Introduction
Breast cancer is the most common malignant disease
affecting women, and a principal cause of cancer morbidity and
mortality in the industrialized world (1). Breast cancer still represents a major
public health problem, with more than 400,000 new cases and 200,000
deaths per years in Europe (2).
Breast cancer is currently regarded as a heterogeneous group of
tumors with diverse morphologic and molecular behavior, outcome and
response to therapy (3). The
prognosis is generally related to the stage of the disease and our
currently applied prognostic parameters include mainly the
following histopathological characteristics: tumor size, lymph node
status and the presence of distant metastases (4). Despite combined treatment with
surgery, radiotherapy and chemotherapy, a great percentage of
breast cancer patients will eventually develop recurrent and
metastatic disease (1). Breast
tumors are well known to be composed of phenotypically diverse
groups of cells; however, it is unclear which of these cell types
contribute to tumor development. In contrast to the hypothesis that
all cell populations have the capacity to become tumorigenic
through accumulation of mutations, another hypothesis limits this
ability to an elite group of cells that share classic features of
stem cells such as the ability to self-renew and to differentiate
(5–7). Evidence suggests that many malignant
tumors contain heterogeneous cell population with diverse
biological properties and within these there is small proportion of
tumor cells termed cancer stem cells (CSCs) (8–13). The
CSCs are thought to have the characteristics of self-renewal and,
therefore, could be responsible for tumor formation and progression
(14). Furthermore, they are
thought to have features, which enable resistance to chemotherapy
and represent the source of recurrence (15,16).
The maintenance of the heterogeneity of cells within a tumor is not
fully understood. The cancer stem cell hypothesis was proposed to
explore breast cancer heterogeneity and the risk of breast cancer
recurrence, and these cell subpopulations may contribute to drug
resistance that drives tumor recurrence or metastasis (17).
Al-Hajj and colleagues (8) observed that human breast cancer cells
that have a strong expression of the adhesion molecule CD44
together with very low levels or no expression of the adhesion
molecule CD24 (CD44+/CD24−/low phenotype)
could efficiently form tumors containing an array of cell types
similar to those found in the original carcinoma samples when
injected into immunocompromised mice. Moreover, the cells showing
this antigenic profile, were able to form mammospheres, and were
resistant to drug administration. Therefore, this
CD44+/CD24−/low phenotype has been associated
with stem cell-like characteristics, with enhanced invasive
properties, with radiation resistance and with distinct genetic
profiles suggesting correlation to adverse prognosis (18–23).
The prevalence of CD44+/CD24−/low cells
within breast tumors, however, has not been significantly
associated with clinical characteristics, although tumors with a
higher fraction of CD44+/CD24−/low cells were
more commonly found in patients diagnosed with distant metastases.
In breast cancer patients, CD44+/CD24−/low
cells were readily detectable in metastatic pleural effusions,
moreover, the presence of these cells, in breast cancer, may not be
associated with clinical outcome but may favor distant metastasis,
related to angiogenic properties (24). However, progress in screening
programs, understanding of cancer biology, and the development of
new treatments, have reduced the likelihood of dying from breast
cancer. Despite this progress and the availability of adjuvant
therapies that have significantly reduced recurrence, breast cancer
recurrence still occurs in a substantial proportion of women after
treatment and mortality rates remain unacceptably high (1). Thus, a more effective treatment of
breast cancer is urgently needed. In particular, it is important to
identify new prognostic markers to accurately establish innovative
therapies to eradicate the metastatic breast cancer cells at the
stage of the primary tumor. In this regard, much effort has been
expended in recent years to delineate biomarkers which enable
identification of CSCs. Biomarkers have the potential to increase
the safety and effectiveness of new therapeutics. The development
of novel markers is therefore urgently needed to better evaluate
prognosis and to designate an appropriate treatment for each
individual case. Breast cancers have been classified based on their
histopathological profile (invasive ductal and lobular carcinoma)
and based on gene expression profiles into luminal A and B,
basal-like, HER2+ and normal breast-like subtypes
(25). Each subtype is associated
with a special natural history and treatment responsiveness. The
luminal subtypes are associated with expression of the estrogen
receptor (ER), while basal-like and normal-like tumors are
essentially all ER-negative, as are the majority of
HER2+ tumors. Multiple studies have demonstrated that
basal-like tumors had a particularly poor prognosis (26), although it is unclear whether
basal-like tumors have a significantly worse clinical outcome than
other ER-negative tumors (27).
Immunohistochemical features have been used to characterize
basal-like tumors as typically negative for ER, for the
progesterone receptor (PgR) and for HER2 but positive for basal
cytokeratin (CK5/6/14/17), for epidermal growth factor receptor
(EGFR) and/or for c-kit (28). In
addition to inter-tumor heterogeneity, there is also a high degree
of intra-tumor diversity. Specifically, a single tumor can contain
tumor cell population with distinct molecular profiles and
biological properties. A correlation of the
CD44+/CD24−/low phenotype to specific breast
cancer subtypes has not yet been reported in human breast tumors.
In the present study, we have determined the expression of CD44 and
CD24 in human breast tumors using double-staining flow cytometry
and have correlated the presence of
CD44+/CD24−/low cells to subgroups of breast
cancer, classified using the expression of ER, PgR and Ki-67, as
well as by following the histopathological characteristics tumor
size, grade and lymph node status.
Materials and methods
Collection of human tissue specimens and
cell cultures
Breast tumor and adjacent non-tumor tissue specimens
were obtained by surgical procedures, after informed consent, from
57 consecutive patients who underwent radical mastectomy for
primary breast cancer without neo-adjuvant radiotherapy or
chemotherapy at the National Cancer Institute of Naples. Diagnosis
was based on clinical and histological parameters [46 invasive
ductal carcinoma (IDC) and 11 invasive lobular carcinoma (ILC)].
The patients ranged in age from 27 to 85 years. Tumor specimens
were subjected to enzymatic dissociation by type IV collagenase (1
mg/ml) in phosphate-buffered saline (PBS) at 37°C in agitation for
60 min. The digestive solution was filtered through 70 μm filters
(Becton-Dickinson, Sunnyvale, CA, USA). After filtration and
washing, the cell suspension was centrifuged at 1,300 rpm for 7 min
and the pellet, in part, was cultured in 5 ml Dulbecco’s modified
Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), and
another part was used for cytometric analyses. Flasks were
incubated at 37°C under 5% CO2 and the medium changed
twice a week. All of the tumors capable of growing after 9–15
culture passages were considered to be breast-stabilized cell
lines, whereas all tumor samples capable of growing only after 1–8
culture passages were considered to be primary cell lines.
Flow cytometric analysis
At least 200,000 cells were tested for a panel of
fluorescent labeled monoclonal antibodies and respective isotype
controls. The antibodies were incubated for 30 min at 4°C. After
washing, the labeled cells were analyzed by flow cytometry using a
FACSAria II cell sorter (Becton-Dickinson, Mountain View, CA, USA).
The antibodies used were: anti-human CD44 FITC (BD Pharmingen),
anti-human CD24 PE (BD Pharmingen) and anti-human CD45 APC (BD
Pharmingen). All data were analyzed using Diva 6.1.1 software.
Mammosphere formation
Single cells are plated at 1,000 cells/ml in
ultra-low attachment plates (Corning) in serum-free DMEM-F12
supplemented with 10 ng/ml bFGF, 20 ng/ml EGF, 5 μg/ml insulin and
0.4% BSA. Cells grew in these conditions as non-adherent spherical
clusters of cells (usually named spheres or mammospheres) and were
enzymatically dissociated by incubation in a trypsin-EDTA solution
or mechanically disaggregated every 3 days for 2 min at 37°C. Fresh
aliquots of EGF and bFGF were added every day. After culture for
48–72 h, mammospheres were visible with an inverted phase-contrast
microscope (Carl Zeiss, Milan, Italy).
Histopathological diagnosis
The histopathological diagnoses were made according
to the criteria of the World Health Organization and were recorded
as invasive ductal or invasive lobular, using standard tissue
sections and appropriate immunohistochemical slides.
Clinicopathological parameters were evaluated for each tumor and
included patient age at initial diagnosis; tumor size, histologic
subtype, histologic grade, nuclear grade, nodal status, number of
positive lymph nodes, tumor stage and type of surgery. In addition,
estrogen receptor (ER), progesterone receptor (PgR), and Ki-67 were
considered. Immunohistochemical analyses for CD44 (Dako
Cytomation), E-cadherin (Abcam) on paraffin-embedded tissue
sections were performed with the Dako AEC kit, according to the
manufacturer’s instructions. The nuclei were stained with
hematoxylin, and the cells were observed under an inverted light
microscope (Carl Zeiss).
Correlation between
CD44+/CD24−/low, clinical and
histopathological parameters
To correlate the presence of
CD44+/CD24−/low cells with the outcomes of
disease, the following clinical parameters were analyzed: age,
histotype, tumor size, grading, lymph node status and clinical
stage. Correlation between presence of
CD44+/CD24−/low cells and clinicopathological
parameters was analyzed by Fisher’s exact test. Levels of
significance were set at P<0.05 and P<0.005.
Results
Collection of human tissue specimen
The clinical and histological characteristics of the
breast cancer patients enrolled in the study are summarized in
Table I. There were 57 females,
with mean age of 56.5 years. Breast cancer histotype was invasive
in all cases (46 invasive ductal carcinoma and 11 invasive lobular
carcinoma).
 | Table ICD44+/CD24−/low
expression and clinicopathological characteristics in primary
breast cancer. |
Table I
CD44+/CD24−/low
expression and clinicopathological characteristics in primary
breast cancer.
| Variables | No. of
patients | Mean
CD44+/CD24−/low (%) | P-value |
|---|
| Total | 57 | 4.86 | |
| Age (years) |
| >50 | 34 | 5.68 | 3.19E-04 |
| ≤50 | 23 | 3.65 | |
| Tumor size
(cm) |
| pT1 (≤2.0) | 27 | 4.43 | 0.004925 |
| pT2 (>2.0) | 30 | 4.86 | |
| Lymph node
metastasis |
| pN0 | 30 | 4.51 | |
| pN1 | 9 | 4.15 | 0.415821 |
| pN2 | 10 | 10.00 | 0.517209 |
| pN3 | 8 | 7.27 | 0.000209 |
| Histological
type |
| IDC | 46 | 5.06 | 0.395637 |
| ILC | 11 | 4.06 | |
| Histological
grade |
| G2 | 18 | 3.08 | 4.90E-05 |
| G3 | 39 | 5.69 | |
| Estrogen
receptor |
| Negative | 12 | 6.25 | 0.037009 |
| Positive | 45 | 4.50 | |
| Progesterone
receptor |
| Negative | 16 | 5.42 | 0.016659 |
| Positive | 41 | 4.65 | |
| Ki-67 |
| Negative
(<20%) | 16 | 4.44 | 0.002914 |
| Positive
(≥20%) | 41 | 5.03 | |
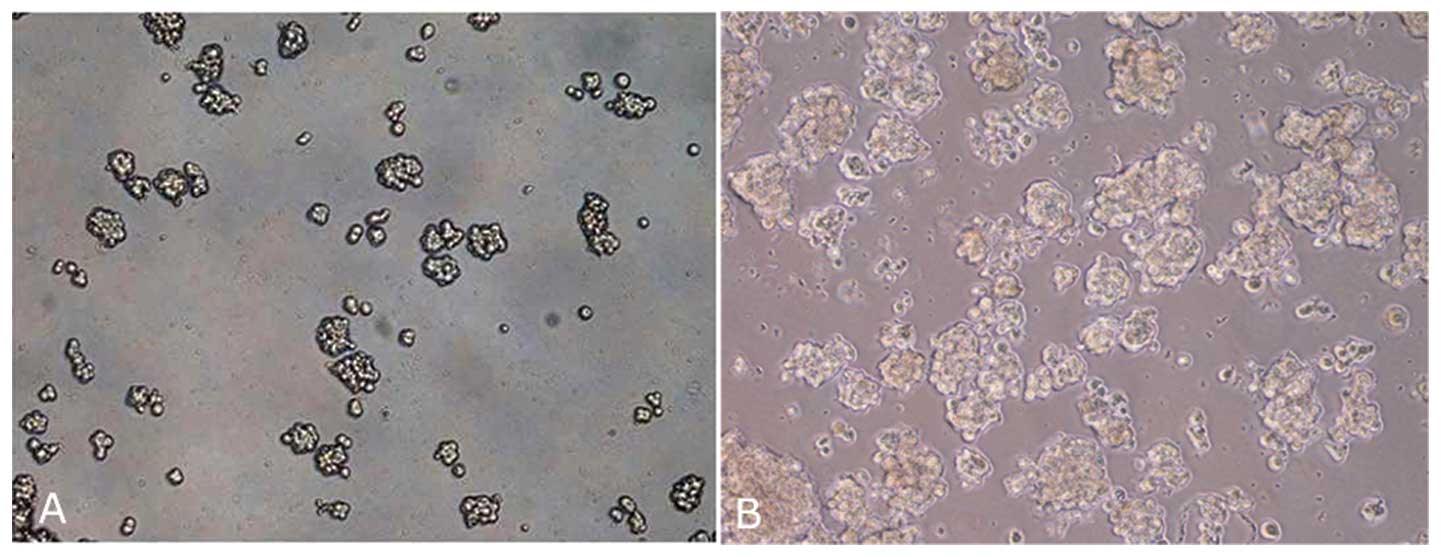
Primary cell culture and mammosphere
formation
Primary cell lines obtained were maintained only for
few passages (from 3 to 5 passages), after which the cells
displayed morphological findings of senescence, namely, enlargement
and flattening. Although mammospheres were obtained for all primary
cell lines, in this case the spheres became senescent between the
second and third culture passage (Fig.
1).
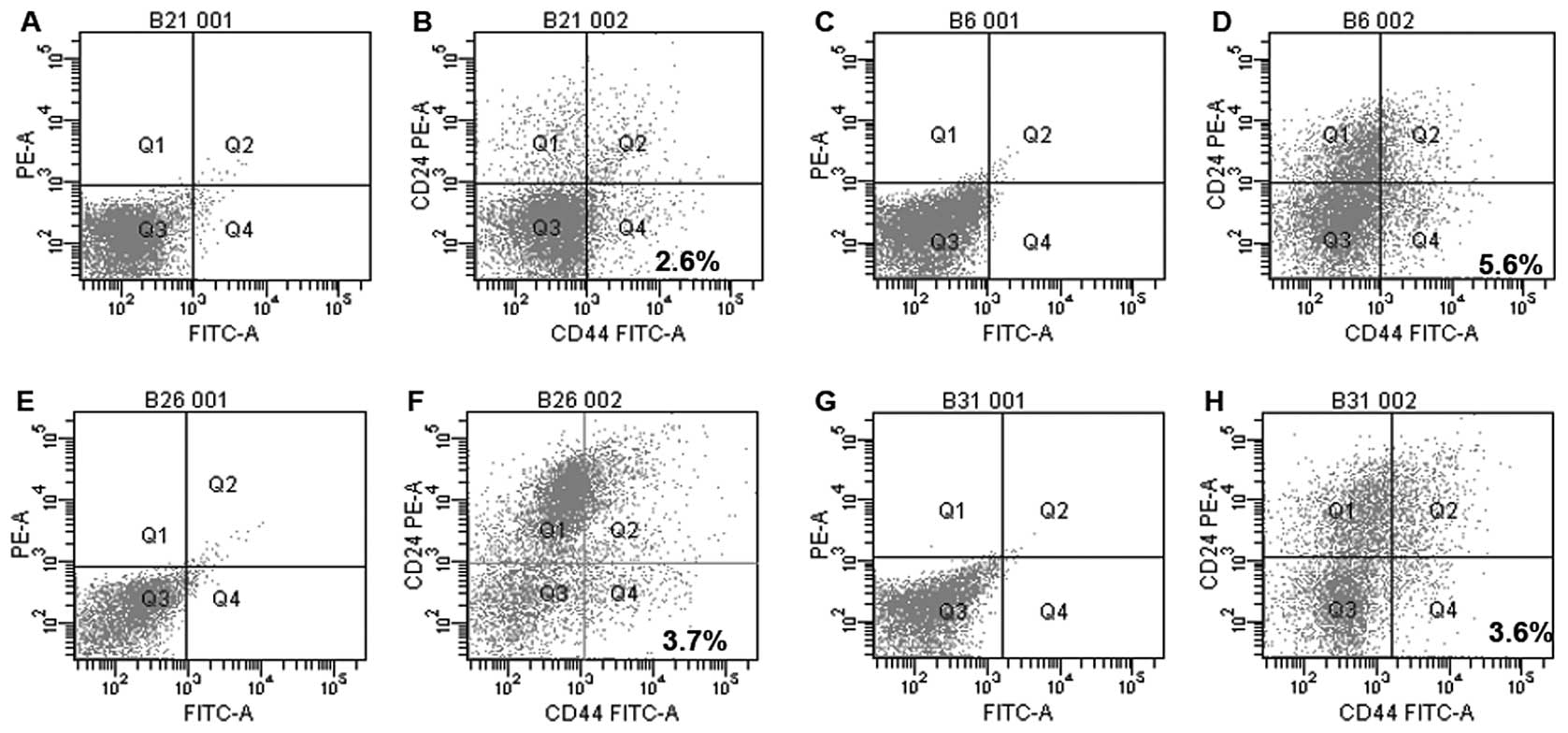
Flow cytometric analysis
Surgical biopsies of IDC and ILC obtained from 57
patients undergoing radical mastectomy were enzymatically
disaggregated and the resulting cell suspensions were analyzed by
flow cytometry to identify the stem phenotypic characteristics of
different cell populations. In order to investigate the possible
presence of a tumor initiating stem cells, we analyzed the
expression of CD44 and CD24 antigens on cell suspensions. All
samples tested, showed CD44+/CD24−/low stem
phenotype with mean percentage of 4.86% of total cell population
(Fig. 2) as confirmed also by
immunohistochemical staining (Fig.
3).
Correlation between
CD44+/CD24−/low, clinical and
histopathological parameters
It has been hypothesized that the CSC content in
tumors may correlate with the more aggressive clinicopathological
features of the disease and clinical parameters and outcome of the
disease. Of the 57 specimens of breast cancer, 57 (100%) were found
to be CD44+/CD24−/low, and these breast
cancers were significantly more likely to be ER+
(P=0.037009), PR+ (P=0.016659), Ki-67+
(P=0.002914) with invasion to lymph nodes (P=0.000209). Statistical
analyses for correlation of CD44+/CD24−/low
expression and clinicopathological parameters in breast cancers
selected for the present study showed that
CD44+/CD24−/low profile was significantly
associated to age (P=3.19E-04). Moreover, significant statistical
association was detectable for tumor size [P=0.004925, tumor grade
(P=4.90E-05) and pN3 (P=0.000209] as showed in Table I and Fig. 4. No correlation was found for
histological type.
CD44+/CD24−/low profile was
found to be strongly expressed in poorly differentiated tumors,
negative for hormone receptors, positive for Ki-67.
Discussion
The hypothesis that the tumors originate from a
small cell population with stem cell characteristics has been
demonstrate by different studies in breast, brain and other solid
tumors (8–13). Studies supporting this theory are
based on xenotransplantation experiments, where human cancer cells
are grown in immunocompromised mice and only CSC generate tumors
and different phenotypes of these cells play a different goal in
the behavior of tumors (7–13). In the present study, we investigate
whether breast cancer could contain CSCs correlating them with
clinicopathological parameters. The recent study of Al-Hajj et
al (8) is believed to have
provided evidence supporting the existence of stem cells in breast
cancer. We have analyzed the presence of CD44 and CD24 antigens in
fresh human breast cancer specimens. In the present study, as
confirmed previously (29),
CD44+/CD24−/low cell subsets were isolated
from the tumor specimens collected using flow cytometry. In
accordance with the concept that CSCs are only a minimal part of
the total tumor cell population (30), we found that the
CD44+/CD24−/low was expressed in a very small
percentage of the total cell population of breast cancer supporting
the hypothesis of the existence of CSCs in breast cancer. CD44 and
CD24 have been shown to regulate invasion and metastasis of breast
cancer cells (23). However, others
have shown inhibition of breast cancer metastasis by these
molecules (31). Association of
CD44+/CD24−/low phenotype with invasion is
correlated with invasive properties of other clinical parameters
such as ER, PR and Ki-67 status.
In the present study, we found that the CSCs ratio
was significantly related to the hormone receptor (HR) status,
Ki-67 status, lymph node status, tumor size, age and especially to
histological grade. The CD44+/CD24−/low cell
population was found to be strongly expressed in young patients
with poorly differentiated tumors, high histological grade, high
tumor size, negative to hormone receptors and high proliferation
rate. For tumor size, although a correlation, statistically
significant is present, no strong difference was detectable. In
addition, no correlation was found for histological type, even
though CD44+/CD24−/low phenotype seems to be
more frequent in patients with invasive ductal carcinoma. Notably,
we found also that CD44+/CD24−/low tumor
cells were associated with the lymph node status pN3. Therefore, we
can assume that the CD44+/CD24−/low
population plays a critical role in metastasis. Metastasis is a
complex process that involves integrated activity of genes, which
function in different steps that include angiogenesis, invasion,
intravasion, survival in circulation, extravasion and homing and
proliferation at sites of metastasis (32,33).
Baumann et al (34) showed
that CD44−/CD24+ tumor cells acquired
enhanced spreading, motility and invasive properties that
facilitated metastasis.
Consequently, this means that the CSCs are not only
able to start the tumor from the transformation of multiple stem
cells and/or progenitor cells in the normal breast, but also to
cause relapse and metastasis. Hence, in this context,
CD44+/CD24−/low cell population could reflect
the characteristics of breast cancer progress, which may be related
to proliferation and invasion of CSCs. Dontu et al (35) suggested a model in which the
transformation of different subsets of stem and progenitor cells
generates the diversity of breast cancer phenotypes, including
estrogen receptor positive (ER+) and negative
(ER−) breast cancer subtypes.
In conclusion, the present study shows new important
insight into breast cancer. In particular, we have confirmed
CD44+/CD24−/low cell population as a
potential breast cancer stem/initiating-cell profile. In addition,
as CD44+/CD24−/low cell subpopulation
correlated with a more aggressive tumor phenotype,
CD44+/CD24−/low profile could be used in
diagnostics, and as a predictive tool indicating poor
prognosis.
Acknowledgements
We thank Dr Alessandra Trocino and the librarians of
the National Cancer Institute, Naples, for providing excellent
bibliographic services and assistance. We thank Dr Virginia Tirino,
Researcher of the Second University of Naples, for providing
excellent technical and scientific support. The present study was
supported by grants from the Current Research of 2011/12 of the
Italian Department of Health to G. Pirozzi.
References
|
1
|
Assi HA, Khoury KE, Dbouk H, Khalil LE,
Mouhieddine TH and El Saghir NS: Epidemiology and prognosis of
breast cancer in young women. J Thorac Dis. 5:S2–S8.
2013.PubMed/NCBI
|
|
2
|
De Santis C, Siegel R, Bandi P and Jemal
A: Breast cancer statistics. CA Cancer J Clin. 61:409–418.
2011.
|
|
3
|
Provenzano E, Brown JP and Pinder SE:
Pathological controversies in breast cancer: classification of
ductal carcinoma in situ, sentinel lymph nodes and low volume
metastatic disease and reporting of neoadjuvant chemotherapy
specimens. Clin Oncol (R Coll Radiol). 25:80–92. 2013.
|
|
4
|
Boyle DP, McCourt CM, Matchett KB and
Salto-Tellez M: Molecular and clinicopathological markers of
prognosis in breast cancer. Expert Rev Mol Diagn. 13:481–498. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tirino V, Desiderio V, Paino F, et al:
Cancer stem cells in solid tumors: an overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
10
|
Fang D, Nguyen TK, Leishear K, et al: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tirino V, Desiderio V, Paino F, et al:
Human primary bone sarcomas contain CD133+ cancer stem
cells displaying high tumorigenicity in vivo. FASEB J.
25:2022–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu A, Feng B, Gu W, Cheng X, Tong T,
Zhang H and Hu Y: The CD133+ subpopulation of the SW982
human synovial sarcoma cell line exhibits cancer stem-like
characteristics. Int J Oncol. 42:1399–1407. 2013.
|
|
13
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Spheroid body-forming cells in the human gastic
cancer cell line MKN-45 possess cancer stem cell properties. Int J
Oncol. 42:453–459. 2013.PubMed/NCBI
|
|
14
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
16
|
Bao B, Ahmad A, Azmi AS, Ali S and Sarkar
FH: Overview of cancer stem cells (CSCs) and mechanisms of their
regulation: implications for cancer therapy. Curr Protoc Pharmacol.
Chapter 14(Unit 14): 252013.PubMed/NCBI
|
|
17
|
Chuthapisith S, Eremin J, El-Sheemey M and
Eremin O: Breast cancer chemoresistance: emerging importance of
cancer stem cells. Surg Oncol. 19:27–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan W, Chen Y, Yao Y, Zhang H and Wang T:
Increased invasion and tumorigenicity capacity of
CD44+/CD24− breast cancer MCF7 cells in
vitro and in nude mice. Cancer Cell Int. 13:622013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Beca FF, Caetano P, Gerhard R,
Alvarenga CA, Gomes M, Paredes J and Schmitt F: Cancer stem cells
markers CD44, CD24 and ALDH1 in breast cancer special histological
types. J Clin Pathol. 66:187–191. 2013.PubMed/NCBI
|
|
20
|
Leth-Larsen R, Terp MG, Christensen AG, et
al: Functional heterogeneity within the CD44 high human breast
cancer stem cell-like compartment reveals a gene signature
predictive of distant metastasis. Mol Med. 18:1109–1121. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei W, Hu H, Tan H, Chow LW, Yip AY and
Loo WT: Relationship of CD44+CD24−/low breast
cancer stem cells and axillary lymph node metastasis. J Transl Med.
10:S1–S6. 2012.
|
|
22
|
Lin Y, Zhong Y, Guan H, Zhang X and Sun Q:
CD44+/CD24− phenotype contributes to
malignant relapse following surgical resection and chemotherapy in
patients with invasive ductal carcinoma. J Exp Clin Cancer Res.
4:31–59. 2012.
|
|
23
|
Sheridan C, Kishimoto H, Fuchs RK, et al:
CD44+/CD24− breast cancer cells exhibit
enhanced invasive properties: an early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
24
|
Abraham BK, Fritz P, McClellan M,
Hauptvogel P, Athelogou M and Brauch H: Prevalence of
CD44+/CD24−/low cells in breast cancer may
not be associated with clinical outcome but may favor distant
metastasis. Clin Cancer Res. 11:1154–1159. 2005.PubMed/NCBI
|
|
25
|
Viale G: The current state of breast
cancer classification. Ann Oncol. 23:207–210. 2012. View Article : Google Scholar
|
|
26
|
Cassol L, Silveira Graudenz M, Zelmanowicz
A, Candela A, Werutsky G, Rovere RK and Garicochea B: Basal-like
immunophenotype markers and prognosis in early breast cancer.
Tumori. 96:966–970. 2010.PubMed/NCBI
|
|
27
|
Lavasani MA and Moinfar F: Molecular
classification of breast carcinomas with particular emphasis on
‘basal-like’ carcinoma: acritical review. J Biophotonics.
5:345–366. 2012.
|
|
28
|
Toft DJ and Cryns VL: Minireview:
basal-like breast cancer: from molecular profiles to targeted
therapies. Mol Endocrinol. 25:199–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehmann C, Jobs G, Thomas M, Burtscher H
and Kubbies M: Established breast cancer stem cell markers do not
correlate with in vivo tumorigenicity of tumor-initiating cells.
Int J Oncol. 41:1932–1942. 2012.PubMed/NCBI
|
|
31
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: an enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seton-Rogers S: Metastasis: signalling in
transit. Nat Rev Cancer. 12:42011.
|
|
33
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baumann P, Cremers N, Kroese F, et al:
CD24 expression causes the acquisition of multiple cellular
properties associated with tumor growth and metastasis. Cancer Res.
65:10783–10793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dontu G, El-Ashry D and Wicha MS: Breast
cancer, stem/progenitor cells and the estrogen receptor.
Trends Endocrinol Metab. 15:193–197. 2004. View Article : Google Scholar
|