Introduction
Glioblastoma multiforme (GBM) is one of the most
common and malignant central nervous system (CNS) tumors in humans.
Median survival of patients with GBM is usually less than 1 year
from the time of diagnosis, and most patients die within 2 years
even in the most favorable circumstances (1–3).
Despite recent advances in diagnostics and clinical management
regimens, the prognosis for patients suffering from malignant
glioma remains very poor (4,5). One
important reason for treatment failure is the uncontrollable
invasion and migration of glioma cells, which ultimately leads to
diffuse growth and recurrence of the tumor. Therefore, new
therapeutic strategies that effectively control the invasion and
migration behavior of glioma cells are urgently required.
The Arp2/3 complex contains seven-subunit proteins
and plays a major role in the regulation of the actin cytoskeleton.
It consists of actin-related protein-2 (ARP2), ARP3, actin-related
protein complex-1 (ARPC1) (p40), ARPC2 (p34), ARPC3 (p21), ARPC4
(p20) and ARPC5 (p16). The activation of the Arp2/3 complex
increases its binding to the sides of actin filaments and induces
the formation of an actin branch, which grows and is considered to
push against the plasma membrane causing lamellipodial protrusions,
which are predicted to be critical for cell motility (6–10).
In mammalian cells, the Arp2/3 complex requires
activation by nucleation promoting factors (NPFs). When engaged by
NPFs, it is activated to initiate the formation of a new (daughter)
filament that emerges from an existing (mother) filament in a
y-branch configuration with a regular 70° branch angle. NPFs are
grouped into 2 categories. Type I NPFs, such as Wiskott-Aldrich
syndrome protein (WASP) and suppressor of cyclic AMP repressor
[SCAR; also known as WASP-family verprolin-homologous protein
(WAVE); there are 3 WAVE homologues, WAVE1, WAVE2 and WAVE3, with
WAVE2 being crucial for lamellipodium formation] directly activate
the Arp2/3 complex by inducing conformational changes in the
complex and supplying the first actin monomer of the new filament.
Type II NPFs, such as cortactin, are weaker NPFs on their own but
potently synergize with type I NPFs (11,12).
In addition, the signal transduction pathways of Rho-family
GTPases, CDC42 and Rac, can activate NPFs (9).
In addition to its role in lamellipodia, the Arp2/3
complex also functions in other important processes. In mouse
oocytes, the Arp2/3 complex takes part in maintenance of asymmetric
meiotic spindle position (13) and
Arp2/3-dependent actin nucleation promotes nuclear movements in the
zygote. Notably, WAVE and Arp2/3 support MT organization in plants
(14). In S. cerevisiae and
S. pombe, inactivating or deleting genes that encode
subunits of the Arp2/3 complex causes severe growth defects or
lethality (6,15,16,18),
while knockdown of Arp2/3 subunits in C. elegans affects
ventral enclosure in the developing worm and results in lethality
(19). Inactivation of Arpc3 in
human HeLa cells by RNAi is lethal (20) and disrupting the activity of the
Arp2/3 complex and its activators in yeasts or mammalian cells can
affect endocytosis (21–25).
Previous data have confirmed the role of the Arp2/3
complex in metastases of non-CNS tumors, and cell migration and
invasion were significantly reduced after Arp2/3 complex disruption
by RNA interference (26).
Moreover, Suraneni et al(27) found that Arp2/3 complex played a
critical role in the lamellipodia extension and directional
fibroblast migration and Mariani et al(28) found that Arp2/3 was upregulated in
migrating glioma cells. However, it is unknown whether the Arp2/3
complex is also functionally important in glioma cells for
lamellipodial protrusions and cell movement. In this study, we
investigated the role of the Arp2/3 complex in the morphology and
motility of glioma cells. We found that inhibition of the Arp2/3
complex by CK666 caused a significant reduction in migration and
invasion of human glioma cells.
Materials and methods
Reagents and specimens
The following reagents were used in this study.
p34-Arc antibody (Millipore, CA, USA), which was specific for the
Arp2/3 complex (in the former research, p34-Arc was usually
mentioned as 1 subunit of ARP2/3 complex, and we did not find any
literature mentioning the freely available p34-Arc); rhodamine
phalloidin (Invitrogen Life Technologies, Carlsbad, CA, USA), used
for actin staining; Alexa Fluor 488 and 555-conjugated secondary
antibodies (Invitrogen Life Technologies); Triton X-100 (Solarbio,
Beijing, China); 4% paraformaldehyde (Solarbio); CK666 (inhibitor
of the Arp2/3 complex) and CK689 (inactive control of CK666) (Merck
KGaA, Darmstadt, Germany), which were dissolved in dimethyl
sulfoxide (DMSO) (Solarbio); DAPI (Sigma, St. Louis, MO, USA); MTT
(Sigma).
Fifty tumor specimens were obtained from patients
with glioma by surgical resection in the Department of
Neurosurgery, Tianjin Medical University General Hospital from July
2011 to December 2012. None of these patients had undergone
radiation or chemotherapy before surgical therapy. The pathological
diagnosis and grading for each glioma was assessed by
neuropathologists according to the 2007 World Health Organization
(WHO) Classification of Nervous System Tumors. Glioma specimens
included 8 cases of pilocytic astrocytoma (WHO grade I), 6 cases of
diffuse astrocytoma (WHO grade II), 8 cases of oligoastrocytoma
(WHO grade II), 10 cases of anaplastic oligodendroglioma (WHO grade
III) and 18 cases of glioblastoma (WHO grade IV). Eight specimens
of non-tumor brain tissue were obtained from patients undergoing
craniotomy for epilepsy. All tissue samples were collected in
accordance with institutional review board-approved protocols.
After surgical resection, tissue specimens were immediately frozen
and stored in liquid nitrogen until use. This study was approved by
the institutional review boards of Tianjin Medical University
General Hospital and written informed consent was obtained from all
patients.
Cell culture
Human glioma cell lines, U251, LN229 and SNB19, were
purchased from the Chinese Academy of Sciences Cell Bank. U251,
LN229 and SNB19 cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS)
(Solarbio), and maintained at 37°C in an atmosphere of 5%
CO2 and routinely passaged at 2–3 day intervals.
Immunohistochemistry assay
Frozen sections were fixed, washed with PBS and
incubated in 5% bovine serum albumin (BSA) to block non-specific
binding, sections were then incubated with p34-Arc antibodies
(rabbit) at a dilution of 1:100 overnight at 4°C, washed in PBS,
and then incubated with Alexa 555-conjugated goat anti-rabbit
secondary antibody at a dilution of 1:1,000 for 1 h at 37°C. Cell
nuclei were stained by DAPI. Immunofluorescence was visualized
using a fluorescence microscope (Olympus DP70, Tokyo, Japan).
Western blot analysis
For each specimen, 50 mg of tissue was broken into
small pieces and transferred into a 1.5 ml microcentrifuge tube. A
total of 500 μl cell lysis buffer was added to the tube. The tissue
was homogenized on ice with 10–15 strokes (3–4 sec/stroke) of a
mini-homogenizer and plastic pestle. The sample was centrifuged at
12,000 × g for 15 min at 4°C and the supernatant then transferred
to a fresh tube. A total of 50 μg protein and an equal volume of 2X
sample buffer were heated at 94°C for 5 min. Proteins were
separated on an 8% sodium dodecyl sulfate-polyacrylamide gel and
then transblotted onto a polyvinylidene difluoride (PVDF) transfer
membrane. The blot was blocked in PBST and 5% skimmed dried milk at
37°C for 1 h. The membrane was then incubated in primary antibody
(p34, rabbit, 1:1,000) at 4°C overnight, followed by treatment with
mouse anti-rabbit secondary antibody (1:5,000). Blots were
developed using enhanced chemiluminescence (ECL) reagents (Amersham
Pharmacia, UK) and visualized using the GeneGenius Imaging System
(Frederick, MD, USA). p34-Arc antibody was used to detect the
expression of Arp2/3 and β-actin was used as the internal
standard.
MTT assay
Briefly, tumor cells (5,000 cells/well) were seeded
in 96-well plates. After 24 h, a different concentration of CK666,
CK689 (50, 100 and 150 μM) or DMSO (0.4, 0.8 and 1.2 μl/ml) was
added to the cells for 0.5–4 h, then followed by a washout. Cells
were then incubated in fresh medium for an additional 48 h. A total
of 20 μl of MTT labeling reagent was then added to each well
containing cells in 150 μl of medium, and cells were incubated for
4 h at 37°C in a CO2 incubator to allow the MTT to be
metabolized. The medium was then removed and 200 μl of DMSO was
added to each well to dissolve the formazan. The absorbance of the
samples was measured at a wavelength of 570 nm by a microplate
reader. Percent viability was calculated relative to DMSO treated
cells.
Confocal microscopy analysis of
F-actin
Glioma cells were grown on glass coverslips for
24–48 h. The cells were preincubated with CK666 (100 μM), CK689
(100 μM) or DMSO (0.8 μl/ml) for 30 min, washed and then fixed with
4% paraformaldehyde for 25 min. Fixed cells were permeabilized by
treatment with 0.5% Triton X-100 for 5 min and blocked by
incubation with 5% BSA in PBS for 1 h. Cells were then incubated
overnight at 4°C with p34-Arc antibodies at a dilution of 1:100.
Cells were washed 3 times with PBS and then incubated for 1 h with
Alexa 488-conjugated goat anti-rabbit secondary antibody at a
dilution of 1:1,000 for 1 h at 37°C. Cells were washed with PBS and
then counterstained with rhodamine phalloidin for 20 min to stain
actin filaments and DAPI to stain DNA. The cells were imaged under
a confocal microscope (Olympus FV1000S, Japan).
Morphological analysis and wound-healing
assays
Glioma cells (1.0×105 cells/ml) were
seeded in 6-well plates and allowed to spread for 24 h. Then cells
in different wells were treated with CK666 (100 μM), CK689 (100 μM)
or DMSO. The changes in morphology were recorded by a microscope
(Olympus, Japan) equipped with a 37°C, 5% CO2 incubator
for a period of 30 min with frames captured every 2 min.
For the wound-healing assay, glioma cells were
seeded in 6-well plates at a density of 2.0×105 cells/ml
and allowed to reach confluency. Before a confluent monolayer was
obtained, cells in different wells were preincubated with CK666
(100 μM), CK689 (100 μM) or DMSO for 30 min, and then wounds were
created using a 200 μl sterile pipette tip. Subsequently, cell
debris was removed by washing the plates twice with PBS and fresh
DMEM supplemented with 3% FBS was added to each well. The cells
were further cultivated for up to 48 h. The wound healing area was
recorded by taking photomicrographs at different time points.
Transwell invasion assay
Glioma cells were preincubated with CK666 (100 μM),
CK689 (100 μM) or DMSO for 30 min, and then seeded in the top
chamber of a Matrigel-coated Boyden chamber (Millipore, USA) at
5.0×104 cells/well without serum. DMEM (600 μl) with 10%
FBS was added to the lower chamber as chemoattractant. Following
incubation for 48 h, non-invading cells were removed from the top
chamber with a cotton swab. The cells on the lower surface were
fixed by replacing the culture medium in the bottom with 4%
formaldehyde. After fixation for 15 min at room temperature, the
chambers were rinsed in PBS and stained with 0.2% crystal violet
for 10 min. For each experimental condition, 10 image fields were
photographed and quantified.
Statistical analysis of data
Statistical analyses were carried out using SPSS
17.0 (Chicago, IL, USA). One-way analysis of variance (ANOVA),
least significant difference and Pearson’s correlation tests were
used. P<0.05 was considered to indicate statistically
significant differences. Values are expressed as means ± standard
deviation (SD). All in vitro experiments were repeated 3
times.
Results
Arp2/3 complex expression in human
gliomas
Hematoxylin and eosin (H&E) staining was
performed to confirm the WHO grading of the tumors. To determine
Arp2/3 complex expression in human gliomas, both immunofluorescence
and western blot analysis were employed. Immunofluorescence of
frozen tissue sections revealed that the Arp2/3 complex was
localized at the cell cytoplasm, and its fluorescence intensity
increased with increasing tumor malignancy (Fig. 1A). Semi-quantitative assessment of
Arp2/3 complex levels by western blot analysis validated the
immunofluorescence results (Fig.
1B). Relative to β-actin, the level of Arp2/3 in tissue
specimens was 15.69±1.04% in non-tumor brain tissue (NB, n=8),
31.17±4.30% in WHO grade I (n=8), 40.51±2.12% in WHO grade II
(n=14), 48.68±2.69% in WHO grade III (n=10) and 55.42±3.45% in WHO
grade IV (n=18) (Fig. 1C). The
expression of Arp2/3 in the glioma specimens was significantly
higher than in non-tumor brain tissue (P<0.05) and there was a
positive correlation between the expression of Arp2/3 and the
malignancy of glioma specimens (r=0.686, P=0.02). These data
indicate that levels of the Arp2/3 complex may be involved in
malignant progression of glioma, including tumor cell migration and
invasion.
Effects of inhibiting the Arp2/3 complex
on cell survival and proliferation
The MTT assay, which is widely used to measure cell
survival and proliferation, is based on reduction of the
tetrazolium salt,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
by actively growing cells to produce a blue formazan product
(29,30). To analyze the effects of the Arp2/3
complex inhibitor, CK666, on survival and proliferation of U251,
LN229 and SNB19 human glioma cells, we performed an MTT assay
(Fig. 2). CK689 and DMSO served as
an inactive compound control and a solvent control, respectively.
Glioma cells were preincubated with CK666, CK689 or DMSO for 0.5–4
h. The viability and proliferation of glioma cells was not clearly
impaired (P<0.05) by treatment with CK666 (50 or 100 μM) for 30
min, but decreased gradually when treated for over 30 min or even
within 30 min when the drug concentration was 150 μM, compared with
inactive and solvent control. This observation demonstrated that
CK666 (50 or 100 μM) treatment for 30 min had no immediate
cytotoxic effect on human glioma cells. In the subsequent
experiment, we chose the higher drug concentration, 100 μM, to
treat the glioma cells.
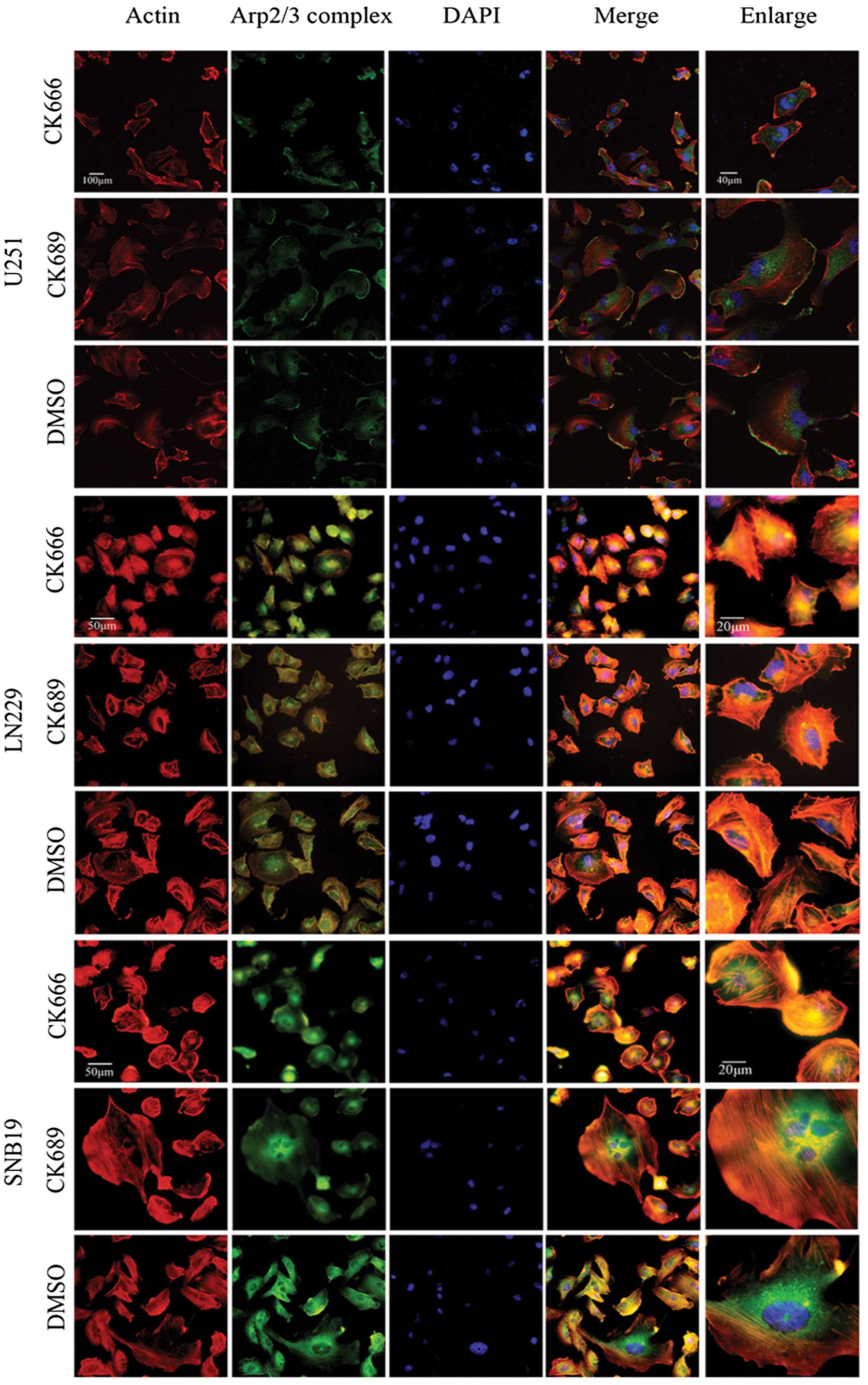
The Arp2/3 complex is localized in
lamellipodia of glioma cells
To determine the localization of the Arp2/3 complex
in lamellipodia, U251, LN229 and SNB19 cells were treated with
CK666 (100 μM), CK689 (100 μM) or DMSO (0.8 μl/ml) for 30 min, then
fixed and stained with rhodamine phalloidin for actin and a p34-Arc
subunit antibody, specific for the Arp2/3 complex (Fig. 3). Phalloidin staining of cells
treated with CK689 or DMSO showed typical lamellipodia, with
F-actin enriched at it. Staining with the anti-p34 antibody
confirmed that the Arp2/3 complex was also localized in the
actin-rich lamellipodia. By contrast, these staining patterns were
absent in CK666-treated cells, suggesting that these protrusions
may be formed through Arp2/3 complex-mediated actin assembly and
that CK666 could inhibit the action of the Arp2/3 complex.
Inhibition of the Arp2/3 complex alters
the morphology of glioma cells
Culture-activated glioma cells developed spreading
lamellipodia and formed stress fibers. When treated with CK666 (100
μM) for 30 min, lamellipodia became increasingly smaller and
finally disappeared (Fig. 4).
Notably, CK666 treatment caused an attenuation of cell polarity. No
such changes were observed in CK689- or DMSO-treated cells. Twelve
hours after the cell culture medium was refreshed with DMEM
supplemented with 10% FBS, cells recovered their morphology (data
not shown). Overall, our results confirmed the obligate role of the
Arp2/3 complex in generating densely branched actin
networks/lamellipodia in human glioma cells.
Inhibition of the Arp2/3 complex reduces
motility of human glioma cells
The wound-healing assay was one of the first methods
to be developed to study cell migration in vitro. Although
not an exact duplication of cell migration in vivo, this
method mimics to some extent migration of cells in wound healing.
To evaluate the inhibitory effect of CK666 on migration, we
performed the assay using human glioma cell lines (Fig. 5). Wound closure was monitored by
capturing photomicrographs at 0 and 48 h after wound creation.
CK666 pretreatment markedly inhibited U251 cell migration to
38.73±3.45% of control, LN229 cells to 57.40±2.16% of control and
SNB19 cells to 34.17±3.82% of control. These data suggested that
inhibition of the Arp2/3 complex by CK666 effectively reduced cell
migration in all 3 human glioma cell lines.
Part of the invasion cascade involves tumor cells
attaching to and penetrating basement membranes. Therefore,
basement membranes are critical barriers to the passage of
disseminating tumor cells. The Transwell chamber with Matrigel has
been used to evaluate the invasive capability of tumor cells
(31). Since both cell migration
and invasion are critical properties for the diffuse growth of
gliomas, we further investigated the role of CK666 in suppressing
tumor cell invasion using the Transwell invasion assay (Fig. 6). CK666 significantly impaired the
invasion of U251, LN229 and SNB19 glioma cells across the Transwell
chamber compared with DMSO by 72.70±4.86 (P<0.05), 39.12±8.42
(P<0.05) and 41.41±4.66% (P<0.05), respectively (Fig. 6B).
These data strongly indicate that the Arp2/3 complex
plays an important role in glioma cell migration and invasion, and
that an Arp2/3 complex inhibitor, targeting lamellipodial actin
assembly, is a potential treatment strategy for glioma.
Discussion
In the current study, we investigated the Arp2/3
complex in 3 glioma cell lines using the inhibitor CK666. Our
results demonstrated that the Arp2/3 complex may play an important
role in human glioblastoma progression and suggested that targeting
the Arp2/3 complex may be a potential anticancer strategy to treat
glioma.
Cancer metastasis is the leading cause of mortality
in most cancer patients due to tumor burden and organ dysfunction.
The central, defining process of cancer metastasis is the ability
of tumor cells to mobilize, invade and cross normally
non-permissive tissue barriers. During cancer metastasis, tumor
cells gain the ability to migrate throughout the body, seed and
proliferate in distant organs to establish secondary tumors within
normal tissues (32). Cell
migration away from the site of the primary tumor is a hallmark of
malignant cancer metastasis, often leading to recurrence and the
failure of existing therapies. This is particularly evident in
malignant gliomas, which are the most challenging tumor of the
central nervous system, characterized by an ability to disperse
through normal neural tissue and recur after initial treatment
(33,34). Histological evidence has shown that
glioma cell dispersal in the brain occurs along preferential
patterns, in many cases following the orientation of thin,
elongated anatomical structures, such as capillaries, white matter
fibers, and unmyelinated axons (35,36).
The biological features of aggressive infiltration
into adjacent tissues generally make glioblastoma incurable, even
in the absence of overt metastasis. Complete resection is virtually
impossible due to the infiltrative nature of the disease (37,38),
while conventional adjuvant therapies such as chemotherapy and
radiotherapy may in fact trigger glioma cell dispersion (39,40).
In addition, motile glioma cells are also more resistant than
non-motile cells to apoptotic stimuli (30,41).
Gliomas produce few metastases in extracranial organs, which is
different from tumors in other organs that are prone to distant
metastasis. If the control of migration/invasion of glioma cells
can be realized, it may open the door for other directed therapies
against an anatomically-restricted neoplasia.
Over the past decades, marked progress has been made
in our understanding of the mechanisms of cell migration. In
general, cell migration involves leading edge protrusion (42,43)
and adhesion to the extracellular matrix (ECM), cell body
translocation, and posterior retraction (6,21,44).
In the migrating cell, the leading edge contains 2 types of actin
structure: branched networks of actin filaments that form
lamellipodia and parallel bundles that form filopodia (45). Lamellipodia are sheet-like
protrusions that contain a distinctive, extensively branched
network of actin filaments and they are the main cellular engine
for locomotion. Filopodia, meanwhile, are believed to be sensory
and guidance organelles, responsible for ‘intelligent’ cell
behavior (46,47). In lamellipodia, activation of the
Arp2/3 complex nucleates new filaments on the side of preexisting
F-actin filaments. The barbed ends of the actin filaments in this
dendritic network push against the membrane at the leading edge and
generate protrusive force by polymerization (48–50).
Arp2/3 mRNA and protein levels, together with those
of N-WASP, WAVE2 and other factors that are functionally associated
with cell motility, are upregulated in some tumor tissues and
invasive cells. Breast cancer cells expressing both WAVE2 and the
Arp2/3 complex frequently appear at the invasive front, and
coexpression of both has been shown to be correlated with poor
clinical outcome (12). In this
study, we detected Arp2/3 complex expression in human glioma
tissues (WHO grade I–IV) and non-tumor brain tissues by
immunofluorescent staining and western blot analysis. The
expression level of the Arp2/3 complex was higher in gliomas than
in non-tumor brain tissues and this elevation was strongly
correlated with the tumor grades. Gliomas of higher grade are
generally easier dispersed through normal neural tissue and have
more opportunities to recur after initial treatment, thus patients
with higher-grade gliomas often have a poor prognosis. This may be
ascribed to glioma cells of higher-grade that have stronger
capability of motility. However, lamellipodia play a critical role
in the cell movement and the Arp2/3 complex mediates the formation
of lamellipodia. We hypothesized that Arp2/3 complex is closely
related to the cell motility and further selected 3 human glioma
cell lines to determine whether the Arp2/3 complex was functionally
important for cell migration in glioma cells. We first found the
Arp2/3 complex is localized to lamellipodia of glioma cells through
the immunofluorescence.
The subsequent steps in this study were designed to
investigate whether lamellipodia and actin formation could be
suppressed if we treated glioma cells with an Arp2/3 complex
inhibitor and how migration and invasion of glioma cells was
consequently affected. Wu et al(51) microinjected bovine Arp2/3 complex
into Arp2/3-inhibited cells, and observed the reappearance of
lamellipodia within 20 min after injection. Their experiment
supplied evidence that the lack of lamellipodia was solely due to
the absence of Arp2/3 activity. The inhibitor of Arp2/3 complex
mentioned for the first time is CK636 and CK548. However, CK666 and
CK869 are more potent inhibitors related to CK636 and CK548. In
particular, CK666 is a better choice to inhibit the Arp2/3 complex,
which binds between Arp2 and Arp3, blocking the formation of an
active conformation (52). Our
study demonstrated that inhibition of Arp2/3 function by CK666 led
to the disappearance of lamellipodia and the significant inhibition
of migration and invasion capability of glioma cells. These results
were consistent with previous findings in melanoblasts (51,53).
The data indicated that CK666 showed moderate effect in U251
migration. However, it showed highest effect in U251 invasion. In
the invasion assay, there were more factors involved in the process
of cell movement except the lamellipodia, such as matrix
metalloproteinases (MMPs). MMPs may have a more important role in
the LN229 and SNB19 invasion assay than in the U251 invasion assay.
This required further investigation. In addition to the effects of
CK666 on lamellipodia, we also found it affected the polarization
of glioma cells, which is also a requirement for cellular movement
(43,54,55).
In summary, the Arp2/3 complex is known to play a
crucial role in the formation of lamellipodia. Our data show that
the Arp2/3 complex is overexpressed in human gliomas and is
involved in the regulation of glioma cell morphology and motility.
Inhibition of Arp2/3 reduced the migration rate and altered the
morphology of glioma cells. These findings encourage us to further
evaluate the role of the Arp2/3 complex in glioma cell migration
and provide a basis for developing Arp2/3 complex therapeutic
targets to inhibit glioma cell dissemination. In future studies, we
will further assess the role of Arp2/3 complex activators, such as
WAVE, in the motility of glioma cells, not only in vitro but
also in vivo. Cell motility in vivo may also involve
invadopodia, which are actin-rich membrane protrusions formed by
invasive cancer cells and mediate the focal degradation of
pericellular ECM by the localized proteolytic activity of MMPs.
However, actin polymerization in invadopodia is also dependent on
Arp2/3 (56,57). Targeting motility may improve
therapy of glioma by preventing further infiltration and expansion
into normal brain tissues.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81272782).
References
|
1
|
Kim KJ, Lee KH, Kim HS, Moon KS, Jung TY,
Jung S and Lee MC: The presence of stem cell marker-expressing
cells is not prognostically significant in glioblastomas.
Neuropathology. 31:494–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30(Suppl 19): 10–14. 2003.
View Article : Google Scholar
|
|
3
|
Peterson K: Brain tumors. Neurol Clin.
19:887–902. 2001. View Article : Google Scholar
|
|
4
|
Sathornsumetee S and Rich JN: New
treatment strategies for malignant gliomas. Expert Rev Anticancer
Ther. 6:1087–1104. 2006. View Article : Google Scholar
|
|
5
|
Sathornsumetee S and Rich JN: Designer
therapies for glioblastoma multiforme. Ann NY Acad Sci.
1142:108–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrell JL, Morphew M and Gould KL: A
mutant of Arp2p causes partial disassembly of the Arp2/3 complex
and loss of cortical actin function in fission yeast. Mol Biol
Cell. 10:4201–4215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollard TD and Cooper JA: Actin, a central
player in cell shape and movement. Science. 326:1208–1212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao G, Simone B and Liu G:
Mis-localization of Arp2 mRNA impairs persistence of directional
cell migration. Exp Cell Res. 317:812–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pollard TD: Regulation of actin filament
assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol
Struct. 36:451–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goley ED and Welch MD: The ARP2/3 complex:
an actin nucleator comes of age. Nat Rev Mol Cell Biol. 7:713–726.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai L, Makhov AM, Schafer DA and Bear JE:
Coronin 1B antagonizes cortactin and remodels Arp2/3-containing
actin branches in lamellipodia. Cell. 134:828–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokotsuka M, Iwaya K, Saito T, Pandiella
A, et al: Overexpression of HER2 signaling to WAVE2-Arp2/3 complex
activates MMP-independent migration in breast cancer. Breast Cancer
Res Treat. 126:311–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi K, Unruh JR, Deng M, Slaughter BD,
Rubinstein B and Li R: Dynamic maintenance of asymmetric meiotic
spindle position through Arp2/3-complex-driven cytoplasmic
streaming in mouse oocytes. Nat Cell Biol. 13:1252–1258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong H, Mohler WA and Soto MC: The
branched actin nucleator Arp2/3 promotes nuclear migrations and
cell polarity in the C. elegans zygote. Dev Biol.
357:356–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lees-Miller JP, Henry G and Helfman DM:
Identification of act2, an essential gene in the fission yeast
Schizosaccharomyces pombe that encodes a protein related to
actin. Proc Natl Acad Sci USA. 89:80–83. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winter D, Podtelejnikov AV, Mann M and Li
R: The complex containing actin-related proteins Arp2 and Arp3 is
required for the motility and integrity of yeast actin patches.
Curr Biol. 7:519–529. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winter DC, Choe EY and Li R: Genetic
dissection of the budding yeast Arp2/3 complex: a comparison of the
in vivo and structural roles of individual subunits. Proc Natl Acad
Sci USA. 96:7288–7293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balasubramanian MK, Feoktistova A,
McCollum D and Gould KL: Fission yeast Sop2p: a novel and
evolutionarily conserved protein that interacts with Arp3p and
modulates profilin function. EMBO J. 15:6426–6437. 1996.PubMed/NCBI
|
|
19
|
Sawa M, Suetsugu S, Sugimoto A, Miki H,
Yamamoto M and Takenawa T: Essential role of the C. elegans
Arp2/3 complex in cell migration during ventral enclosure. J Cell
Sci. 116:1505–1518. 2003.
|
|
20
|
Harborth J, Elbashir SM, Bechert K, Tuschl
T and Weber K: Identification of essential genes in cultured
mammalian cells using small interfering RNAs. J Cell Sci.
114:4557–4565. 2001.PubMed/NCBI
|
|
21
|
Mendoza MC, Er EE, Zhang W, Ballif BA,
Elliott HL, Danuser G and Blenis J: ERK-MAPK drives lamellipodia
protrusion by activating the WAVE2 regulatory complex. Mol Cell.
41:661–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreau V, Galan JM, Devilliers G,
Haguenauer-Tsapis R and Winsor B: The yeast actin-related protein
Arp2p is required for the internalization step of endocytosis. Mol
Biol Cell. 8:1361–1375. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaerer-Brodbeck C and Riezman H:
Functional interactions between the p35 subunit of the Arp2/3
complex and calmodulin in yeast. Mol Biol Cell. 11:1113–1127. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonsdottir GA and Li R: Dynamics of yeast
Myosin I: evidence for a possible role in scission of endocytic
vesicles. Curr Biol. 14:1604–1609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benesch S, Polo S, Lai FP, Anderson KI,
Stradal TE, Wehland J and Rottner K: N-WASP deficiency impairs EGF
internalization and actin assembly at clathrin-coated pits. J Cell
Sci. 118:3103–3115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laurila E, Savinainen K, Kuuselo R, Karhu
R and Kallioniemi A: Characterization of the 7q21–q22 amplicon
identifies ARPC1A, a subunit of the Arp2/3 complex, as a regulator
of cell migration and invasion in pancreatic cancer. Genes
Chromosomes Cancer. 48:330–339. 2009.
|
|
27
|
Suraneni P, Rubinstein B, Unruh JR, Durnin
M, Hanein D and Li R: The Arp2/3 complex is required for
lamellipodia extension and directional fibroblast cell migration. J
Cell Biol. 197:239–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mariani L, Beaudry C, McDonough WS, et al:
Glioma cell motility is associated with reduced transcription of
proapoptotic and proliferation genes: a cDNA microarray analysis. J
Neurooncol. 53:161–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imamura H, Takao S and Aikou T: A modified
invasion-3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium
bromide assay for quantitating tumor cell invasion. Cancer Res.
54:3620–3624. 1994.PubMed/NCBI
|
|
30
|
Berridge MV and Tan AS: Characterization
of the cellular reduction of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT):
subcellular localization, substrate dependence, and involvement of
mitochondrial electron transport in MTT reduction. Arch Biochem
Biophys. 303:474–482. 1993. View Article : Google Scholar
|
|
31
|
Repesh LA: A new in vitro assay for
quantitating tumor cell invasion. Invasion Metastasis. 9:192–208.
1989.PubMed/NCBI
|
|
32
|
Wu TL and Zhou D: Viral delivery for gene
therapy against cell movement in cancer. Adv Drug Deliv Rev.
63:671–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agudelo-Garcia PA, De Jesus JK, Williams
SP, et al: Glioma cell migration on three-dimensional nanofiber
scaffolds is regulated by substrate topography and abolished by
inhibition of STAT3 signaling. Neoplasia. 13:831–840.
2011.PubMed/NCBI
|
|
35
|
Louis DN: Molecular pathology of malignant
gliomas. Annu Rev Pathol. 1:97–117. 2006. View Article : Google Scholar
|
|
36
|
Yu SP, Yang XJ, Zhang B, et al: Enhanced
invasion in vitro and the distribution patterns in vivo of
CD133+ glioma stem cells. Chin Med J (Engl).
124:2599–2604. 2011.PubMed/NCBI
|
|
37
|
Brandes AA, Tosoni A, Franceschi E, Reni
M, Gatta G and Vecht C: Glioblastoma in adults. Crit Rev Oncol
Hematol. 67:139–152. 2008. View Article : Google Scholar
|
|
38
|
Stark AM, van de Bergh J, Hedderich J,
Mehdorn HM and Nabavi A: Glioblastoma: clinical characteristics,
prognostic factors and survival in 492 patients. Clin Neurol
Neurosurg. 114:840–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lamszus K, Kunkel P and Westphal M:
Invasion as limitation to anti-angiogenic glioma therapy. Acta
Neurochir Suppl. 88:169–177. 2003.PubMed/NCBI
|
|
40
|
Zhai GG, Malhotra R, Delaney M, et al:
Radiation enhances the invasive potential of primary glioblastoma
cells via activation of the Rho signaling pathway. J Neurooncol.
76:227–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joy AM, Beaudry CE, Tran NL, Ponce FA,
Holz DR, Demuth T and Berens ME: Migrating glioma cells activate
the PI3-K pathway and display decreased susceptibility to
apoptosis. J Cell Sci. 116:4409–4417. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanisch J, Kolm R, Wozniczka M, Bumann D,
Rottner K and Stradal TE: Activation of a RhoA/myosin II-dependent
but Arp2/3 complex-independent pathway facilitates
Salmonella invasion. Cell Host Microbe. 9:273–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shao D, Levine H and Rappel WJ: Coupling
actin flow, adhesion, and morphology in a computational cell
motility model. Proc Natl Acad Sci USA. 109:6851–6856. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Insall RH and Machesky LM: Actin dynamics
at the leading edge: from simple machinery to complex networks. Dev
Cell. 17:310–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Campellone KG, Webb NJ, Znameroski EA and
Welch MD: WHAMM is an Arp2/3 complex activator that binds
microtubules and functions in ER to Golgi transport. Cell.
134:148–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Svitkina TM and Borisy GG: Arp2/3 complex
and actin depolymerizing factor/cofilin in dendritic organization
and treadmilling of actin filament array in lamellipodia. J Cell
Biol. 145:1009–1026. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vignjevic D and Montagnac G:
Reorganisation of the dendritic actin network during cancer cell
migration and invasion. Semin Cancer Biol. 18:12–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Watanabe N: Inside view of cell locomotion
through single-molecule: fast F-/G-actin cycle and G-actin
regulation of polymer restoration. Proc Jpn Acad Ser B Phys Biol
Sci. 86:62–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mullins RD, Heuser JA and Pollard TD: The
interaction of Arp2/3 complex with actin: nucleation, high affinity
pointed end capping, and formation of branching networks of
filaments. Proc Natl Acad Sci USA. 95:6181–6186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mogilner A and Oster G: Cell motility
driven by actin polymerization. Biophys J. 71:3030–3045. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu C, Asokan SB, Berginski ME, et al:
Arp2/3 is critical for lamellipodia and response to extracellular
matrix cues but is dispensable for chemotaxis. Cell. 148:973–987.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nolen BJ, Tomasevic N, Russell A, et al:
Characterization of two classes of small molecule inhibitors of
Arp2/3 complex. Nature. 406:1031–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li A, Ma Y, Yu X, et al: Rac1 drives
melanoblast organization during mouse development by orchestrating
pseudopod-driven motility and cell-cycle progression. Dev Cell.
21:722–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Verkhovsky AB, Svitkina TM and Borisy GG:
Self-polarization and directional motility of cytoplasm. Curr Biol.
9:11–20. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rottner K and Stradal TE: Actin dynamics
and turnover in cell motility. Curr Opin Cell Biol. 23:569–578.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sibony-Benyamini H and Gil-Henn H:
Invadopodia: the leading force. Eur J Cell Biol. 91:896–901. 2012.
View Article : Google Scholar
|
|
57
|
Yamaguchi H: Pathological roles of
invadopodia in cancer invasion and metastasis. Eur J Cell Biol.
91:902–907. 2012. View Article : Google Scholar : PubMed/NCBI
|