Introduction
In response to various types of stress, cells
activate a highly conserved heat shock response in which a set of
heat shock proteins (Hsps) are induced. These proteins play
important roles in cellular repair and protective mechanisms. There
are 2 types of Hsps, i.e., stress-inducible and constitutive types.
In addition, it is well known that Hsps act as molecular chaperones
to maintain the homeostasis of organisms (1). Recently, much information has become
available on the specific role of individual Hsps. In particular,
there are many significant reports regarding Hsp70 family member
proteins that are closely involved in cell death. It has been
reported that Hsp70 inhibits apoptosis by hindering the activation
of JNK (2), or by preventing
recruitment of procaspase-9 to the Apaf-1 apoptosome (3). In contrast, Hsp70 promotes apoptosis.
For example, overexpression of Hsp70 was found to enhance TCR and
fas-mediated apoptotic cell death (4). Among the Hsp70 family, Hsp70 and the
constitutively expressed 73-kDa heat shock cognate protein (Hsc)70
have been found to be located in the cytosol and to migrate to the
nucleus after specific stress (5),
but are also expressed on the cell surface and interact with
various types of receptors (6–8). It
has also been reported that Hsps such as Hsp70 and Hsc70 are
expressed in the glycosphingolipid-enriched microdomain (GEM) on
the cell surface (9,10).
Sialic acid-binding lectin (SBL) isolated from
Rana catesbeiana oocytes was identified as a lectin, since
SBL agglutinated certain types of tumor cells and the agglutination
was inhibited by glycoprotein or ganglioside-containing sialic acid
(11–13). Agglutination induced by SBL was
observed only in tumor cells but not in normal red blood cells and
fibroblasts (13). The amino acid
sequence of SBL shows that it has homology to members of the RNase
A superfamily, and it has been revealed that SBL has pyrimidine
base-specific ribonuclease activity (14–17).
The antitumor effect of SBL was reported using P388 and L1210
murine leukemia cells in vitro and sarcoma 180 cells and
Ehrlich and Mep 2 ascites cells in vivo(18–20).
We recently reported that SBL had a cytotoxic effect on various
leukemia cells including MDR cells and showed that this
cytotoxicity was induced through multiple apoptotic signaling
pathways (21). Furthermore, data
indicate that the SBL receptor (SBLR) may exist in the GEM on the
cell surface (Tatsuta et al, unpublished data). In the
present study, we investigated the involvement of Hsps in
SBL-induced apoptosis, focusing on Hsp70 and Hsc70 that have been
reported to exist in the GEM on the cell surface.
Materials and methods
Materials
SBL was isolated by sequential chromatography on
Sephadex G-75, DEAE-cellulose, hydroxyapatite and SP-Sepharose as
described previously (13). The
anti-SBL antibody was established in our laboratory. The
anti-caspase-3 antibody was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The anti-Hsp70 and Hsc70 antibodies were purchased
from Stressgen (Kampenhout, Belgium). The horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG antibody and the fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit antibody were
purchased from Cedarlane (Hornby, Ontario, Canada). Quercetin was
from Cayman Chemical Company (Ann Arbor, MI, USA).
Cell culture
Mouse leukemia P388 cells were obtained from the
Cell Resource Center of Biomedical Research, Institute of
Development, Aging and Cancer, Tohoku University (Sendai, Japan).
Cells were routinely maintained in RPMI-1640 medium (Nissui
Pharmaceutical Co. Ltd., Tokyo, Japan) supplemented with 10% fetal
calf serum (FCS), penicillin (100 U/ml) and streptomycin (100
μg/ml) at 37°C in a 95% air and 5% CO2 atmosphere.
Detection of DNA fragmentation
The cells (2×105 cells/ml) were cultured
in 96-well plates (100 μl/well). After treatment with SBL, the
cells were collected by centrifugation, washed with PBS, then lysed
with cell lysis buffer [50 mM Tris-HCl (pH 6.8), 10 mM EDTA, 0.5%
(w/v) sodium N-lauroylsarcosinate]. The samples were incubated with
RNase A (final concentration, 500 μg/ml) for 30 min at 50°C, before
being digested with proteinase K (final concentration, 500 μg/ml)
for 30 min at 50°C. After the samples were electrophoresed on 1.8%
agarose gel, DNA bands were visualized by ethidium bromide (EtBr)
staining.
Western blotting
Whole cell lysate was prepared with extraction
buffer [10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 5 mM
EDTA (pH 8.0), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1
tablet/10 ml protease inhibitor cocktail (Roche Applied Science,
Indianapolis, IN, USA)]. Lysates from the membrane, cytosol and
nuclear fractions were prepared by ProteoExtract Subcellular
Proteome Extraction kit (Merck Millipore, Billerica, MA, USA).
Soluble proteins were collected, and concentrations were measured
by the DC protein assay kit (Bio-Rad, Richmond, CA, USA) in
accordance with the manufacturer’s instructions. Proteins were
separated by SDS-PAGE and transferred to a polyvinylidene
difluoride (PVDF) membrane (GE Healthcare, Little Chalfont, UK).
The membrane was blocked using 5% fat-free skim milk for 1 h. After
the membrane was washed with TBST [20 mM Tris-HCl (pH 7.6), 137 mM
NaCl, 0.05% Tween-20], primary and secondary antibodies were added
to the membrane, respectively. The proteins on the membrane were
detected using ECL western blotting detection reagents (GE
Healthcare). The intensity of the bands was calculated by Quantity
One software (Bio-Rad).
Flow cytometric analysis of SBLR, Hsp70
and Hsc70
For detection of SBLR, cells were treated with SBL
for 30 min at 4°C, washed with PBS, and incubated with the anti-SBL
antibody for 30 min at 4°C. Cells were washed with PBS, and then
FITC-labeled anti-rabbit IgG was added and incubated for 30 min at
4°C. For detection of Hsp70 and Hsc70, cells were treated with
anti-Hsp70 and anti-Hsc70 antibodies for 30 min at 4°C, washed with
PBS and incubated with FITC-labeled anti-rabbit IgG for 30 min at
4°C, respectively. Fluorescence intensity was determined using a
FACSCalibur flow cytometer (Becton-Dickinson).
Reverse transcription and polymerase
chain reaction (RT-PCR)
Total cellular RNA was isolated from the cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
Reverse transcription (RT) was performed using ReverTra Ace
(Toyobo, Osaka, Japan) with total RNA (1 μg) and
oligo(dT)12–18 primers. The RT reaction mixture (1 μl)
was subjected to PCR for 25, 40 and 30 cycles for GAPDH, Hsp70 and
Hsc70, respectively, in a final volume of 20 μl of Taq DNA
polymerase (1.25 U) (ABgene, Epsom, UK) and gene-specific forward
and reverse primers for each gene. After initial denaturation at
94°C for 2 min, each of the cycles consisted of 94°C for 30 sec,
50°C for 30 sec and 72°C for 30 sec. The PCR products were
separated on 1.5% agarose gel, and the bands were visualized with
EtBr staining. The intensity of bands was calculated by Quantity
One software.
Measurement of cell viability
Cell viability was determined by the trypan blue dye
exclusion assay. The cells (2×105 cells/ml) were
cultured with SBL (2 μM) and/or quercetin (5 μM) in 96-well plates.
After treatment with SBL and/or quercetin, the cells were stained
with 0.25% trypan blue, and both viable and nonviable cells were
counted.
Statistical analysis
Each experiment was performed at least in
triplicate. The results are expressed as the means ± standard
deviation. Statistical analysis was performed using unpaired
Student’s t-tests; P<0.05 was considered to indicate a
statistically significant difference.
Results
SBL-induced apoptosis in P388 cells
We recently reported that SBL induces apoptosis in
various leukemia cell lines. In human leukemia Jurkat cells,
typical apoptotic morphological change such as karyorrhexis,
nuclear condensation and fragmentation, or apoptotic biological
changes such as phosphatidylserine (PS) externalization, activation
of caspases, DNA fragmentation were observed after treatment with
SBL (21). In the present study,
the apoptosis-inducing effect of SBL in P388 cells was analyzed by
the detection of activated caspase-3. Caspase-3 activity was
monitored by use of DEVD-pNA. The activity of caspase-3 was
observed and maximized at 6 h of treatment (Fig. 1). As a concequence, SBL-induced
apoptosis in P388 cells and the execution process may start as
early as 6 h.
Expression of SBLR, Hsp70 and Hsc70 on
the P388 cell membrane
It has been suggested that SBL binds to the cell
membrane to exert its antiproliferative effects which indicates the
existence of SBLR. We analyzed the involvement of Hsps in
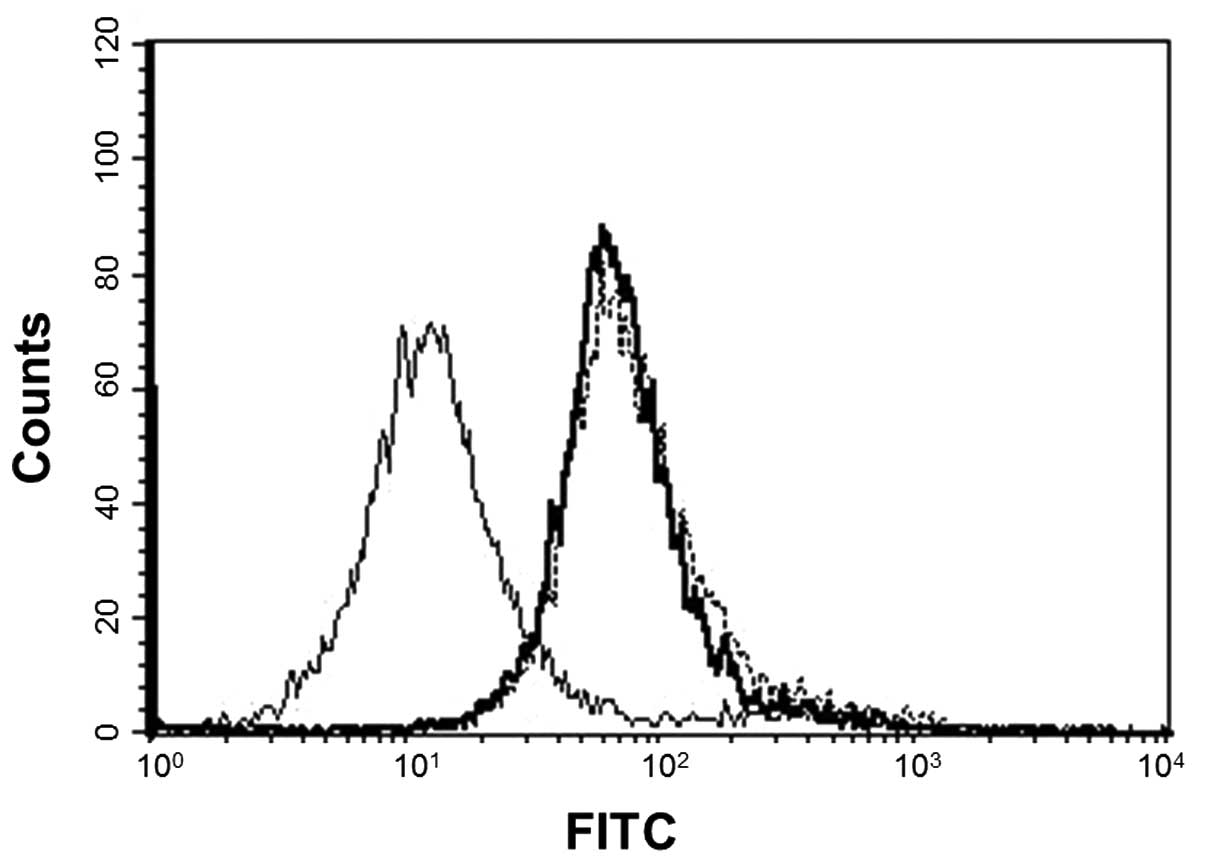
SBL-induced apoptosis. We analyzed the expression of SBLR, Hsp70
and Hsc70 on the cell membrane by flow cytometric analysis. The
results showed that both Hsp70 and Hsc70 were expressed on the cell
membrane as well as SBLR (Fig.
2).
Distribution of Hsp70 and Hsc70 in
SBL-treated P388 cells
Our next experiment was designed to elucidate the
localization of Hsp70 and Hsc70 after stimulation of SBL in P388
cells. After treatment with SBL, the cells were lysed and
fractionated into the membrane, cytosol and nuclear fractions, and
then the amounts of Hsp70 or Hsc70 were analyzed by western
blotting. The results showed that Hsp70 in the membrane fraction at
6 h of treatment was decreased 34% when compared to the 0 h-control
and then gradually increased (Fig.
3A). In the cytosol fraction, both Hsp70 and Hsc70 were
transiently increased at 3 h of treatment, and a transient increase
was also observed in the nuclear fraction at 9 h of treatment
(Fig. 3B and C). After treatment
with SBL, the localization of Hsp70 and Hsc70 was altered when
compared to that in the cells without SBL treatment. Notably,
SBL-induced activation of caspase-3 began to be observed, just
after the amounts of Hsp70 and Hsc70 in the cytosol fraction
reached maximum levels.
Effect of quercetin on expression of
Hsp70 or Hsc70
To clarify the relationship between Hsp70 and Hsc70
in SBL-induced apoptosis, we treated P388 cells with quercetin, a
known bioflavonoid which decreases the expression of Hsp70.
Treatment with quercetin for 12 h resulted in a 60% reduction in
Hsp70 at the mRNA level and a 50% reduction at the protein level
(Fig. 4). By contrast, quercetin
did not affect the expression of Hsc70 at the mRNA or protein
level.
Effect of quercetin on the binding of SBL
to P388 cells
As we confirmed that quercetin decreases the
expression of Hsp70, sialidase treatment of cells was found to
abolish tumor cell agglutination and the antiproliferative effect
induced by SBL, and the existence of SBLR in the GEM on the cell
surface has been suggested (13).
In the present study, we investigated the possible involvement of
Hsp70 and Hsc70 known Hsps which exist on the GEM, in SBL-induced
apoptosis.
We found that Hsp70 and Hsc70 were expressed on the
P388 cell surface as well as SBLR (Fig.
2). Distribution of Hsp70 and Hsc70 was analyzed after
treatment with SBL, and expression of Hsp70 and Hsc70 in the
cytosol was dramatically increased immediately prior to the
execution of apoptosis in SBL-treated P388 cells (Figs. 1 and 3). Next we studied the functional
relationship of Hsps and the cytotoxic activity of SBL by the use
of quercetin. Quercetin was previously found to decrease the
expression of Hsp70 at the mRNA level by inhibiting the activation
of HSF1 (23). The expression of
Hsp70 was decreased at both the mRNA and protein levels in the
quercetin-treated cells (Fig. 4).
We found that binding of SBL to P388 cells was not affected by a
decrease in Hsp70 expression by quercetin. The result indicates
that Hsp70 itself may not be the receptor for SBL (Fig. 5). However, further study revealed
that a decrease in Hsp70 by quercetin inhibited both SBL-induced
cytotoxicity and apoptosis (Fig.
6), suggesting the important role that Hsp70 plays in
SBL-induced apoptosis.
Various reports have indicated a relationship
between Hsps, particularly the Hsp70 family, and various receptors
on the cell surface. It was reported that Hsp70 and Hsc70 possibly
interact with CD14, CD40 and the toll-like receptor family members
(24–26). Notably, Guerrero and Moreno
(27) reported that Hsc70 and
integrin αvβ3 formed a complex in the GEM and act as a receptor for
rotavirus and may participate in the process of adsorption and
penetration of the viruses into cells. In the present study, we
showed that binding of SBL was not affected by a decrease in the
expression of Hsp70, but an attenuated induction of apoptosis was
noted. It is possible that Hsps on the P388 cell surface may
interact with SBLR or participate in the penetration of SBL into
cells, and may affect the cytotoxicity of SBL, as cell
susceptibility to RNase can be affected by binding as well as the
internalization or translocation of RNases as described above.
Since the expression of Hsc70 was not affected by quercetin,
studies to clarify the possible involvement of Hsc70 or other Hsps
in the function of SBL will be undertaken.
In summary, the present study demonstrated that
Hsp70 and Hsc70 are expressed on the P388 cell surface as well as
SBLR, and their expression levels are markedly increased in the
cytosol immediately prior to the execution of apoptosis following
SBL treatment. A functional study of Hsp70 revealed that decreased
expression of Hsp70 suppressed the apoptosis induced by SBL. It is
suggested, for the first time, that Hsps participate in the
antitumor effect of cytotoxic RNases.
Acknowledgements
This study was supported in part by a Grant-in-Aid
from the ‘Academic Frontier’ Project for Private Universities from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan.
References
|
1
|
Snoeckx LH, Cornelussen RN, Van
Nieuwenhoven FA, Reneman RS and Van Der Vusse GJ: Heat shock
proteins and cardiovascular pathophysiology. Physiol Rev.
81:1461–1497. 2001.PubMed/NCBI
|
|
2
|
Mosser DD, Caron AW, Bourget L,
Denis-Larose C and Massie B: Role of the human heat shock protein
hsp70 in protection against stress-induced apoptosis. Mol Cell
Biol. 17:5317–5327. 1997.PubMed/NCBI
|
|
3
|
Beere HM, Wolf BB, Cain K, Mosser DD,
Mahboubi A, Kuwana T, Tailor P, et al: Heat-shock protein 70
inhibits apoptosis by preventing recruitment of procaspase-9 to the
Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liossis SN, Ding XZ, Kiang JG and Tsokos
GC: Overexpression of the heat shock protein 70 enhances the
TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat
T cells. J Immunol. 158:5668–5675. 1997.PubMed/NCBI
|
|
5
|
Welch WJ: Mammalian stress response: cell
physiology, structure/function of stress proteins, and implications
for medicine and disease. Physiol Rev. 72:1063–1081.
1992.PubMed/NCBI
|
|
6
|
Bausero MA, Page DT, Osinaga E and Asea A:
Surface expression of Hsp25 and Hsp72 differentially regulates
tumor growth and metastasis. Tumour Biol. 25:243–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calderwood SK, Mambula SS, Gray PJ Jr and
Theriault JR: Extracellular heat shock proteins in cell signaling.
FEBS Lett. 581:3689–3694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugawara S, Kawano T, Omoto T, Hosono M,
Tatsuta T and Nitta K: Binding of Silurus asotus lectin to
Gb3 on Raji cells causes disappearance of membrane-bound form of
HSP70. Biochim Biophys Acta. 1790:101–109. 2009.
|
|
9
|
Chen S, Bawa D, Besshoh S, Gurd JW and
Brown IR: Association of heat shock proteins and neuronal membrane
components with lipid rafts from the rat brain. J Neurosci Res.
81:522–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Broquet AH, Thomas G, Masliah J, Trugnan G
and Bachelet M: Expression of the molecular chaperone Hsp70 in
detergent-resistant microdomains correlates with its membrane
delivery and release. J Biol Chem. 278:21601–21606. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawauchi H, Sakakibara F and Watanabe K:
Agglutinins of frog eggs: a new class of proteins causing
preferential agglutination of tumor cells. Experientia. 31:364–365.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakakibara F, Kawauchi H, Takayanagi G and
Ise H: Egg lectin of Rana japonica and its receptor
glycoprotein of Ehrlich tumor cells. Cancer Res. 39:1347–1352.
1979.
|
|
13
|
Nitta K, Takayanagi G, Kawauchi H and
Hakomori S: Isolation and characterization of Rana
catesbeiana lectin and demonstration of the lectin-binding
glycoprotein of rodent and human tumor cell membranes. Cancer Res.
47:4877–4883. 1987.PubMed/NCBI
|
|
14
|
Titani K, Takio K, Kuwada M, Nitta K,
Sakakibara F, Kawauchi H, Takayanagi G and Hakomori S: Amino acid
sequence of sialic acid binding lectin from frog (Rana
catesbeiana) eggs. Biochemistry. 26:2189–2194. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamiya Y, Oyama F, Oyama R, Sakakibara F,
Nitta K, Kawauchi H, Takayanagi Y and Titani K: Amino acid sequence
of a lectin from Japanese frog (Rana japonica) eggs. J
Biochem. 108:139–143. 1990.PubMed/NCBI
|
|
16
|
Nitta K, Oyama F, Oyama R, Sekiguchi K,
Kawauchi H, Takayanagi Y, Hakomori S and Titani K: Ribonuclease
activity of sialic acid-binding lectin from Rana catesbeiana
eggs. Glycobiology. 3:37–45. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okabe Y, Katayama N, Iwama M, Watanabe H,
Ohgi K, Irie M, Nitta K, et al: Comparative base specificity,
stability, and lectin activity of two lectins from eggs of Rana
catesbeiana and R. japonica and liver ribonuclease from
R. catesbeiana. J Biochem. 109:786–790. 1991.PubMed/NCBI
|
|
18
|
Nitta K, Ozaki K, Ishikawa M, Furusawa S,
Hosono M, Kawauchi H, Sasaki K, et al: Inhibition of cell
proliferation by Rana catesbeiana and Rana japonica
lectins belonging to the ribonuclease superfamily. Cancer Res.
54:920–927. 1994.PubMed/NCBI
|
|
19
|
Nitta K, Ozaki K, Tsukamoto Y, Furusawa S,
Ohkubo Y, Takimoto H, Murata R, et al: Characterization of a
Rana catesbeiana lectin-resistant mutant of leukemia P388
cells. Cancer Res. 54:928–934. 1994.
|
|
20
|
Nitta K, Ozaki K, Tsukamoto Y, Hosono M,
Ogawakonno Y, Kawauchi H, Takayanagi Y, et al: Catalytic lectin
(leczyme) from bullfrog (Rana catesbeiana) eggs: Mechanism
of tumoricidal activity. Int J Oncol. 9:19–23. 1996.PubMed/NCBI
|
|
21
|
Tatsuta T, Hosono M, Sugawara S, et al:
Sialic acid-binding lectin (leczyme) induces caspase-dependent
apoptosis-mediated mitochondrial perturbation in Jurkat cells. Int
J Oncol. 43:1402–1412. 2013.
|
|
22
|
Haigis MC, Kurten EL and Raines RT:
Ribonuclease inhibitor as an intracellular sentry. Nucleic Acids
Res. 31:1024–1032. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hosokawa N, Hirayoshi K, Kudo H, Takechi
H, Aoike A, Kawai K and Nagata K: Inhibition of the activation of
heat shock factor in vivo and in vitro by flavonoids. Mol Cell
Biol. 12:3490–3498. 1992.PubMed/NCBI
|
|
24
|
Asea A, Kraeft SK, Kurt-Jones EA,
Stevenson MA, Chen LB, Finberg RW, Koo GC and Calderwood SK: HSP70
stimulates cytokine production through a CD14-dependant pathway,
demonstrating its dual role as a chaperone and cytokine. Nat Med.
6:435–442. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker T, Hartl FU and Wieland F: CD40, an
extracellular receptor for binding and uptake of Hsp70-peptide
complexes. J Cell Biol. 158:1277–1285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asea A, Rehli M, Kabingu E, Boch JA, Bare
O, Auron PE, Stevenson MA and Calderwood SK: Novel signal
transduction pathway utilized by extracellular HSP70: role of
toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 277:15028–15034.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guerrero CA and Moreno LP: Rotavirus
receptor proteins Hsc70 and integrin αvβ3 are located in the lipid
microdomains of animal intestinal cells. Acta Virol. 56:63–70.
2012.
|