 | |
| >55 | 19 | 10/5/3/1 | |
| Gender | | | 0.258 |
| Male | 19 | 8/5/5/1 | |
| Female | 20 | 12/5/1/2 | |
| ECOG performance
status | | | 0.159 |
| ≤2 | 11 | 4/3/2/2 | |
| >2 | 28 | 16/7/4/1 | |
| Ki-67 index | | | 0.011 |
| ≤25 | 26 | 10/7/6/3 | |
| >25 | 13 | 10/3/0/0 | |
Numb localization on the membrane or in the
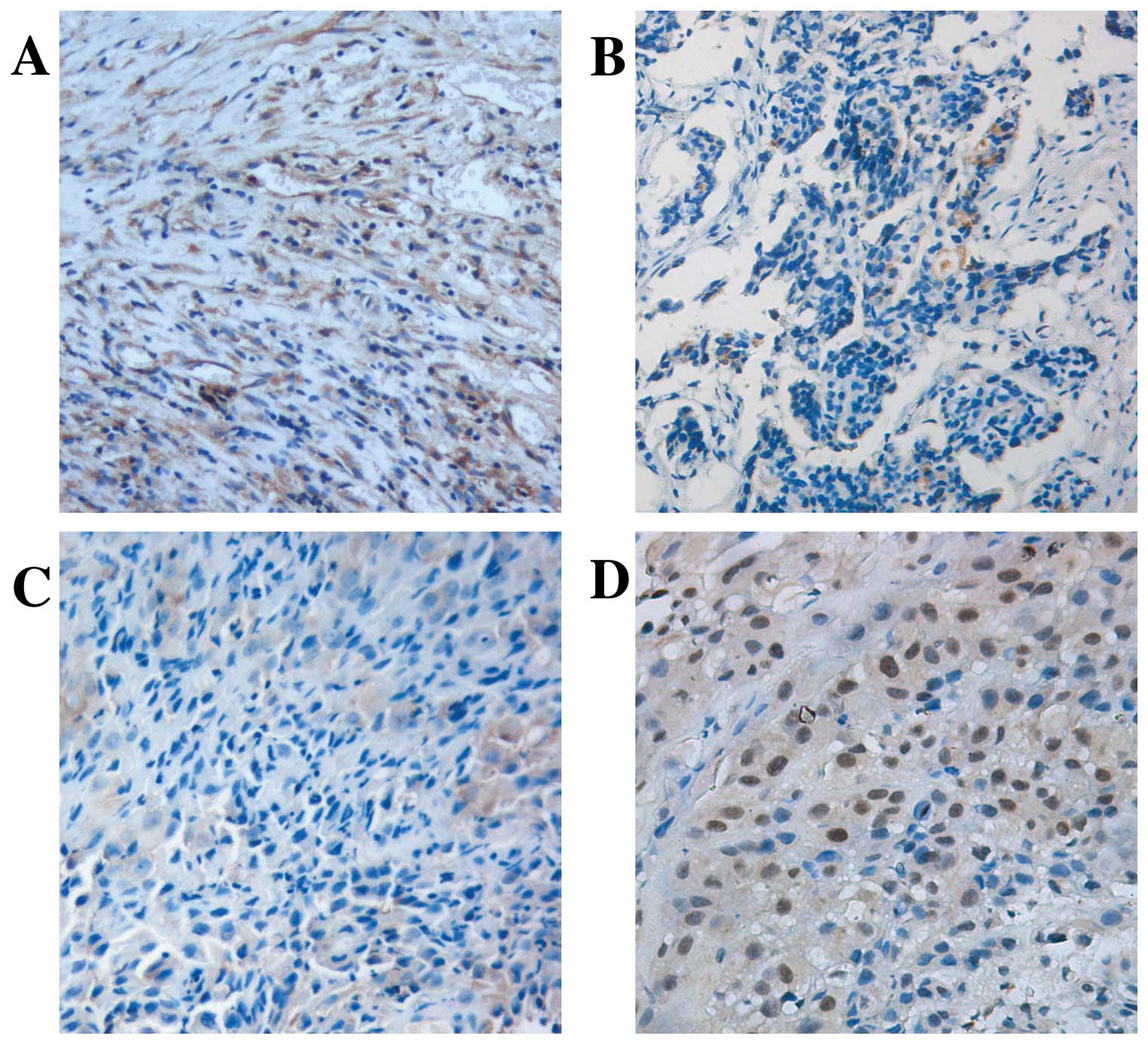
cytoplasm was demonstrated in tumor samples [51.3% (20/39)
positive] with a slighter intensity compared with [86.4% (19/22)]
the normal pleural specimens (P<0.05; Fig. 1A and B). A marked inverse
correlation was found between Numb expression levels and Ki-67
labeling index (P<0.05; Fig. 1C and
D, Table I). There was no
correlation between loss of Numb expression and gender, age and
ECOG performance status (P>0.05).
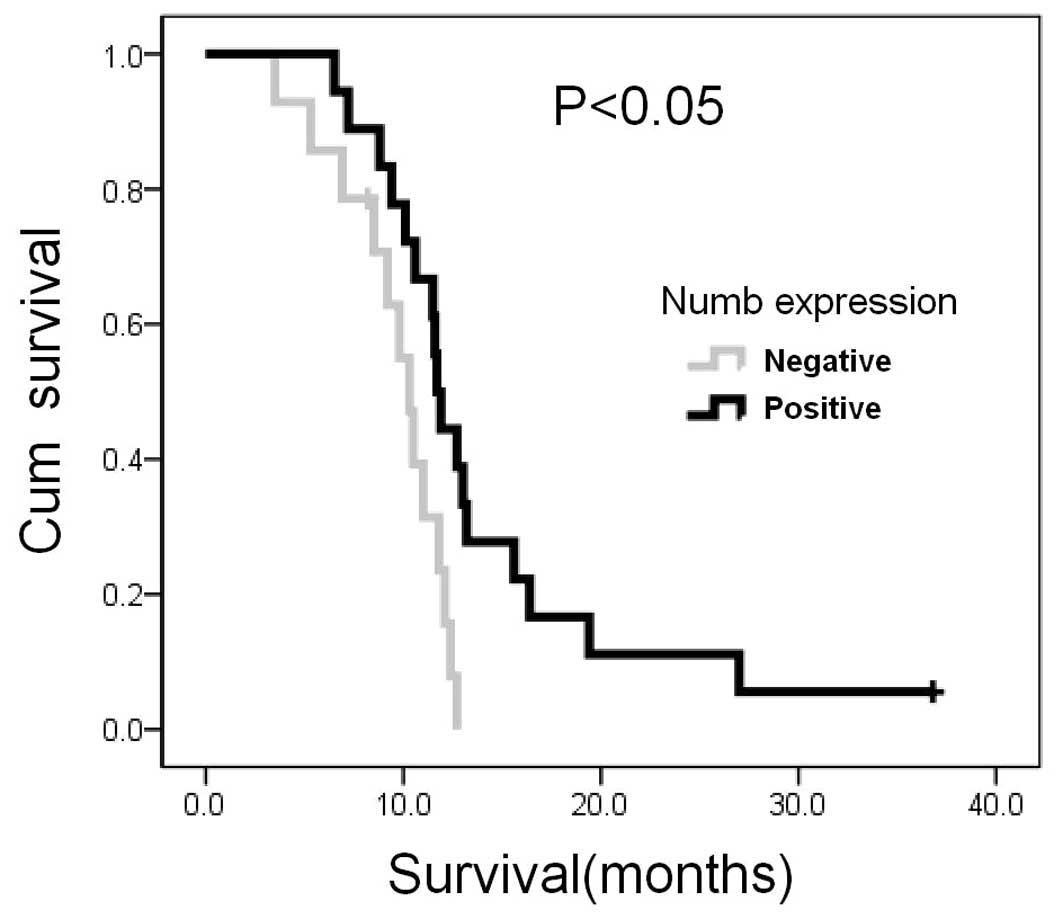
Complete clinical follow-up data were obtained from
32 patients allowing retrospective survival analysis. At the
completion of the study in July 2011, 30 patients had died, with a
median follow-up of 11.3 months. Univariate analysis indicated that
overall survival was influenced by Numb expression (log-rank test
P<0.05; Fig. 2), comparing
negativity (grade 0; median survival, 10.3 months; 95% CI,
9.1–11.5) vs. weak-moderate-strong expression (grade 1–3; median
survival, 11.7 months; 95% CI, 11.1–12.3).
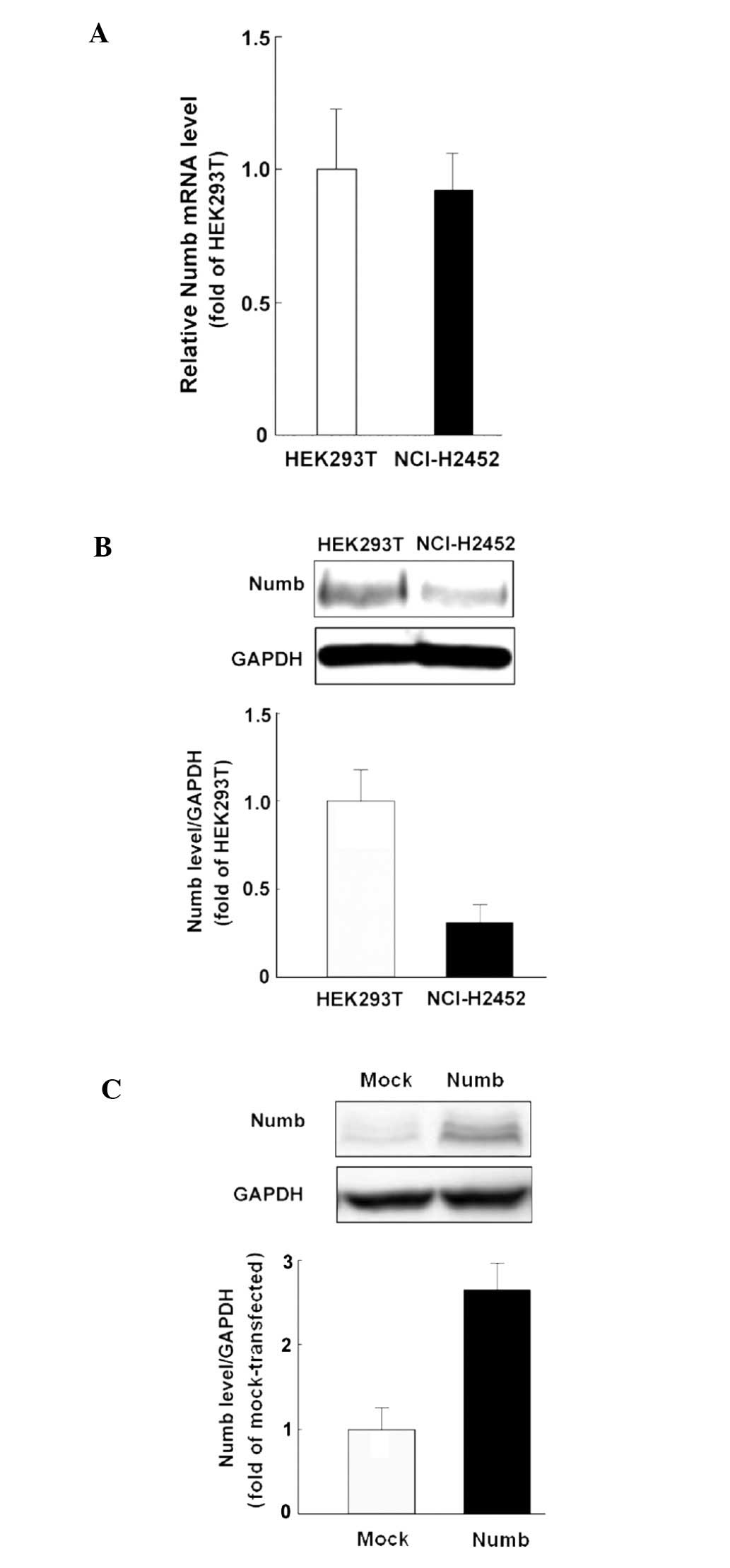
Numb protein expression is increased in
NCI-H2452 cells by transfection
Before transfection, NCI-H2452 cells displayed
levels of Numb mRNA expression comparable to that detected in
HEK-293T cells, but low protein expression was noted (Fig. 3A and B). The NCI-H2452 cells
transfected with pcDNA3.1-Numb showed higher Numb protein
expression compared with the mock-transfected control (Fig. 3C).
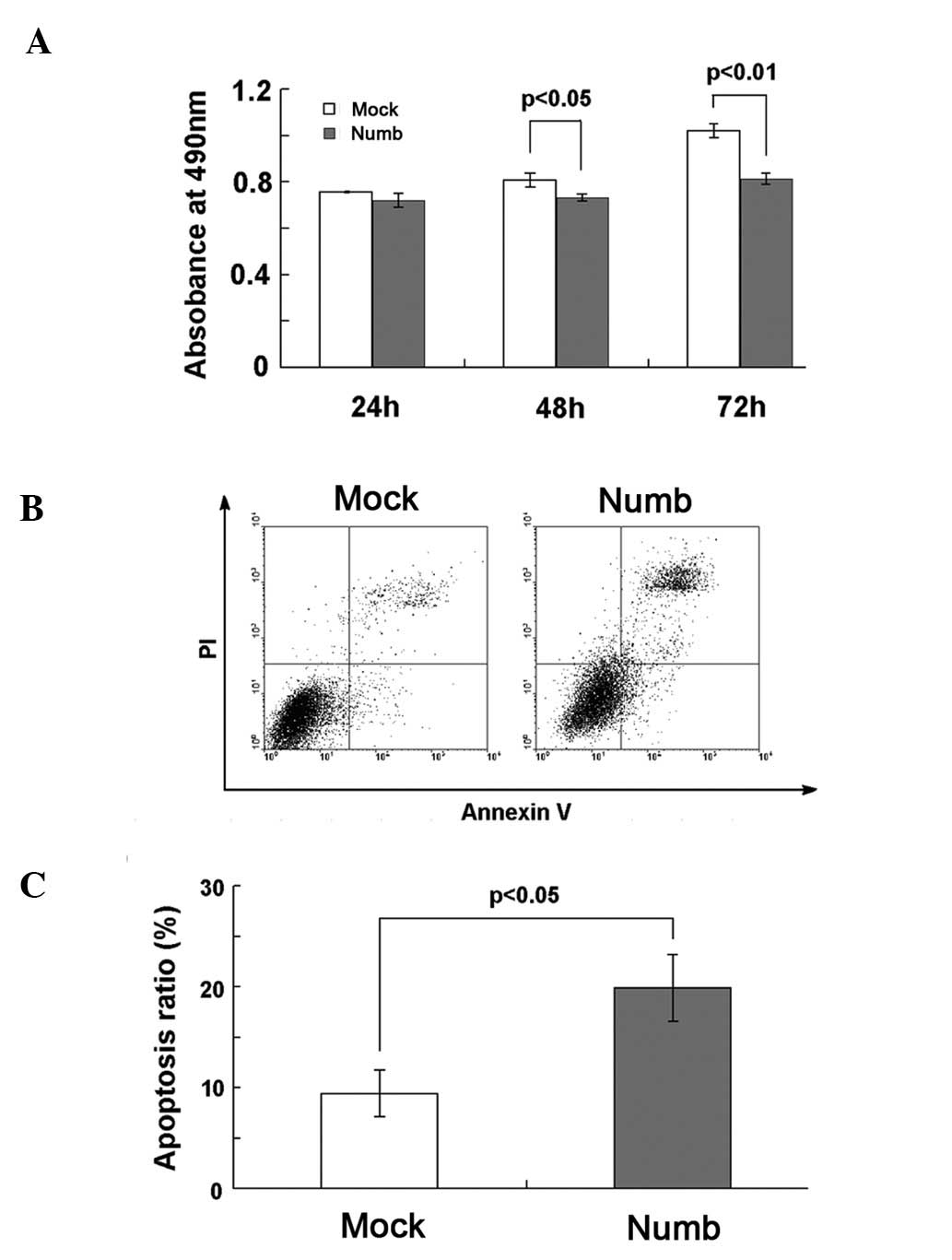
Numb inhibits proliferation and promotes
apoptosis in the NCI-H2452 cells
The absorbance of Numb-transfected cells, as
indicated by MTT, was markedly lower at 48 and 72 h compared to
that of the mock-transfected cells (P<0.05, P<0.01; Fig. 4A), which indicates that Numb
overexpression inhibited the growth of NCI-H2452 cells. There were
no significant differences between the two groups at 24 h
(P>0.05). Apoptosis was investigated at 72 h after transfection.
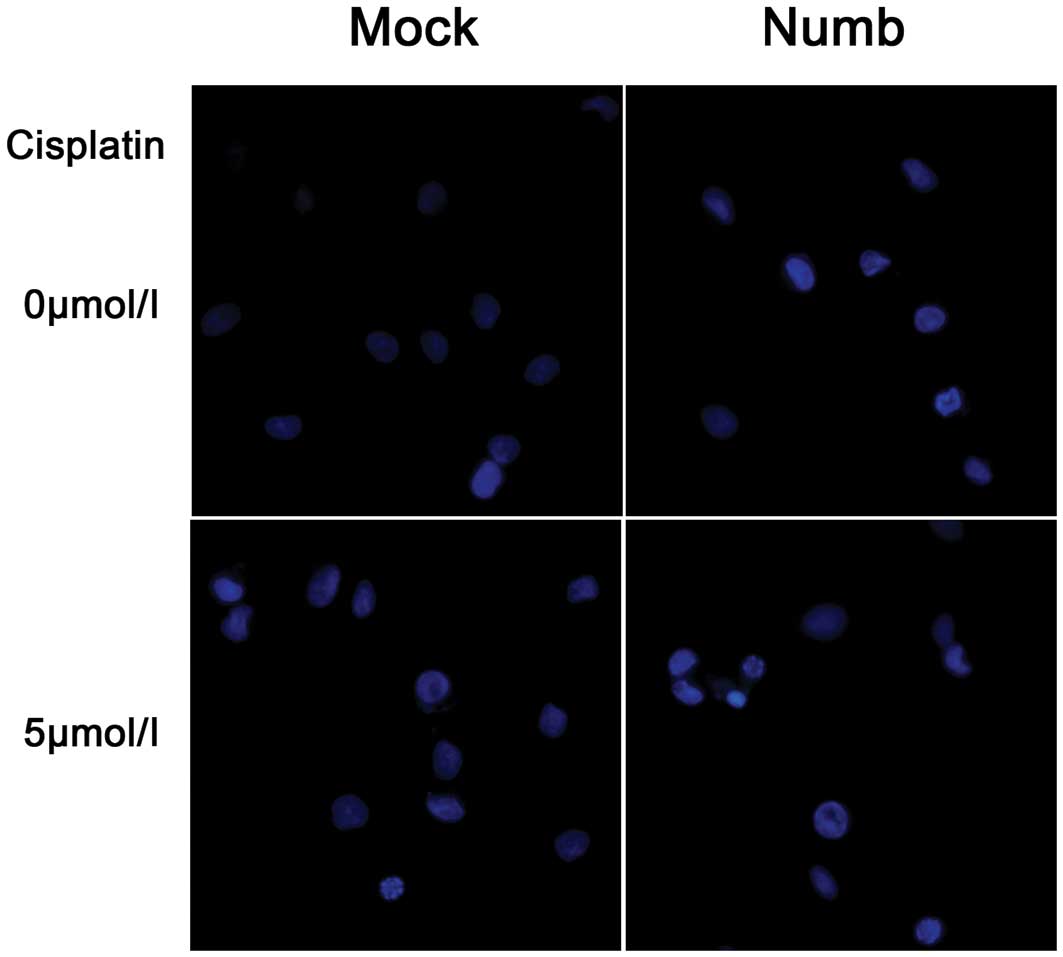
Hoechst staining showed that Numb overexpression increased the
number of apoptotic cells, which were characterized by several
unique morphological nuclear changes, such as chromatin
condensation and nuclear fragmentation (Fig. 5). The apoptosis rate was assayed
using flow cytometry. The percentage of early and late apoptotic
cells was notably higher in the Numb-transfected (19.88±3.31%) when
compared to the percentage in the mock-transfected cells
(9.41±2.34%, P<0.05; Fig. 4B and
C).
Overexpression of Numb induces activation
of caspase-3 and caspase-9, as well as cytochrome c release and
XIAP and survivin degradation
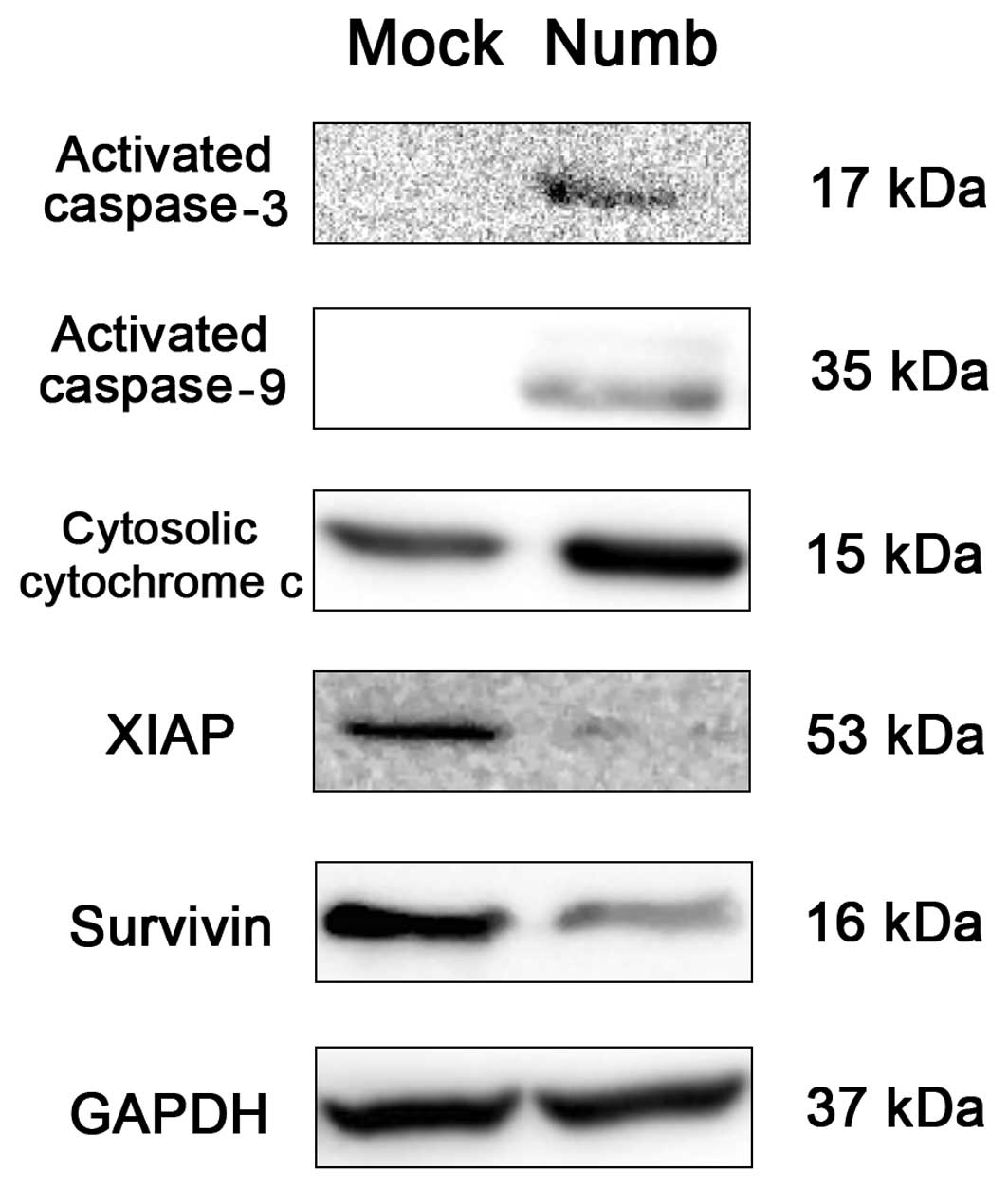
At 48–72 h after transfection, we analyzed various
proteins related to apoptosis in the NCI-H2452 cells by western
blot analysis. Numb-transfected cells showed increased activated
caspase-3 (17 kDa) and caspase-9 (35 kDa) bands compared with the
mock-transfected cells. In addition, we found release of cytochrome
c into the cytoplasm as well as downregulated levels of XIAP
and survivin in the Numb-transfected cells (Fig. 6).
Numb sensitizes NCI-H2452 cells to
cisplatin
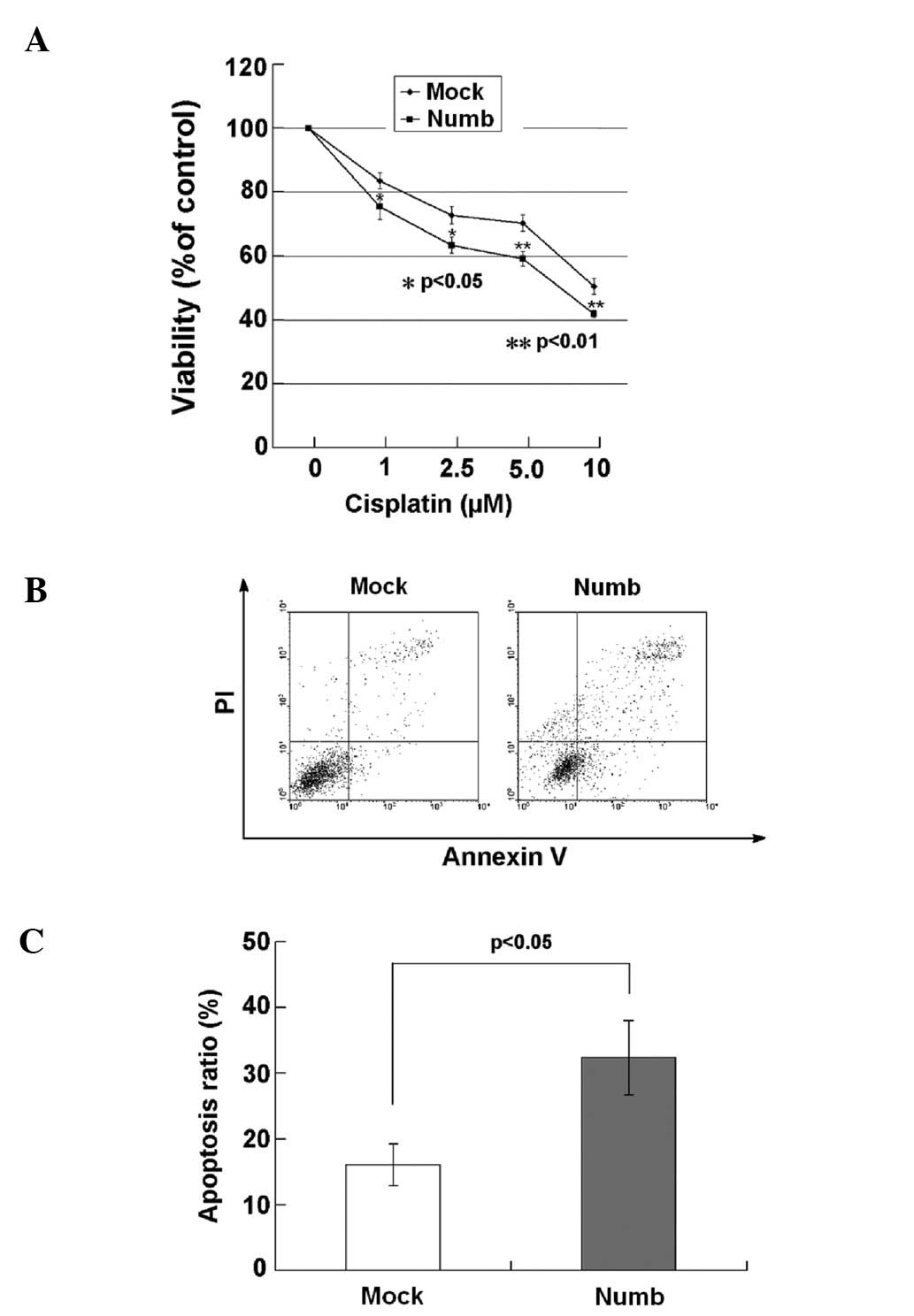
MTT assay was used to determine the cell growth and
proliferation of the NCI-H2452 cells after treatment with cisplatin
for 24 h. Significant decreases in cell viability were observed in
Numb-transfected cells treated with cisplatin at concentrations of
1–10 μmol/l for 24 h compared with the mock-transfected cells
(P<0.05, P<0.01; Fig. 7A). We
next sought to determine whether Numb plays a role in sensitizing
NCI-H2452 cells to cisplatin-induced apoptosis. The apoptosis rate
of the cells was assayed using flow cytometry after treatment with
5 μmol/l cisplatin for 24 h. The percentage of early and late
apoptotic cells was notably higher in the Numb-transfected cells
(32.33±5.64%) when compared with the percentage in the
mock-transfected cells (16.04±3.20%; P<0.05) (Fig. 7B and C).
Discussion
In the present study, we reported the role of Numb
as a tumor suppressor in epithelioid MPM, and revealed that it
enhances the sensitivity of the human MPM cell line NCI-H2452 to
cisplatin.
Our data demonstrated that Numb staining in normal
pleural specimens was intense, but in tumors it was weak and in
many cases completely absent. In accordance with the present study,
the expression of Numb was found to be completely lost in breast
cancers and non-small cell lung carcinomas (NSCLCs) (20,21).
In addition, Numb expression was inversely correlated with the
Ki-67 labeling index, an indicator of tumor aggressiveness. This
finding is consistent with a study concerning breast cancer
(20). High Ki-67 index is the
marker of rapid cell proliferation which is related to high
malignant potential, high recurrence rate and poor survival
(28). This suggests that loss of
Numb expression is involved in the formation and development of
epithelioid MPM. Negative Numb immunohistochemistry with poor
prognosis was previously reported in human salivary gland
carcinomas and breast tumors (12,24).
Our data also showed that the reduction in Numb expression was
associated with poor long term outcome in epithelioid MPM patients.
Numb plays an important role in tumorigenesis due to its crucial
feature of asymmetric cellular distribution. Moreover, due to its
ability to function as an adaptor for E3-enzymes, Numb is involved
in a complex network of ubiquitin-regulated pathways which are
related to the development of cancer. Numb does not live up to its
name as ‘fate determinant’.
We selected NCI-H2452, an epithelioid human MPM cell
line to investigate the expression and function of Numb in
vitro. We detected comparable levels of Numb mRNA in NCI-H2452
cells vs. HEK-293T cells, and this contrasted with the markedly
different levels of Numb protein in the same cultures. Thus, we
speculated that loss of Numb expression in NCI-H2452 cells is
determined at the posttranslational level, through enhanced protein
degradation, similarly to what was shown in breast cancers
(20) and NSCLCs (21), but the exact mechanism needs to be
further explored.
In Numb-negative breast tumor cells, re-expression
of Numb was found to selectively suppress growth (20). Our results also showed that
overexpression of Numb inhibits proliferation and promotes
apoptosis in NCI-H2452 cells. Malignant cells are frequently
resistant to apoptotic stimuli, and the inhibition of apoptosis may
enhance tumor development. Caspase-3 activity is often used as a
definite marker for apoptosis, and cytochrome c/caspase-9
activation is an initial event in the intrinsic pathway for
apoptosis. In the present study, activated caspase-3 and caspase-9
bands as well as release of cytochrome c to the cytoplasm
were observed in Numb-transfected cells. This suggests that
overexpression of Numb induced apoptosis perhaps through cytochrome
c/caspase-9/caspase-3 signaling. Inhibitors of apoptosis
proteins, IAPs, are a family of proteins that regulate the
cytochrome c/caspase activating pathway (29). XIAP is involved in the inactivation
of caspase-3, -7 and -9 and has been found to be elevated in
mesothelioma (30,31). Several studies have now indicated
that higher levels of XIAP may contribute to chemotherapy
resistance in mesothelioma cells (32,33).
Survivin was also found actively expressed in MPM (32,34)
and it has an important role in maintaining apoptosis resistance.
It can partially block activation of caspases due to its ability to
bind to activated caspases (35,36).
Survivin inhibition increases the rates of both spontaneous and
radiation-induced apoptosis (37)
as well as decreased survival of MPM cells (38). In our study, Numb overexpression
decreased the expression of XIAP and survivin, yet the mechanism in
unknown. It has been shown that IAPs contain a RING finger domain
which confers ubiquitin protease ligase (E3) activity, and these
IAPs having E3 activity are able to catalyze their own
ubiquitination and degradation. As we know, Numb is at the
intersection of a complex network of E3-ligases for it contains a
functional phosphotyrosine-binding (PTB) domain. We hypothesized
that Numb downregulates IAPs directly through promoting
ubiquitination and protease degradation, but further study is
needed to explore the exact mechanism. However, we speculate that
IAPs play important roles in the apoptosis promoted by Numb.
Overexpression of Numb to downregulate IAPs may be an important
strategy for overcoming chemotherapy and apoptosis resistance in
MPM.
The standard first-line treatment used for MPM is
cisplatin-based chemotherapy, either as a single agent or in
combination with pemetrexed. Its cytotoxicity is mediated through
platinum-DNA adducts and subsequent induction of apoptosis
(39). However, in MPM the
chemotherapy resistance leads to low efficacy and high doses of
cisplatin cause prohibitive toxicity such as nephrotoxicity
(40). In the present study, we
showed that overexpression of Numb significantly enhanced
sensitivity to cisplatin-induced apoptosis and suppressed growth in
NCI-H2452 cells. These data indicate that Numb may be involved in
conferring cisplatin sensitivity to MPM cells. Decreased caspase-3
and caspase-9 activation have been described as an additional
mechanism of cisplatin resistance in various types of cancers
(41–44). To confirm whether or not Numb
sensitizes MPM cells to cisplatin through the caspase-dependent
apoptotic pathway, further investigation is needed.
In conclusion, our results revealed that Numb was
frequently downregulated in epithelioid MPM cases, which was also
associated with poor prognosis. Overexpression of Numb in NCI-H2452
cells inhibited proliferation and promoted apoptosis, and we
speculated that cytochrome c/caspase signaling was a
possible mechanism through which Numb enhanced apoptosis. Moreover,
Numb sensitized NCI-H2452 cells to cisplatin. These results have
important implications for Numb in the development of novel
strategies for drug-resistant MPM therapy, and further
investigation is warranted.
Acknowledgements
We thank the doctors from the Department of
Respiratory Medicine and the Department of Pathology of Shandong
Provincial Hospital for their technical support.
References
|
1
|
Peto J, Decarli A, La Vecchia C, Levi F
and Negri E: The European mesothelioma epidemic. Br J Cancer.
79:666–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianchi C and Bianchi T: Malignant
mesothelioma: global incidence and relationship with asbestos. Ind
Health. 45:379–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson BWS, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar
|
|
4
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uemura T, Shepherd S, Ackerman L, Jan LY
and Jan YN: numb, a gene required in determination of cell
fate during sensory organ formation in Drosophila embryos.
Cell. 58:349–360. 1989. View Article : Google Scholar
|
|
6
|
Rhyu MS, Jan LY and Jan YN: Asymmetric
distribution of numb protein during division of the sensory organ
precursor cell confers distinct fates to daughter cells. Cell.
76:477–491. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrison SJ and Spradling AC: Stem cells
and niches: mechanisms that promote stem cell maintenance
throughout life. Cell. 132:598–611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rašin MR, Gazula VR, Breunig JJ, et al:
Numb and Numbl are required for maintenance of cadherin-based
adhesion and polarity of neural progenitors. Nat Neurosci.
10:819–827. 2007.PubMed/NCBI
|
|
9
|
Nishimura T and Kaibuchi K: Numb controls
integrin endocytosis for directional cell migration with aPKC and
PAR-3. Dev Cell. 13:15–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGill MA and McGlade CJ: Mammalian numb
proteins promote Notch1 receptor ubiquitination and degradation of
the Notch1 intracellular domain. J Biol Chem. 278:23196–23203.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Marcotullio L, Ferretti E, Greco A, et
al: Numb is a suppressor of Hedgehog signalling and targets Gli1
for Itch-dependent ubiquitination. Nat Cell Biol. 8:1415–1423.
2006.PubMed/NCBI
|
|
12
|
Colaluca IN, Tosoni D, Nuciforo P, et al:
NUMB controls p53 tumour suppressor activity. Nature. 451:76–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pece S, Confalonieri S, Romano PR and Di
Fiore PP: NUMB-ing down cancer by more than just a NOTCH. Biochim
Biophys Acta. 1815:26–43. 2011.PubMed/NCBI
|
|
14
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lanzetti L and Di Fiore PP: Endocytosis
and cancer: an ‘insider’ network with dangerous liaisons. Traffic.
9:2011–2021. 2008.
|
|
17
|
Vaccari T and Bilder D: At the crossroads
of polarity, proliferation and apoptosis: the use of
Drosophila to unravel the multifaceted role of endocytosis
in tumor suppression. Mol Oncol. 3:354–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldstein B and Macara IG: The PAR
proteins: fundamental players in animal cell polarization. Dev
Cell. 13:609–622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aranda V, Nolan ME and Muthuswamy SK: Par
complex in cancer: a regulator of normal cell polarity joins the
dark side. Oncogene. 27:6878–6887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pece S, Serresi M, Santolini E, et al:
Loss of negative regulation by Numb over Notch is relevant to human
breast carcinogenesis. J Cell Biol. 167:215–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Westhoff B, Colaluca IN, D’Ario G, et al:
Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci
USA. 106:22293–22298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caussinus E and Gonzalez C: Induction of
tumor growth by altered stem-cell asymmetric division in
Drosophila melanogaster. Nat Genet. 37:1125–1129. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bric A, Miething C, Bialucha CU, et al:
Functional identification of tumor-suppressor genes through an in
vivo RNA interference screen in a mouse lymphoma model. Cancer
Cell. 16:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maiorano E, Favia G, Pece S, et al:
Prognostic implications of NUMB immunoreactivity in salivary gland
carcinomas. Int J Immunopathol Pharmacol. 20:779–789.
2007.PubMed/NCBI
|
|
25
|
Marino S: Medulloblastoma: developmental
mechanisms out of control. Trends Mol Med. 11:17–22. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan B, Omar FM, Das K, et al:
Characterization of Numb expression in astrocytomas.
Neuropathology. 28:479–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Travis W, Brambilla E, Muller-Hermelink H
and Harris C; World Health Organization Classification of Tumours.
Pathology & Genetics. Tumours of the Lung, Pleura, Thymus and
Heart. IARC Press; Lyon: 2004
|
|
28
|
Heidebrecht H, Buck F, Endl E, Kruse M,
Adam K and Andresen K: Ki-67-Mcm6, a new MoAb specific to Mcm6:
comparison of the distribution profile of Mcm6 and Ki-67 antigen.
Lab Invest. 81:1163–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleinberg L, Lie AK, Flørenes VA, Nesland
JM and Davidson B: Expression of inhibitor-of-apoptosis protein
family members in malignant mesothelioma. Hum Pathol. 38:986–994.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu M, Yuan S, Szporn AH, Gan L, Shtilbans
V and Burstein DE: Immunocytochemical detection of XIAP in body
cavity effusions and washes. Mod Pathol. 18:1618–1622.
2005.PubMed/NCBI
|
|
32
|
Gordon G, Mani M, Mukhopadhyay L, et al:
Expression patterns of inhibitor of apoptosis proteins in malignant
pleural mesothelioma. J Pathol. 211:447–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gordon G, Mani M, Mukhopadhyay L, et al:
Inhibitor of apoptosis proteins are regulated by tumour necrosis
factor-α in malignant pleural mesothelioma. J Pathol. 211:439–446.
2007.
|
|
34
|
Hmeljak J, Erčulj N, Dolžan V, Kern I and
Cör A: BIRC5 promoter SNPs do not affect nuclear survivin
expression and survival of malignant pleural mesothelioma patients.
J Cancer Res Clin Oncol. 137:1641–1651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|
|
36
|
Kobayashi K, Hatano M, Otaki M, Ogasawara
T and Tokuhisa T: Expression of a murine homologue of the inhibitor
of apoptosis protein is related to cell proliferation. Proc Natl
Acad Sci USA. 96:1457–1462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KW, Mutter RW, Willey CD, et al:
Inhibition of survivin and aurora B kinase sensitizes mesothelioma
cells by enhancing mitotic arrests. Int J Radiat Oncol Biol Phys.
67:1519–1525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia C, Xu Z, Yuan X, et al: Induction of
apoptosis in mesothelioma cells by antisurvivin oligonucleotides.
Mol Cancer Ther. 1:687–694. 2002.PubMed/NCBI
|
|
39
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level (Review). Oncol Rep.
10:663–682. 2003.PubMed/NCBI
|
|
41
|
Ikuta K, Takemura K, Kihara M, et al:
Defects in apoptotic signal transduction in cisplatin-resistant
non-small cell lung cancer cells. Oncol Rep. 13:1229–1234.
2005.PubMed/NCBI
|
|
42
|
Okouoyo S, Herzer K, Ucur E, et al: Rescue
of death receptor and mitochondrial apoptosis signaling in
resistant human NSCLC in vivo. Int J Cancer. 108:580–587. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang X, Zheng F, Xing H, et al: Resistance
to chemotherapy-induced apoptosis via decreased caspase-3 activity
and overexpression of antiapoptotic proteins in ovarian cancer. J
Cancer Res Clin Oncol. 130:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mueller T, Voigt W, Simon H, et al:
Failure of activation of caspase-9 induces a higher threshold for
apoptosis and cisplatin resistance in testicular cancer. Cancer
Res. 63:513–521. 2003.PubMed/NCBI
|