Introduction
Ovarian cancer is a fatal gynecological cancer and a
major cause of cancer-related mortality worldwide (1). Regrettably, current chemotherapeutics
(platinum/taxane-based drugs) have not markedly prolonged
recurrence-free survival of this deadly disease. Thus, there is an
urgent need for the development of novel treatment strategies
(2).
Rencently, there has been a growing interest in the
use of herbs as a source of new drugs for cancer (3). Pseudolaric acid B (PAB) is a diterpene
acid isolated from the root and trunk bark of Pseudolarix
kaempferi Gordon (Pinaceae), known as ‘Tu-Jin-Pi’
(4). PAB contains a structural
framework that has never been found in any other natural products
including a unique poly-hydroazulene with a trans-substitution
pattern at the junction sites (5).
It has been demonstrated that PAB significantly delayed tumor
growth of a taxol-resistant liver cancer without showing obvious
toxicity to the animals in vivo, and possessed selective
anti-proliferative effects in human cancer cells but not in normal
cells in vitro (5).
Apoptosis, a process of programmed cell death (PCD),
is crucial during development and to maintain homeostasis. However,
dysregulation of this process is implicated in various diseases
including cancer (6,7). Cell death inhibition is a very
successful strategy that cancer cells employ to combat the immune
system and various anticancer therapies (8). Recent evidence has shown that PAB
treatment leads to apoptosis in many cancer cells (9–11).
However, such an effect of PAB on human ovarian cancer cells has
not been reported, and the molecular mechanisms are still not fully
understood.
Several genes critical in the regulation of
apoptosis have been identified, including XIAP, a member of the IAP
family. X-linked inhibitor of apoptosis resistance by effectively
inhibiting caspase-3, -7 and -9 (12), IAP gene amplification and increased
protein expression occur in many types of cancers, and is an
important pathway by which cancer cells acquire resistance to
chemotherapy and radiation therapy (13).
In the present study, we analyzed the effect of PAB
on cell death and apoptosis in ovarian cancer HO-8910 and A2780
cells. The contribution of caspase-3 and -9, XIAP and Bcl-2 family
members, and cytochrome c (cyto c) and apoptotic
protease activating facter-1 (Apaf-1) in PAB-induced cell death was
also investigated.
Materials and methods
Reagents
Monoclonal anti-β-actin antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Anti-p53, anti-Bax, anti-Bcl-xL, anti-Bcl-2, anti-Bid, anti-XIAP,
anti-cIAP1, anti-cIAP2, anti-Smac, anti-survivin, anti-cyto
c and anti-Apaf-1 were obtained from New England Biolabs
(Beverly, MA, USA). Stocks of the selective XIAP inhibitor Embelin
were obtained from Calbiochem Behring (La Jolla, CA, USA).
RPMI-1640 and fetal bovine serum (FBS) were purchased from
Gibco-BRL (Grand Island, NY, USA). An Annexin V apoptosis detection
kit was purchased from R&D Systems (Abingdon, UK). Cell
isolation and tissue culture reagents were obtained from
Invitrogen-Life Technologies (Lidingö, Sweden). All other reagents
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Ovarian cancer cell lines HO-8910 and A2780 were
obtained from China Medical University. The cells were grown in
Roswell Park Memorial Institute (RPMI)-1640 medium supplemented
with 10% fetal calf serum (FCS; Gibco), 50 μg/ml penicillin, 50
μg/ml streptomycin and 10 μg/ml neomycin. The cells were incubated
at 37°C in a humidified CO2 (5%) incubator. HO-8910 and
A2780 cells in 24-well flat-bottomed plates were incubated with PAB
at different concentrations (0, 2, 4 and 8 μmol/l) for 48 h or at a
concentration of 4 μmol/l for 0, 24, 48, 72 and 96 h, respectively.
In some experiments, Embelin (XIAP inhibitor) was used 30 min prior
to PAB induction.
Cell viability
To assess the overall viability of HO-8910 and A2780
cells following PAB treatment, the cells were treated as described
above. At particular time-points, the HO-8910 or A2780 cells were
washed two times with PBS and treated with a 0.4% solution of
trypan blue and visualized as clear cells under a microscope.
HO-8910 and A2780 cells that were no longer viable, which had
damaged membranes that allowed entry of the dye, were stained blue.
Assays were performed in triplicate and repeated at least three
times. The number of intact viable cells was expressed as a
percentage of total cells and was assessed at different
time-points. The percentage of viable cells was calculated as
follows: Viable cells (%) = (total number of viable cells per ml of
aliquot/total number of cells per ml of aliquot) × 100.
Acridine orange staining
Twenty-five microliters of cell suspension
(0.5×106 to 2.0×106 cells/ml) was incubated
with 1 μl of Acridine orange (AO) solution, and mixed gently. Each
sample was mixed just prior to microscopy and quantification. The
cell suspension (10 μl) was placed onto a microscopic slide,
covered with a glass coverslip and at least 500 cells were examined
by fluorescence microscopy using a fluorescein filter.
Hoechst 33258 staining
The cells were stained with Hoechst 33258 (Molecular
Probes Inc., Eugene, OR, USA) at a dilution of 1:600 (stock
solution, 1 mg/ml) for 5 min in the dark. The samples were observed
under a fluorescence microscope. Five hundred cells were counted
from each coverslip in turn, and the results were confirmed by
visualization of the apoptotic nuclei. There were five coverslips
in each group.
Transmission electron microscopy
The cells treated with 0.1 μmol/l paclitaxel were
trypsinized and harvested after 24 h. Subsequently the cells were
fixed in 4% glutaral and immersed in Epon 821, embedded in capsules
and converged for 72 h at 60°C. The cells were then prepared and
placed onto an ultrathin section (60 nm) and stained with uranyl
acetate and lead citrate. Cell morphology was examined by
transmission electron microscopy.
Flow cytometric analysis
The apoptosis rates of HO-8910 and A2780 cells were
quantified by flow cytometry using FITC-conjugated Annexin V and
PI. Specific binding of Annexin V was achieved by incubating
106 cells in 60 μl of binding buffer saturated with
Annexin V for 15 min at 4°C in the dark. To discriminate between
early apoptosis and necrosis, the cells were simultaneously stained
with Annexin V and PI prior to analysis. The binding of Annexin
V-FITC and PI to the cells was measured by flow cytometry
(FACSCalibur; BD Biosciences) using CellQuest software. At least
10,000 cells were counted in each sample. Experiments were
performed and interpreted as follows: cells that were Annexin
V(−)/PI(−) (lower left quadrant) were considered as living cells,
Annexin V(+)/PI(−) cells (lower right quadrant) as apoptotic cells,
Annexin V(+)/PI(+) (upper right quadrant) cells as necrotic or
advanced apoptotic cells, and Annexin V(−)/PI(+) (upper left
quadrant) cells may be bare nuclei, were considered as cells in
late necrosis or cellular debris.
Measurement of cyto c and Apaf-1 release
from mitochondria
Cells were treated with 0.1% DMSO or different
concentrations of PAB (0, 2, 4 and 8 μmol/l) for 48 h. Mitochondria
and the cytosol were separated using a cyto c-releasing
apoptosis assay kit. Cells were suspended in cytosol extraction
buffer. The cell suspension in extraction buffer was homogenized
using a Dounce homogenizer and then centrifuged (700 × g, 10 min)
after 10 min on ice. Then, the collected supernatant was
re-centrifuged (10,000 × g, 30 min, 4°C). The resulting supernatant
(cytosolic fraction) and pellet (mitochondrial fraction) were
processed for western blot analysis.
Western blot analysis
Western blot analysis using rabbit polyclonal
antibody for p53 (1:2,000 dilution), Bcl-2 (1:2,000 dilution),
Bcl-xL (1:2,000 dilution), Bid (1:2,000 dilution), Bax (1:2,000
dilution), XIAP (1:2,000 dilution), cIAP1 (1:2,000 dilution), cIAP2
(1:2,000 dilution), Smac (1:2,000 dilution), Survivin (1:2,000
dilution), cyto c (1:2,000 dilution) and Apaf-1 (1:2,000
dilution) was performed according to standard protocols. β-actin
(1:2,000) was used to control for equal protein loading. The
immunoblots were then washed three times with TBS-T buffer,
incubated with a horseradish peroxidase-conjugated secondary
antibody (goat anti-rabbit IgM; Santa Cruz Biotechnology), and
developed using chemiluminescent substrate (Pierce, Rockford, IL,
USA).
Measurement of caspase-3 and -9
activity
HO-8910 and A2780 apoptotic cells were harvested and
centrifuged at 1,500 rpm for 10 min. Cells were washed two times
with PBS (pH 7.4) and then resuspended with 50 μl lysis buffer at
4°C and incubated on ice for 10 min. All subsequent steps were
performed on ice. After centrifugation, cell extracts were
transferred to fresh tubes, and protein concentrations were
measured. Each 50 μl of cell extract containing 100 μg of protein
was combined with equal volumes of 2X reaction buffer in a
microplate followed by the addition of 5 μl of the peptide
substrates of caspase-3 and -9. After overnight incubation in the
dark at 37°C, samples were read in a microplate reader at 405 nm.
Caspase-3 and -9 activity was evaluated by the absorbance ratio of
treated/control samples. In some experiments, inhibitors for
caspase-3 (Z-DEVD-FMK) or caspase-9 (Z-LEHD-FMK) were added into
fresh medium of HO-8910 or A2780 cells at 1 h before PAB was
added.
Statistical analysis
Each experiment was carried out in duplicate or
triplicate, and three or four independent experiments were
performed. Results are expressed as means ± standard deviation (SD)
and analyzed with SPSS 11.5 software. Results were compared using
analysis of variance (ANOVA). When ANOVA showed a statistically
significant difference, a group-by-group comparison was performed
using a t-test with Tukey’s correction for multiple comparisons.
Statistical significance was set at P<0.05.
Results
Morphologic analysis of apoptotic HO-8910
cells under light microscopy and transmission electron
microscopy
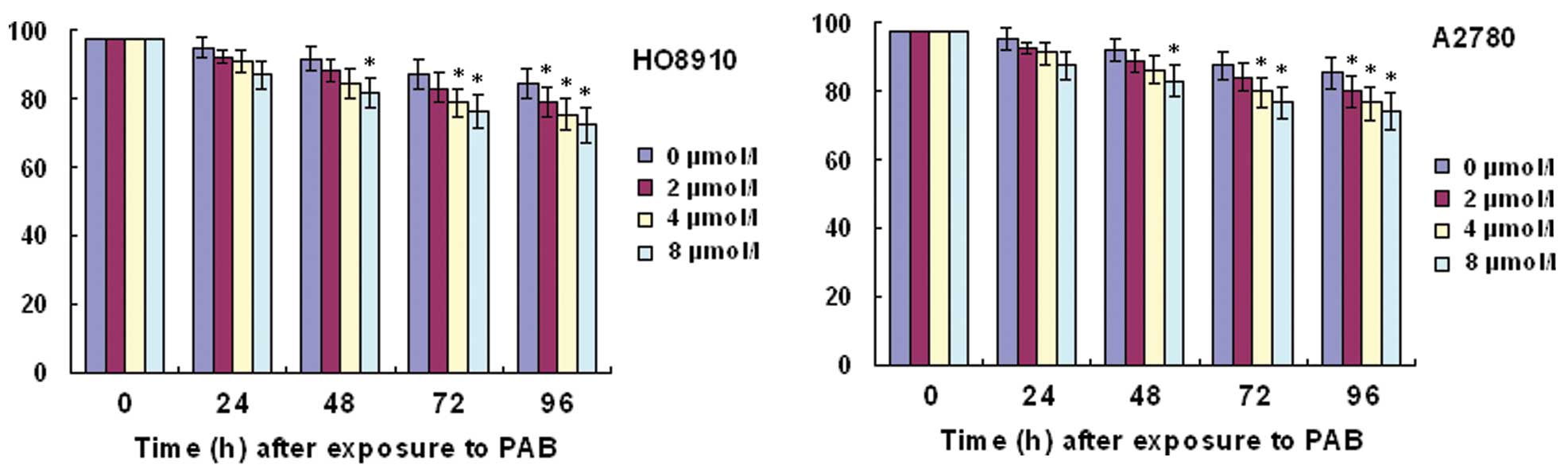
In order to detect HO-8910 or A2780 viability, we
performed a trypan blue exclusion assay. Trypan blue staining
showed that the percentage of cell viability was decreased with
increasing time and concentrations of PAB (Fig. 1).
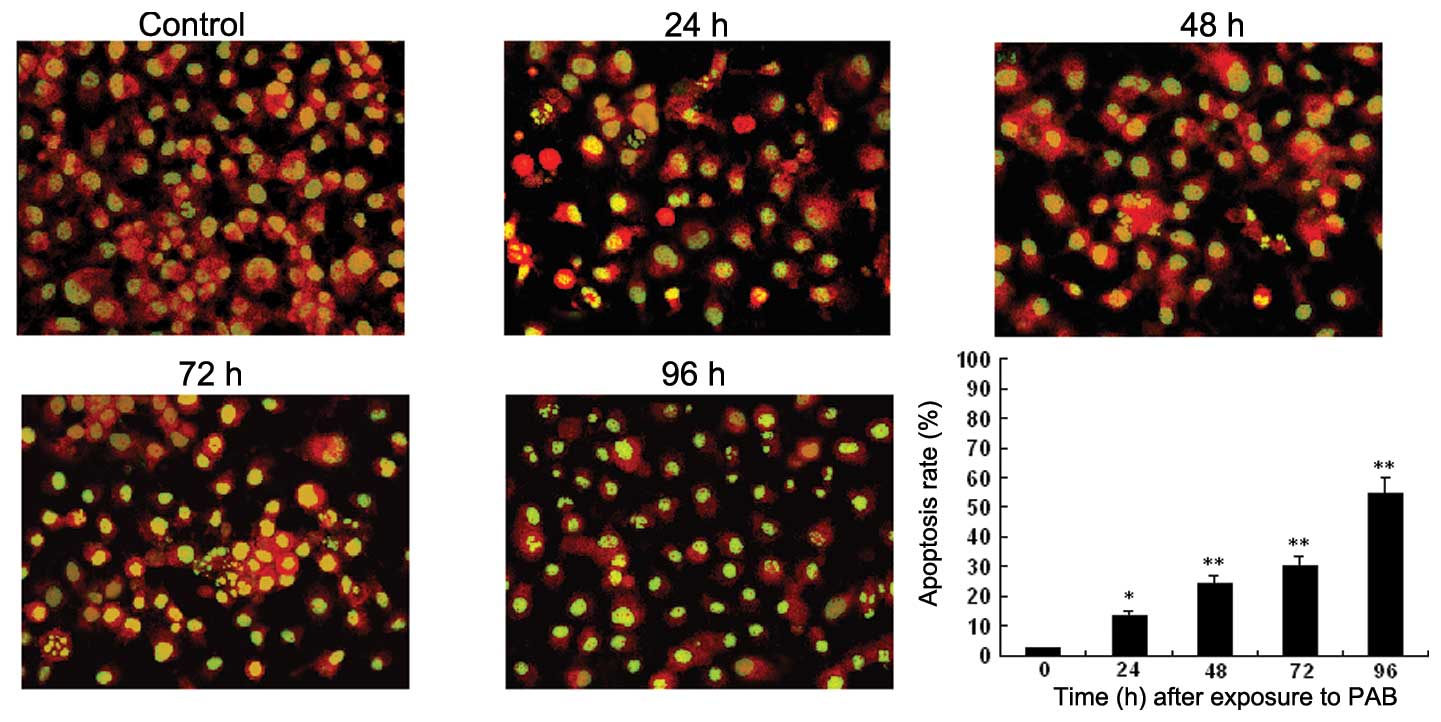
To investigate whether the growth inhibitory effect
was mediated through the induction of apoptosis, we examined the
apoptotic morphology of control and PAB-treated HO-8910 cells by
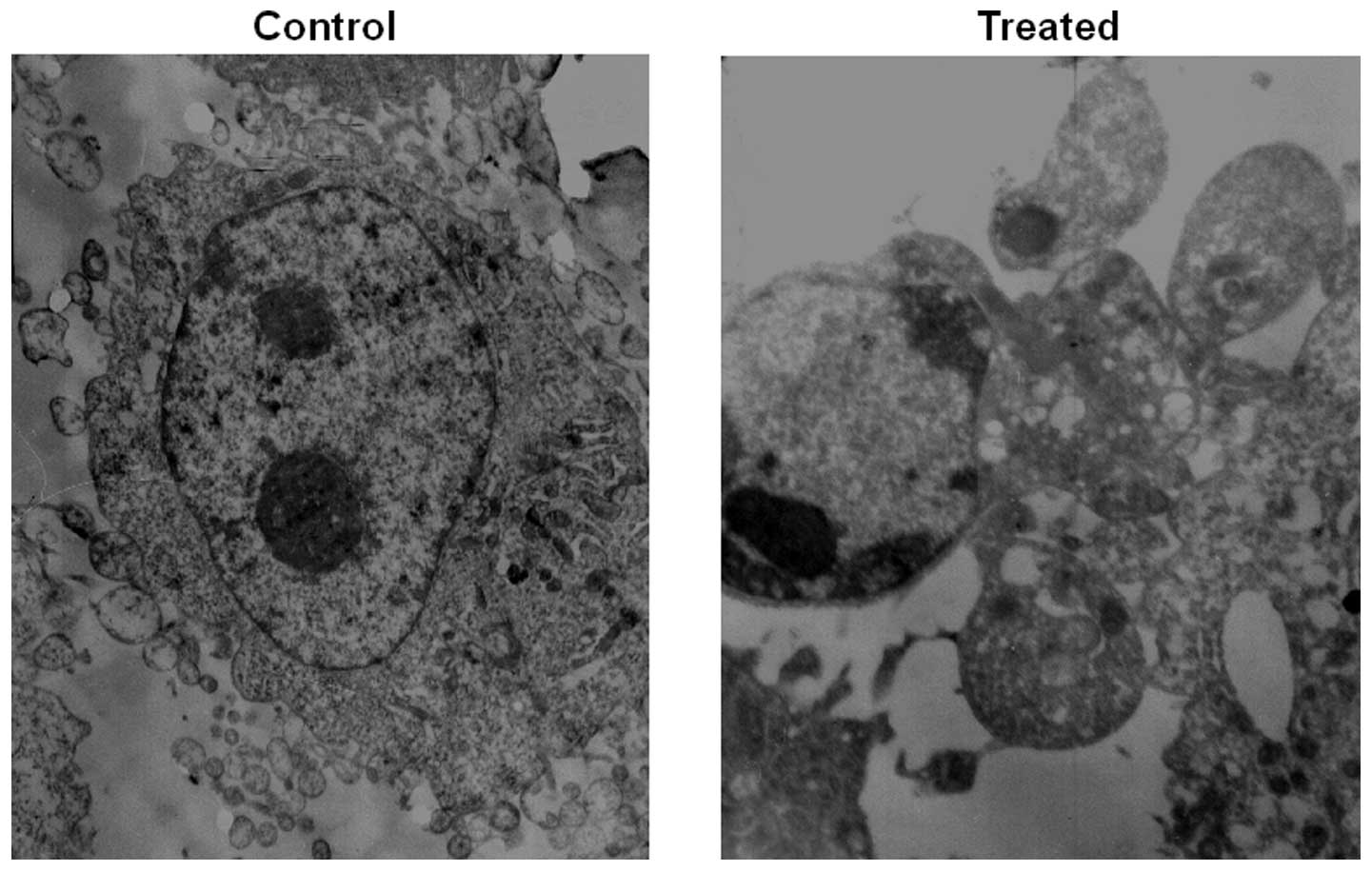
Acridine orange staining and transmission electron microscopy.
Microscopy of PAB-treated HO-8910 cells revealed morphological
changes compared to the control, and the apoptosis rate of HO-8910
increased in a time- and dose-dependent manner. Apoptotic cells
were characterized by membrane blebbing and nuclear condensation,
while necrotic cells were typically larger and lighter with plasma
membrane lesions (Fig. 2). The
percentage of apoptotic cells was calculated by observing 500
cells. Transmission electron microscope imaging is considered the
gold standard in identifying cellular apoptosis due to of its
standard and reliable method (14)
(Fig. 3).
PAB induces apoptosis in human HO-8910
and A2780 cells
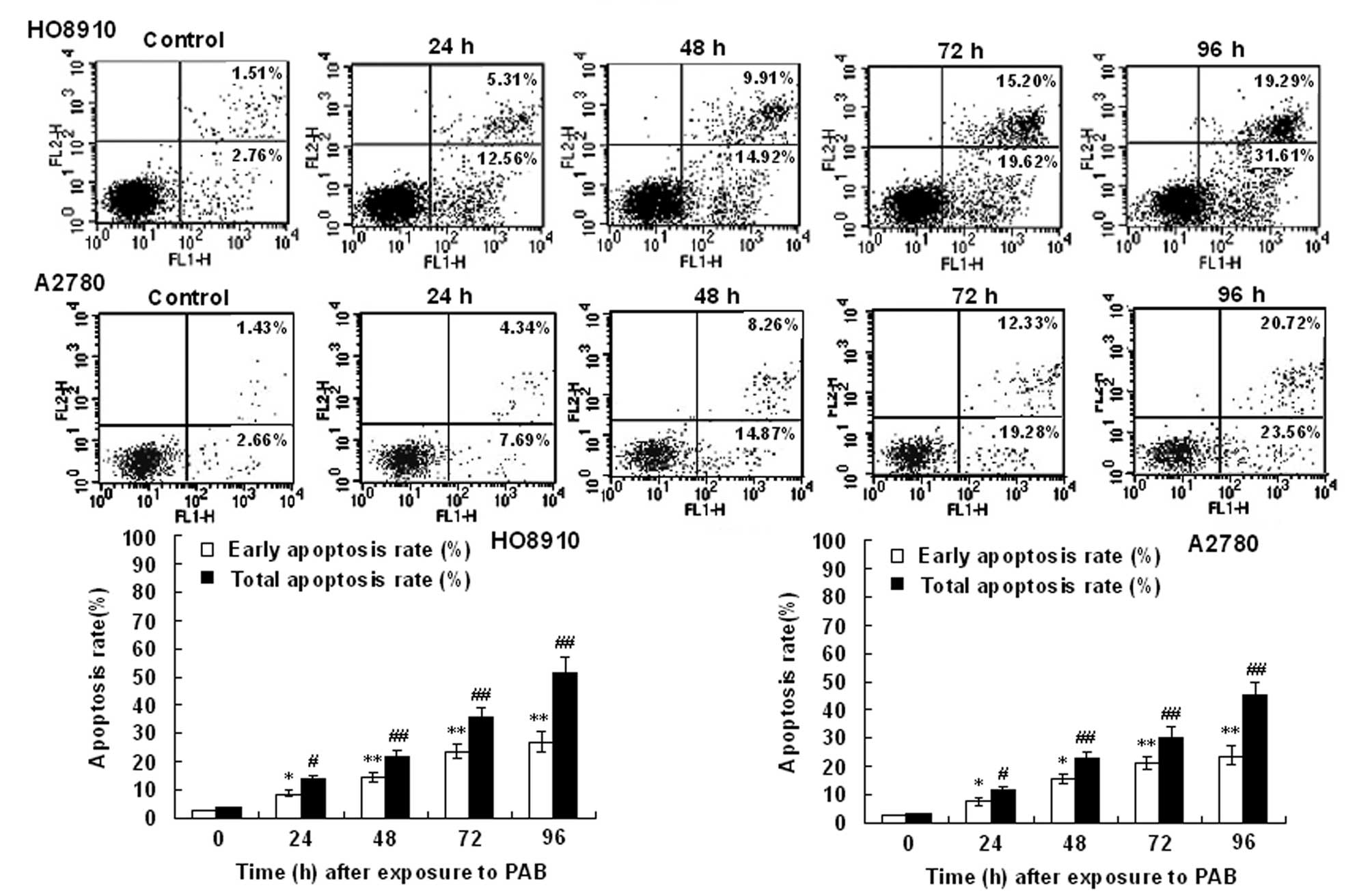
Flow cytometry using FITC-conjugated Annexin V
revealed that HO-8910 and A2780 cells exposed to PAB underwent
rapid apoptosis (Fig. 4). This
effect was positively correlated with the exposure time in the
HO-8910 and A2780 cells, and excessive apoptosis was associated
with loss of membrane integrity in an increased portion of HO-8910
and A2780 cells, which indicated necrosis or late apoptosis.
PAB treatment modulates the Bax/Bcl-2
ratio in HO-8910 and A2780 cells
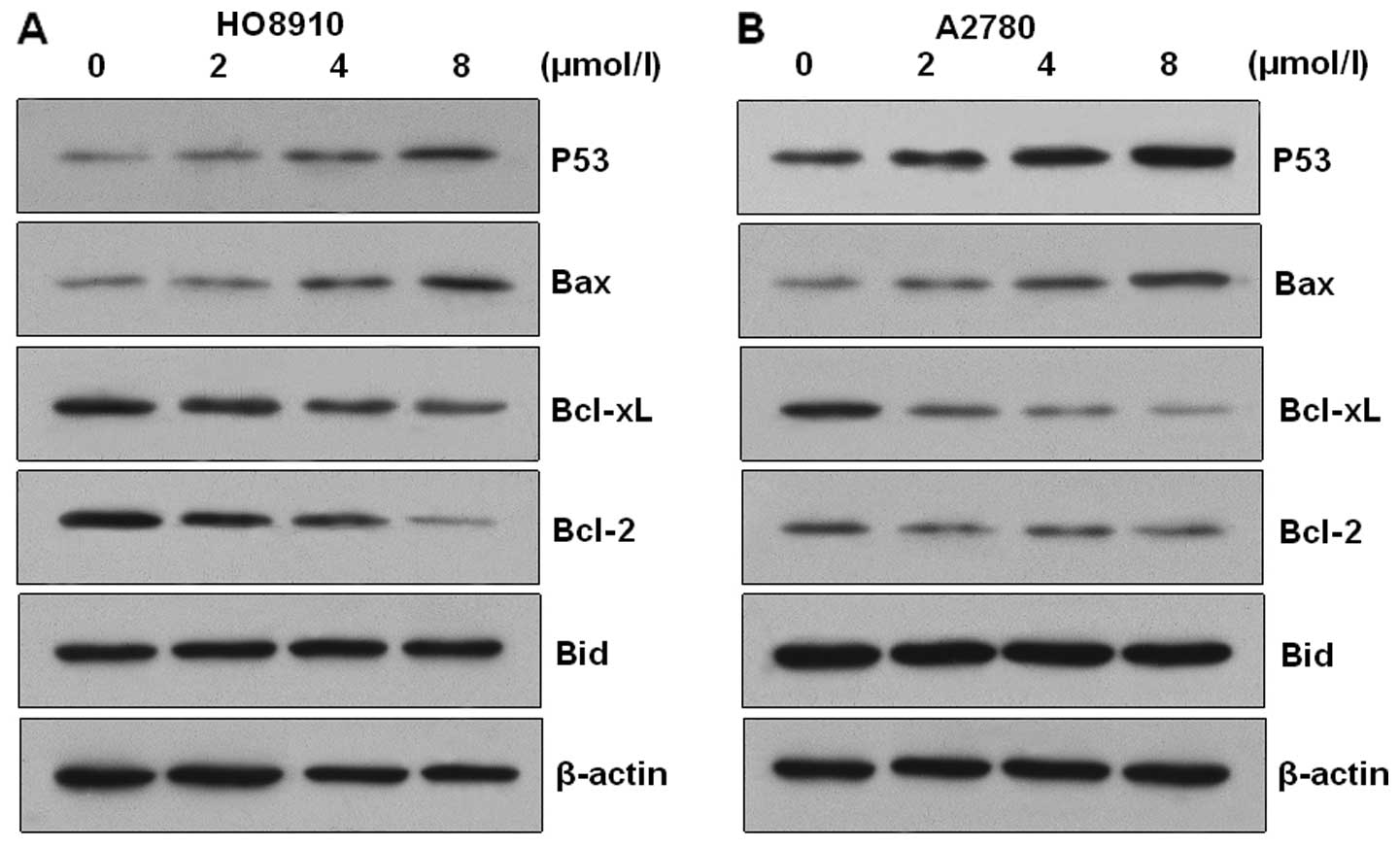
Western blot analysis was carried out to verify the
involvement of Bax and Bcl-2 proteins during PAB-induced apoptosis
of HO-8910 and A2780 cells. The expression of Bcl-2 and Bcl-xL
proteins was downregulated and the expression of p53 and Bax
proteins was upregulated in the PAB-treated cells (Fig. 5). However, the expression of Bid was
not altered in the two types of cells. These results suggest that
PAB induced apoptosis via alteration of the Bax/Bcl-2 ratio in
HO-8910 and A2780 cells.
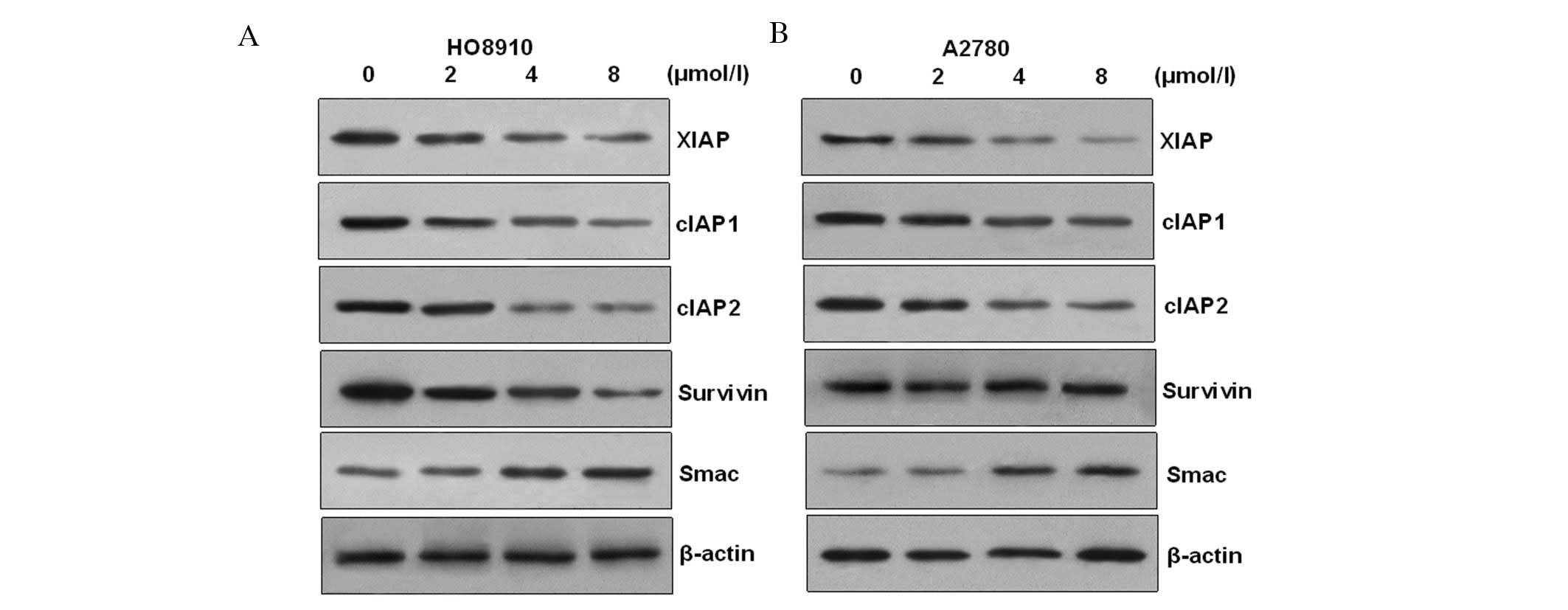
PAB induces HO-8910 and A2780 apoptotic
cell death via modulation of XIAP family proteins
In order to ascertain the apoptotic mechanism in
HO-8910 cells induced by PAB, we examined anti-apoptotic protein
expression. Members of the mammalian IAP family mainly include
XIAP, cIAP-1 and cIAP-2. The results showed that the transcription
and expressions of cIAP1/2, XIAP and survivin were decreased in a
dose-dependent manner after challenge with PAB in HO-8910 and A2780
cells. However, the transcription and expression of Smac (second
mitochondria-derived activator of caspase), which is an intrinsic
antagonist of XIAP, were increased in a time-dependent manner
(Fig. 6). These results suggest
that changes in expression of cIAP1/2, survivin, Smac and XIAP may
contribute to PAB-induced apoptogenesis in HO-8910 and A2780
cells.
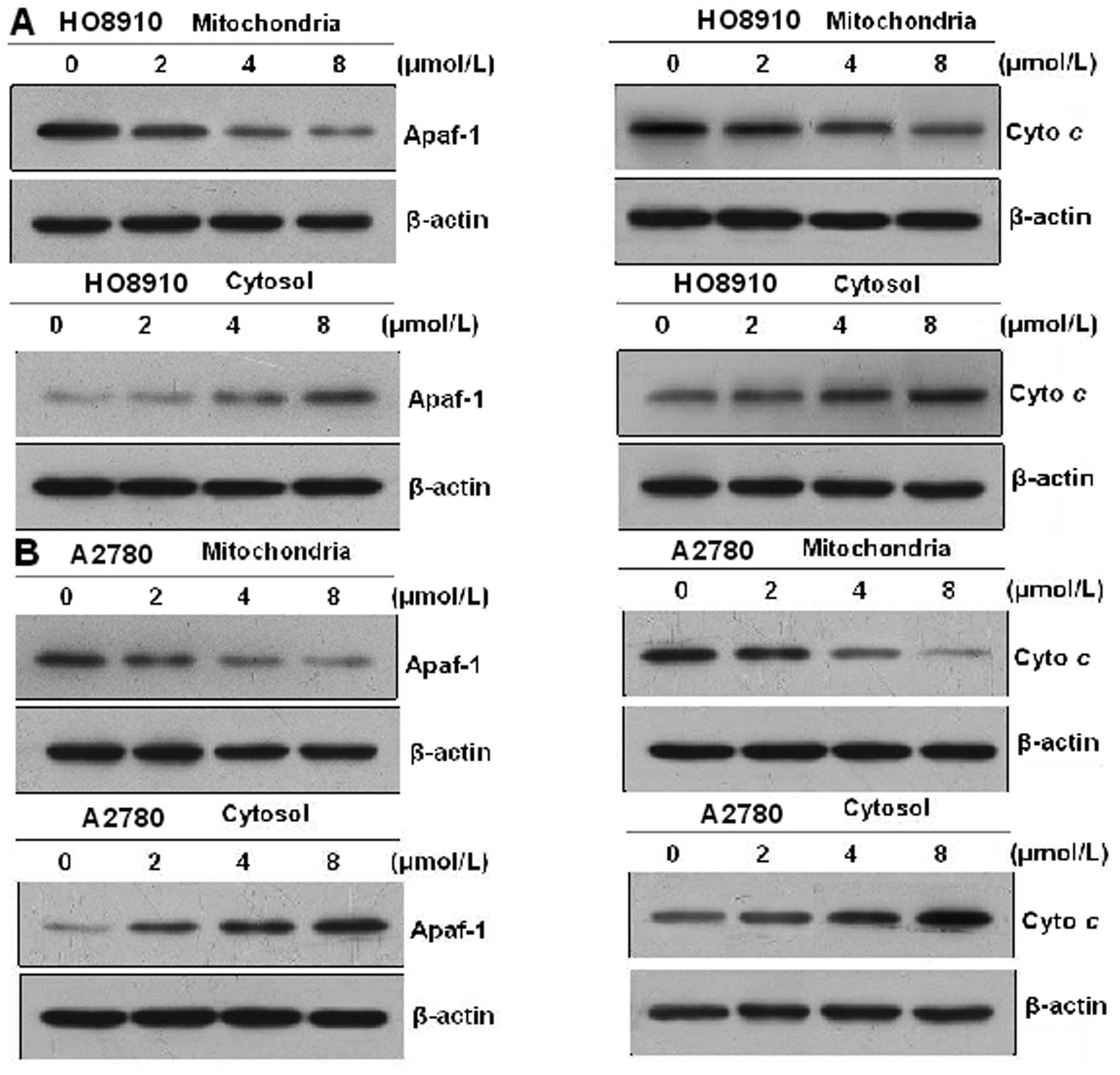
PAB induces cyto c and Apaf-1 release
from the mitochondria
Mitochondria play an essential role in the apoptosis
triggered by chemical (anticancer) agents. The mitochondrial
response includes the release of cyto c and Apaf-1 into the
cytosol. Therefore, we tested the effect of PAB on cyto c
and Apaf-1 release. To analyze the involvement of mitochondria in
HO-8910 and A2780 cells, proteins from the cytosolic fraction were
prepared and analyzed using western blot analysis. Treatment of
HO-8910 and A2780 cells with 0, 2, 4 and 8 μmol/l PAB,
respectively, for 48 h resulted in an increase in cyto c and
Apaf-1 levels in a dose-dependent manner. These results indicate
that PAB promotes cyto c and Apaf-1 release from
mitochondria into the cytosol (Fig.
7).
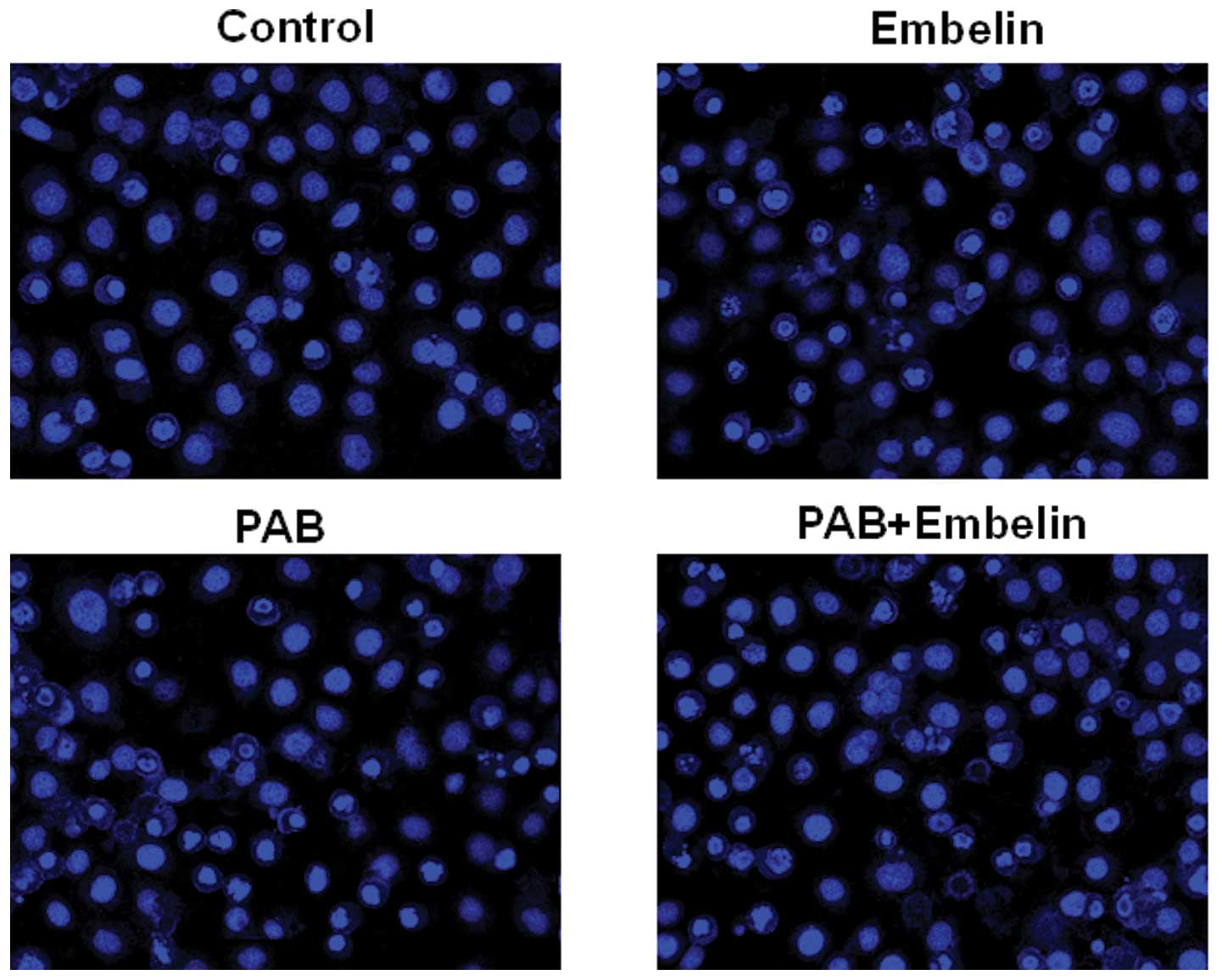
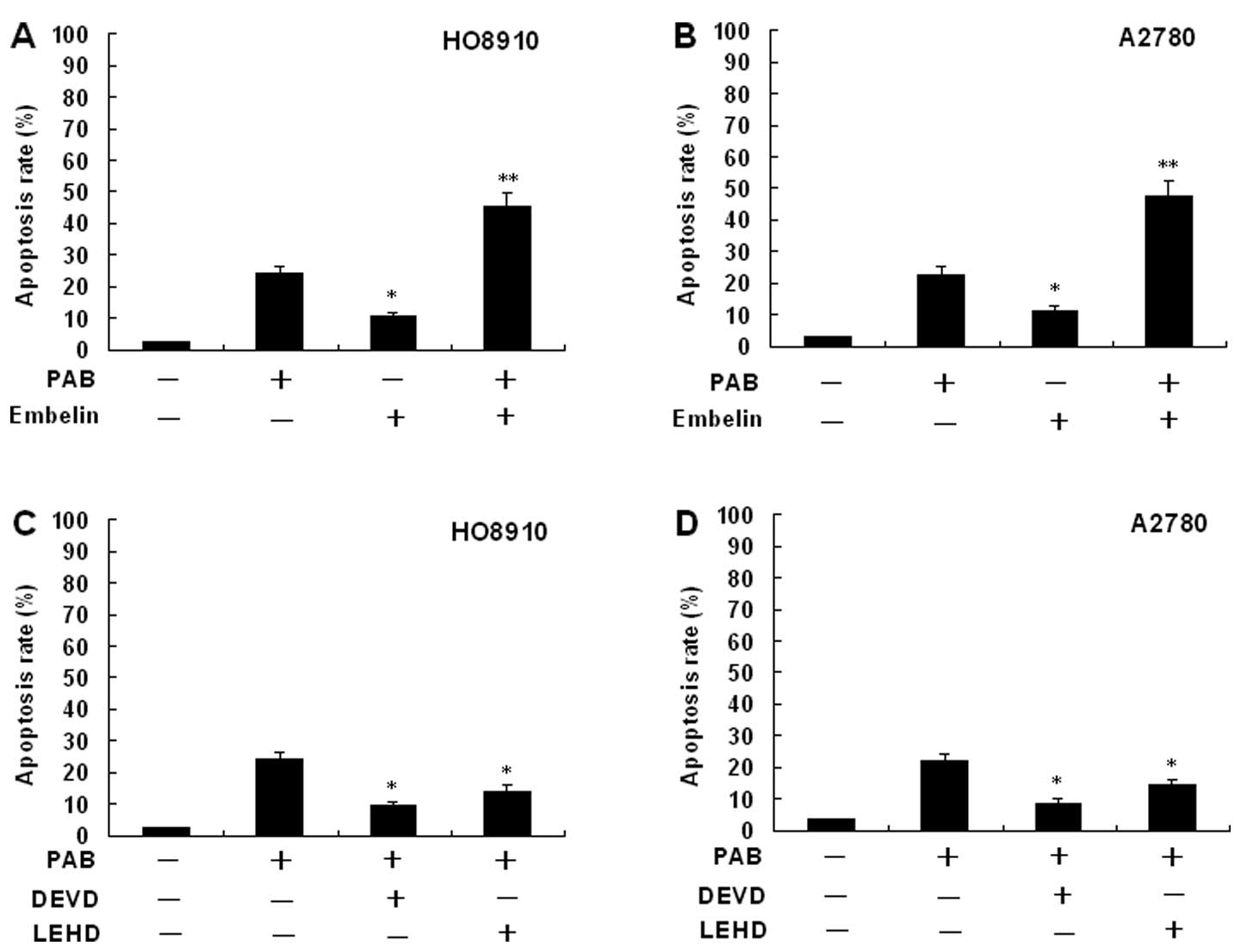
Effects of an inhibitor of XIAP on
PAB-induced HO-8910 and A2780 cell apoptosis
To identify the relevance of the XIAP signaling
pathway in controlling the apoptotic cell death by PAB, inhibition
assays were performed with Embelin (a specific inhibitor of XIAP).
The percentage of apoptosis was determined by flow cytometery.
HO-8910 and A2780 cells were pretreated with 20 μM Embelin for 30
min, and then cultured with 4 μmol/l PAB for 48 h. The cells were
stained with Hoechst 33258 and the samples were observed under a
fluorescence microscope. The results showed that Embelin
significantly increased the apoptosis rate (Fig. 8). The same results were also showed
by flow cytometric analysis (Fig.
9).
Expression of caspase-3 and -9
activity
The expression of caspase-3 and -9 activity in
HO-8910 and A2780 cells incubated in the presense of PAB is
presented in Fig. 10. Treatment of
HO-8910 and A2780 cells with PAB for 48 h at concentrations of 0,
2, 4 and 8 μmol/l, respectively, or for different times at a
concentration of 4 μmol/l showed marked increase in caspase-3 and
-9 activation. Activity of caspase-3 and -9 in HO-8910 and A2780
cells following PAB treatment showed dose- and time-dependent
upregulation. Inhibition of XIAP with Embelin potentiated the
PAB-induced caspase-3 and -9 activity (data not shown). In order to
assess whether PAB-induced cell death occurred due to caspase
activation, we used the caspase inhibitor Z-DEVD-FMK (specific for
caspase-3) or Z-LEHD-FMK (specific for caspase-9) (Fig. 9). PAB induced cell death in HO-8910
and A2780 cells. The pretreatment of HO-8910 and A2780 cells with
Z-DEVD-FMK or Z-LEHD-FMK inhibited PAB-induced apoptosis,
suggesting the involvement of caspase(s) in PAB-induced cell
death.
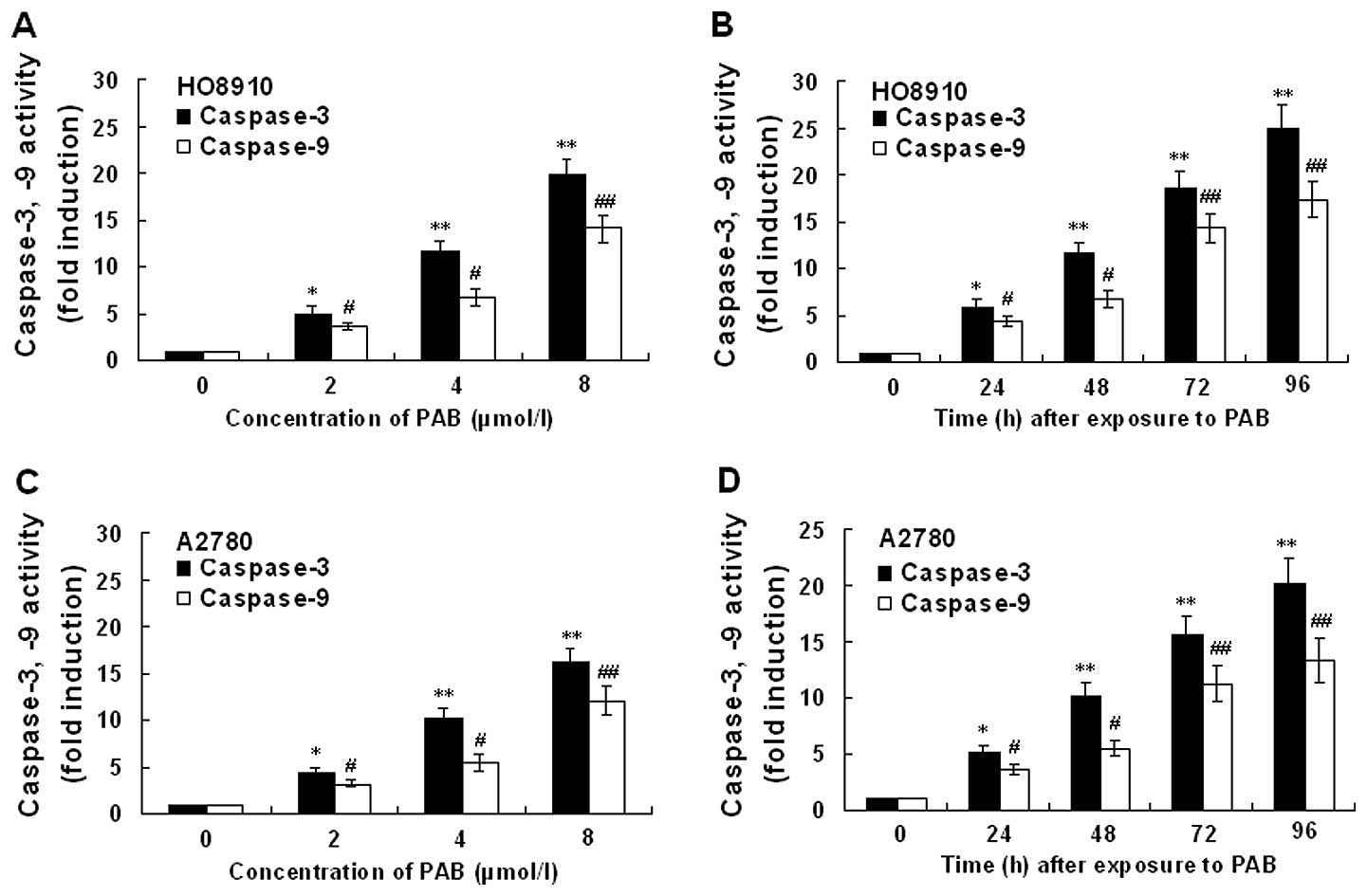 | Figure 10Effect of PAB on the activity of
caspase-3 and -9 in HO-8910 and A2780 cells. The cells were treated
with 0, 2, 4 and 8 μmol/l PAB, respectively, for 48 h or with 4
μmol/l PAB for 0, 24, 48, 72 and 96 h, respectively. (A)
Dose-dependent effect of PAB-induced caspase-3 and -9 activity in
HO-8910 cells. (B) Time-dependent effect of PAB-induced caspase-3
and -9 activity in HO-8910 cells. (C) Dose-dependent effect of
PAB-induced caspase-3 and -9 activity in A2780 cells. (D)
Time-dependent effect of PAB-induced caspase-3 and -9 activity in
A2780 cells. Values represent means ± SD of five experiments
performed in duplicate. *,#P<0.05,
**,##P<0.001 compared with that of control. |
Discussion
Several trials are currently being performed to
investigate the effect of pseudolaric acid B on various types of
solid tumors, including ovarian cancer (4,15).
However, knowledge concerning the mechanism by which this compound
induces cell death is still limited. Thus, our research was
designed to determine whether PAB induces apoptosis in ovarian
cancer cells. To the best of our knowledge, the present study for
the first time demonstrated that PAB induced HO-8910 and A2780 cell
apoptosis in a time- and dose-dependent manner.
A characteristic feature of human cancers is the
inability to mount a proper apoptotic response during tumor
progression or upon treatment with cytotoxic therapies (16). Therefore, evasion of apoptosis
constitutes a critical cause of primary or acquired treatment
resistance that frequently occurs in various types of human cancers
(17). The molecular pathways
leading to apoptosis are evolutionarily conserved and controlled
(18). Apoptotic signaling pathways
are the most promising therapeutic targets for cancer treatment
(19,20).
The Bcl-2 family proteins play an essential role in
the apoptotic process. They are regulators of mitochondrial
membrance permeability and intermembrane space protein efflux
according to the opposing fractions of anti-apoptosis members and
pro-apoptosis members (21). The
ratio of anti- and pro-apoptotic protein expression, such as
Bcl-2/Bax, is crucial for the induction of apoptosis, and it
decides the susceptibility of cells to undergo apoptosis (22). Bcl-2 and Bcl-xL act as
anti-apoptotic factors, and Bax acts as a pro-apoptotic factor. In
the present study, treatment of HO-8910 and A2780 cells with PAB
markedly downregulated Bcl-2 and Bcl-xL expression, upregulated Bax
and P53, whereas the expression of Bid did not change.
The mitochondrion is generally believed to be the
key regulatory element of cell death and the target of many
pro-apoptotic signaling pathways (23). Smac/DIABLO (second
mitochondria-derived activator of caspases or direct IAP binding
protein with low pI), a mitochondrial protein that is released
together with cyto c from the mitochondria in response to
apoptotic stimuli, was found to promote caspase activation by
binding and neutralizing the IAPs via its N-terminal (24,25)
Smac release from mitochondria (26) is a general feature of apoptosis. In
the present study, we showed that PAB upregulated the expression of
Smac in HO-8910 and A2780 cells. Our results also indicate that PAB
promotes cyto c and Apaf-1 release from mitochondria into
the cytosol.
Bax/Bak mediate mitochondrial outer membrane
permeabilization (MOMP), with consequent release of apoptogenic
factors from mitochondria into the cytosol (cyto c,
Smac/DIABLO). These apoptogenic factors precipitate activation of
the caspase cascade and cell-killing via Apaf-1-mediated activation
of the initiator caspase, caspase-9, as well as by derepression of
effector caspases by blocking their antagonist XIAP (27,28).
Inhibitor of apoptosis (IAP) proteins are a family of endogenous
anti-apoptotic proteins (29).
Elevated expression of IAP proteins combined with their
well-established functional importance for survival of tumor
tissues and resistance to anticancer therapies makes IAP proteins
attractive targets for therapeutic intervention (8). Among the IAPs, cellular IAP1 (cIAP1)
and cIAP2 play a key role in the regulation of death
receptor-mediated apoptosis, whereas X-linked IAP (XIAP) inhibits
both death receptor-mediated and mitochondrial-mediated apoptosis
by binding to and inhibiting caspase-3/7 and caspase-9; three
cysteine proteases critical for execution of apoptosis (30). In the present study, we showed that
PAB downregulated the expression of XIAP, survivin, cIAP-2 and
Bcl-2 and upregulated the expression of Bax. It was also shown that
PAB promoted cyto c and Apaf-1 release from mitochondria
into the cytosol.
Embelin (2,5-dihydroxy-3-undercyl-1,4-benzoquinone;
C17H26O2; molecular weight,
294.39) is a type of extract from Japanese Ardisia Herb, and its
traditional use in Chinese herbal medicine is to dispel intestinal
parasites. It was later identified as a novel cell permeable
inhibitor of XIAP (31). To more
directly link the XIAP signaling pathway with caspase activation,
we examined PAB-mediated caspase-3 and -9. In our study, inhibition
of XIAP significantly increased caspase-3 and -9 activitiy.
Moreover, Embelin significantly increased the apoptosis rate.
In conclusion, we found that PAB inhibited cell
growth and induced the apoptosis of HO-8910 and A2780 cells. We
also studied the underlying mechanisms involved in PAB-induced
apoptosis. In the present study, there was a tendency of
alterations showing a decreased expression level of Bcl-2, cIAP1/2,
survivin and XIAP, and also with an increased expression level of
Smac and activation of caspase-3 and -9. Moreover, PAB induced cyto
c and Apaf-1 release from the mitochondria.
Acknowledgements
The present study was supported by the Provincial
Natural Science Foundation of Liaoning Province (201202270), the
Outstanding Scientific Fund of Shengjing Hospital (201206), Tackle
Key Problems in Science and Technology of Liaoning Province
(2011225020), and Tackle Key Problems in Science and Technology of
Shenyang City (F12-193-9-20).
References
|
1
|
Yin F, Liu X, Li D, Wang Q, Zhang W and Li
L: Tumor suppressor genes associated with drug resistance in
ovarian cancer. Oncol Rep. 30:3–10. 2013.PubMed/NCBI
|
|
2
|
Kodigepalli KM, Dutta PS, Bauckman KA and
Nanjundan M: SnoN/SkiL expression is modulated via arsenic
trioxide-induced activation of the PI3K/AKT pathway in ovarian
cancer cells. FEBS Lett. 587:5–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishii T, Kira N, Yoshida T and Narahara H:
Cucurbitacin D induces growth inhibition, cell cycle arrest, and
apoptosis in human endometrial and ovarian cancer cells. Tumour
Biol. 34:285–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu JH, Liu CY, Zheng GB, et al:
Pseudolaric acid B induced cell cycle arrest, autophagy and
senescence in murine fibrosarcoma l929 cell. Int J Med Sci.
10:707–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma G, Chong L, Li XC, Khan IA, Walker LA
and Khan SI: Selective inhibition of human leukemia cell growth and
induction of cell cycle arrest and apoptosis by pseudolaric acid B.
J Cancer Res Clin Oncol. 136:1333–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fulda S: Apoptosis pathways and their
therapeutic exploitation in pancreatic cancer. J Cell Mol Med.
7:1221–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varfolomeev E and Vucic D: Inhibitor of
apoptosis proteins: fascinating biology leads to attractive tumor
therapeutic targets. Future Oncol. 7:633–648. 2011. View Article : Google Scholar
|
|
9
|
Tong J, Yin S, Dong Y, Guo X, Fan L, Ye M
and Hu H: Pseudolaric acid B induces caspase-dependent apoptosis
and autophagic cell death in prostate cancer cells. Phytother Res.
27:885–891. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan M, Zheng B, Yi F, et al: Pseudolaric
acid B induces caspase-dependent and caspase-independent apoptosis
in u87 glioblastoma cells. Evid Based Complement Alternat Med.
2012:9575682012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carter BZ, Mak DH, Wang Z, Ma W, Mak PY,
Andreeff M and Davis RE: XIAP downregulation promotes
caspase-dependent inhibition of proteasome activity in AML. Leuk
Res. 37:974–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Z, Zhang R, Li J, et al: X-linked
inhibitor of apoptosis protein (XIAP) regulation of cyclin D1
protein expression and cancer cell anchorage-independent growth via
its E3 ligase-mediated protein phosphatase 2A/c-Jun axis. J Biol
Chem. 288:20238–20247. 2013. View Article : Google Scholar
|
|
14
|
Yao SQ, Rojanasakul LW, Chen ZY, et al:
Fas/FasL pathway-mediated alveolar macrophage apoptosis involved in
human silicosis. Apoptosis. 16:1195–1204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Supiot S, Gouraud W, Campion L, et al:
Early dynamic transcriptomic changes during preoperative
radiotherapy in patients with rectal cancer: a feasibility study.
World J Gastroenterol. 19:3249–3254. 2013. View Article : Google Scholar
|
|
16
|
Fulda S: Tumor resistance to apoptosis.
Int J Cancer. 124:511–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fulda S: Inhibitor of apoptosis (IAP)
proteins as therapeutic targets for radiosensitization of human
cancers. Cancer Treat Rev. 38:760–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paramasivam A, Sambantham S, Shabnam J, et
al: Anti-cancer effects of thymoquinone in mouse neuroblastoma
(Neuro-2a) cells through caspase-3 activation with down-regulation
of XIAP. Toxicol Lett. 213:151–159. 2012. View Article : Google Scholar
|
|
19
|
Galluzzi L, Morselli E, Kepp O, et al:
Mitochondrial gateways to cancer. Mol Aspects Med. 31:1–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu CX, Jin H and Cho MH: Apoptosis and
apoptosis-based therapy in lung cancer. Anticancer Agents Med Chem.
9:952–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang C, Chen X, Guo B, et al: Induction
of apoptosis by Icariside II through extrinsic and intrinsic
signaling pathways in human breast cancer MCF7 cells. Biosci
Biotechnol Biochem. 76:1322–1328. 2012. View Article : Google Scholar
|
|
22
|
Zeng C, Ke Z, Song Y, et al: Annexin A3 is
associated with a poor prognosis in breast cancer and participates
in the modulation of apoptosis in vitro by affecting the Bcl-2/Bax
balance. Exp Mol Pathol. 95:23–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
24
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verhagen AM, Ekert PG, Pakusch M, et al:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adrain C, Creagh EM and Martin SJ:
Apoptosis-associated release of Smac/DIABLO from mitochondria
requires active caspases and is blocked by Bcl-2. EMBO J.
20:6627–6636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fulda S and Vucic D: Targeting IAP
proteins for therapeutic intervention in cancer. Nat Rev Drug
Discov. 11:109–124. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salvesen GS and Duckett CS: Apoptosis: IAP
proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol.
3:401–410. 2002.
|
|
31
|
Ahn KS, Sethi G and Aggarwal BB: Embelin,
an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein,
blocks nuclear factor-κB (NF-κB) signaling pathway leading to
suppression of NF-κB-regulated antiapoptotic and metastatic gene
products. Mol Pharmacol. 71:209–219. 2007.
|